Exploring Psoriasis Inflammatory Microenvironment by NanoString Technologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. RNA Extraction and Quantification
2.3. Transcriptomic Analysis with NanoString Technologies
3. Results
3.1. Case Series
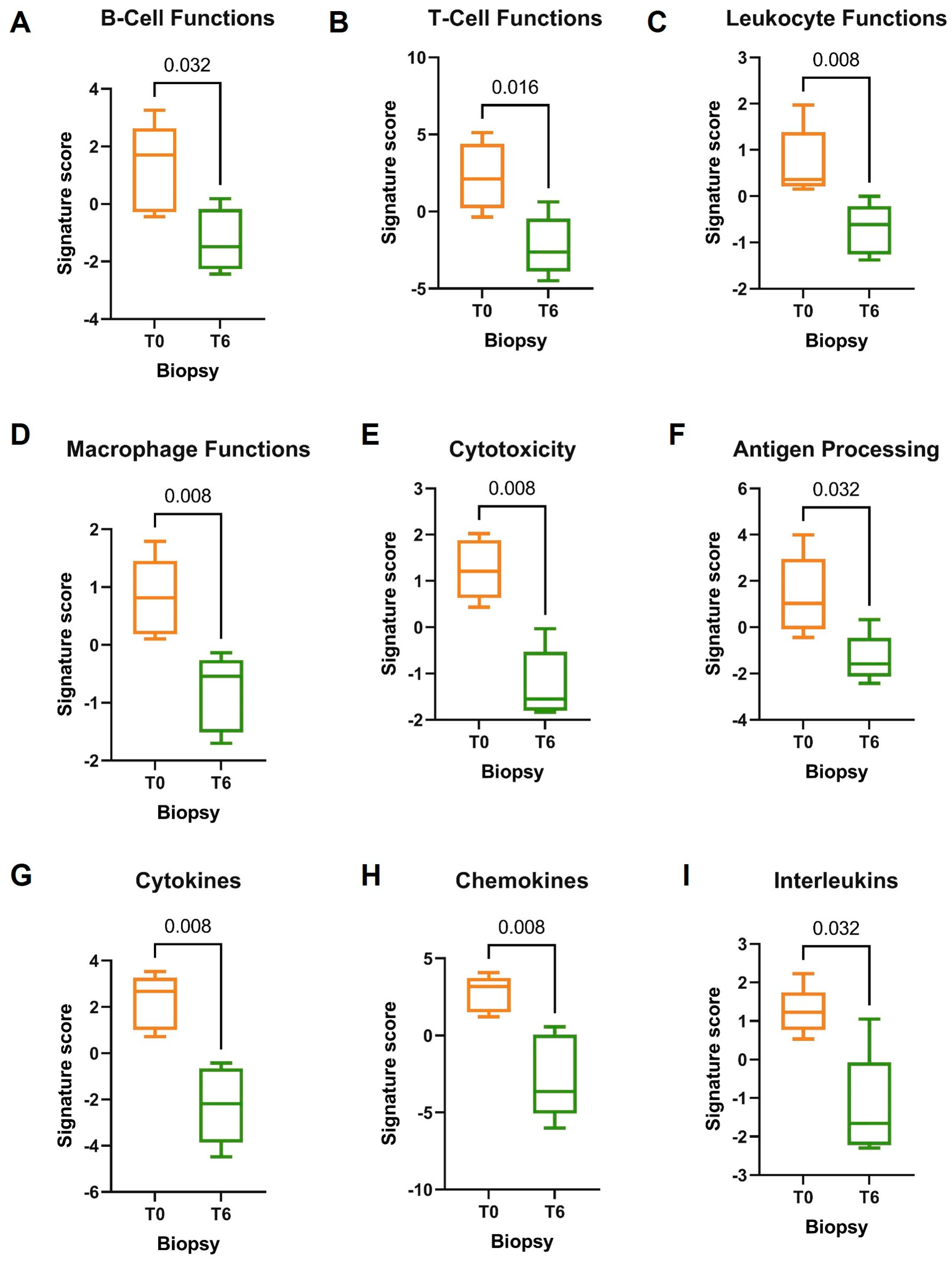
3.2. NanoString Molecular Analysis
3.3. Molecular Profile of DMF-Treated Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meglio, D.; Villanova, F.; Nestle, F.O. Psoriasis. Cold Spring Harb. Perspect. Med. 2014, 4. [Google Scholar] [CrossRef]
- Chandran, V.; Raychaudhuri, S.P. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J. Autoimmun. 2010, 34, J314–J321. [Google Scholar] [CrossRef]
- Griffiths, C.E.; Christophers, E.; Barker, J.N.; Chalmers, R.J.; Chimenti, S.; Krueger, G.G.; Leonardi, C.; Menter, A.; Ortonne, J.P.; Fry, L. A classification of psoriasis vulgaris according to phenotype. Br. J. Dermatol. 2007, 156, 258–262. [Google Scholar] [CrossRef]
- Henseler, T.; Christophers, E. Psoriasis of early and late onset: Characterization of two types of psoriasis vulgaris. J. Am. Acad. Dermatol. 1985, 13, 450–456. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Stuart, P.E.; Tian, C.; Gudjonsson, J.E.; Das, S.; Zawistowski, M.; Ellinghaus, E.; Barker, J.N.; Chandran, V.; Dand, N.; et al. Large scale meta-analysis characterizes genetic architecture for common psoriasis associated variants. Nat. Commun. 2017, 8, 15382. [Google Scholar] [CrossRef]
- Elder, J.T.; Bruce, A.T.; Gudjonsson, J.E.; Johnston, A.; Stuart, P.E.; Tejasvi, T.; Voorhees, J.J.; Abecasis, G.R.; Nair, R.P. Molecular dissection of psoriasis: Integrating genetics and biology. J. Investig. Dermatol. 2010, 130, 1213–1226. [Google Scholar] [CrossRef]
- Kim, G.K.; Del Rosso, J.Q. Drug-provoked psoriasis: Is it drug induced or drug aggravated? Understanding pathophysiology and clinical relevance. J. Clin. Aesthet. Dermatol. 2010, 3, 32–38. [Google Scholar]
- Naldi, L.; Chatenoud, L.; Linder, D.; Fortina, A.B.; Peserico, A.; Virgili, A.R.; Bruni, P.L.; Ingordo, V.; Scocco, G.L.; Solaroli, C.; et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: Results from an italian case-control study. J. Investig. Dermatol. 2005, 125, 61–67. [Google Scholar] [CrossRef]
- Lowes, M.A.; Russell, C.B.; Martin, D.A.; Towne, J.E.; Krueger, J.G. The il-23/t17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013, 34, 174–181. [Google Scholar] [CrossRef]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M.; on behalf of the Global Psoriasis Atlas. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The il-17 family of cytokines in health and disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, C.L.; Kimball, A.B.; Papp, K.A.; Yeilding, N.; Guzzo, C.; Wang, Y.; Li, S.; Dooley, L.T.; Gordon, K.B. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (phoenix 1). Lancet 2008, 371, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Harper, E.G.; Guo, C.; Rizzo, H.; Lillis, J.V.; Kurtz, S.E.; Skorcheva, I.; Purdy, D.; Fitch, E.; Iordanov, M.; Blauvelt, A. Th17 cytokines stimulate ccl20 expression in keratinocytes in vitro and in vivo: Implications for psoriasis pathogenesis. J. Investig. Dermatol. 2009, 129, 2175–2183. [Google Scholar] [CrossRef]
- Quesada, J.R.; Gutterman, J.U. Psoriasis and alpha-interferon. Lancet 1986, 1, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Fruh, K.; Yang, Y. Antigen presentation by mhc class i and its regulation by interferon gamma. Curr. Opin. Immunol. 1999, 11, 76–81. [Google Scholar] [CrossRef]
- Henry, C.M.; Sullivan, G.P.; Clancy, D.M.; Afonina, I.S.; Kulms, D.; Martin, S.J. Neutrophil-derived proteases escalate inflammation through activation of il-36 family cytokines. Cell Rep. 2016, 14, 708–722. [Google Scholar] [CrossRef]
- Xing, X.; Liang, Y.; Sarkar, M.K.; Wolterink, L.; Swindell, W.R.; Voorhees, J.J.; Harms, P.W.; Kahlenberg, J.M.; Johnston, A.; Gudjonsson, J.E. Il-17 responses are the dominant inflammatory signal linking inverse, erythrodermic, and chronic plaque psoriasis. J. Investig. Dermatol. 2016, 136, 2498–2501. [Google Scholar] [CrossRef]
- Nestle, F.O.; Conrad, C.; Tun-Kyi, A.; Homey, B.; Gombert, M.; Boyman, O.; Burg, G.; Liu, Y.J.; Gilliet, M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005, 202, 135–143. [Google Scholar] [CrossRef]
- Johnston, A.; Xing, X.; Wolterink, L.; Barnes, D.H.; Yin, Z.; Reingold, L.; Kahlenberg, J.M.; Harms, P.W.; Gudjonsson, J.E. Il-1 and il-36 are dominant cytokines in generalized pustular psoriasis. J. Allergy Clin. Immunol. 2017, 140, 109–120. [Google Scholar] [CrossRef]
- Kryczek, I.; Bruce, A.T.; Gudjonsson, J.E.; Johnston, A.; Aphale, A.; Vatan, L.; Szeliga, W.; Wang, Y.; Liu, Y.; Welling, T.H.; et al. Induction of il-17+ t cell trafficking and development by ifn-gamma: Mechanism and pathological relevance in psoriasis. J. Immunol. 2008, 181, 4733–4741. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Wang, Y.; Xiao, Y.; Li, W. Applications of single-cell rna sequencing in atopic dermatitis and psoriasis. Front. Immunol. 2022, 13, 1038744. [Google Scholar] [CrossRef]
- Amatore, F.; Villani, A.P.; Tauber, M.; Viguier, M.; Guillot, B.; Psoriasis Research Group of the French Society of Dermatology. French guidelines on the use of systemic treatments for moderate-to-severe psoriasis in adults. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 464–483. [Google Scholar] [CrossRef] [PubMed]
- Allegra, C.J.; Drake, J.C.; Jolivet, J.; Chabner, B.A. Inhibition of phosphoribosylaminoimidazolecarboxamide transformylase by methotrexate and dihydrofolic acid polyglutamates. Proc. Natl. Acad. Sci. USA 1985, 82, 4881–4885. [Google Scholar] [CrossRef] [PubMed]
- Belge, K.; Bruck, J.; Ghoreschi, K. Advances in treating psoriasis. F1000Prime Rep. 2014, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Brownstone, N.D.; Hong, J.; Mosca, M.; Hadeler, E.; Liao, W.; Bhutani, T.; Koo, J. Biologic treatments of psoriasis: An update for the clinician. Biologics 2021, 15, 39–51. [Google Scholar] [CrossRef]
- Gladman, D.D.; Antoni, C.; Mease, P.; Clegg, D.O.; Nash, P. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann. Rheum. Dis. 2005, 64 (Suppl. S2), ii14–ii17. [Google Scholar] [CrossRef]
- Sulaimani, J.; Cluxton, D.; Clowry, J.; Petrasca, A.; Molloy, O.E.; Moran, B.; Sweeney, C.M.; Malara, A.; McNicholas, N.; McGuigan, C.; et al. Dimethyl fumarate modulates the treg-th17 cell axis in patients with psoriasis. Br. J. Dermatol. 2021, 184, 495–503. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Bruck, J.; Kellerer, C.; Deng, C.; Peng, H.; Rothfuss, O.; Hussain, R.Z.; Gocke, A.R.; Respa, A.; Glocova, I.; et al. Fumarates improve psoriasis and multiple sclerosis by inducing type ii dendritic cells. J. Exp. Med. 2011, 208, 2291–2303. [Google Scholar] [CrossRef]
- Mrowietz, U.; Morrison, P.J.; Suhrkamp, I.; Kumanova, M.; Clement, B. The pharmacokinetics of fumaric acid esters reveal their in vivo effects. Trends Pharmacol. Sci. 2018, 39, 1–12. [Google Scholar] [CrossRef]
- Burlando, M.; Campione, E.; Cuccia, A.; Malara, G.; Naldi, L.; Prignano, F.; Zichichi, L. Real-world use of dimethyl fumarate in patients with plaque psoriasis: A delphi-based expert consensus. Dermatol. Rep. 2023, 15, 9613. [Google Scholar] [CrossRef]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast cells and neutrophils release il-17 through extracellular trap formation in psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, K.; Dudeck, A.; Hasenberg, M.; Nye, E.; van Rooijen, N.; Hartmann, K.; Gunzer, M.; Roers, A.; Hogg, N. Mast cell and macrophage chemokines cxcl1/cxcl2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013, 121, 4930–4937. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, S.; Gardiner, C.M. Nk cells and psoriasis. J. Biomed. Biotechnol. 2011, 2011, 248317. [Google Scholar] [CrossRef] [PubMed]
- Kamata, M.; Tada, Y. Dendritic cells and macrophages in the pathogenesis of psoriasis. Front. Immunol. 2022, 13, 941071. [Google Scholar] [CrossRef]
- Kapp, A.; Wokalek, H.; Schopf, E. Involvement of complement in psoriasis and atopic dermatitis--measurement of c3a and c5a, c3, c4 and c1 inactivator. Arch. Dermatol. Res. 1985, 277, 359–361. [Google Scholar] [CrossRef]
- Singh, S.; Kalb, R.E.; de Jong, E.; Shear, N.H.; Lebwohl, M.; Langholff, W.; Hopkins, L.; Srivastava, B.; Armstrong, A.W. Effect of age of onset of psoriasis on clinical outcomes with systemic treatment in the psoriasis longitudinal assessment and registry (psolar). Am. J. Clin. Dermatol. 2018, 19, 879–886. [Google Scholar] [CrossRef]
- Litjens, N.H.; Rademaker, M.; Ravensbergen, B.; Rea, D.; van der Plas, M.J.; Thio, B.; Walding, A.; van Dissel, J.T.; Nibbering, P.H. Monomethylfumarate affects polarization of monocyte-derived dendritic cells resulting in down-regulated th1 lymphocyte responses. Eur. J. Immunol. 2004, 34, 565–575. [Google Scholar] [CrossRef]
- Treumer, F.; Zhu, K.; Glaser, R.; Mrowietz, U. Dimethylfumarate is a potent inducer of apoptosis in human t cells. J. Investig. Dermatol. 2003, 121, 1383–1388. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.Q.; Cheng, J.; Hui, R.S.; Gao, T.W. Increased th17 cells are accompanied by foxp3(+) treg cell accumulation and correlated with psoriasis disease severity. Clin. Immunol. 2010, 135, 108–117. [Google Scholar] [CrossRef]
- Bovenschen, H.J.; van Vlijmen-Willems, I.M.; van de Kerkhof, P.C.; van Erp, P.E. Identification of lesional cd4+ cd25+ foxp3+ regulatory t cells in psoriasis. Dermatology 2006, 213, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Yun, W.J.; Lee, D.W.; Chang, S.E.; Yoon, G.S.; Huh, J.R.; Won, C.H.; Lee, M.W.; Kim, S.E.; Kim, B.J.; Moon, K.C.; et al. Role of cd4cd25foxp3 regulatory t cells in psoriasis. Ann. Dermatol. 2010, 22, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.X.; Fang, X.; Han, L.; Zhang, Z.H.; Kang, K.F.; Zheng, Z.Z.; Huang, Q. Foxp3+ regulatory t cells and related cytokines differentially expressed in plaque vs. Guttate psoriasis vulgaris. Br. J. Dermatol. 2010, 163, 48–56. [Google Scholar] [CrossRef] [PubMed]
- van Hezik, D.F.C.; Bovenschen, H.J. Association of lymphopenia and eosinophilia with dimethylfumarate treatment efficacy and tolerability in psoriasis: A retrospective study. J. Dermatol. Treat. 2020, 31, 378–381. [Google Scholar] [CrossRef]
- Wain, E.M.; Darling, M.I.; Pleass, R.D.; Barker, J.N.; Smith, C.H. Treatment of severe, recalcitrant, chronic plaque psoriasis with fumaric acid esters: A prospective study. Br. J. Dermatol. 2010, 162, 427–434. [Google Scholar] [CrossRef]
- Hoxtermann, S.; Nuchel, C.; Altmeyer, P. Fumaric acid esters suppress peripheral cd4- and cd8-positive lymphocytes in psoriasis. Dermatology 1998, 196, 223–230. [Google Scholar] [CrossRef]
- Harries, M.J.; Chalmers, R.J.; Griffiths, C.E. Fumaric acid esters for severe psoriasis: A retrospective review of 58 cases. Br. J. Dermatol. 2005, 153, 549–551. [Google Scholar] [CrossRef]

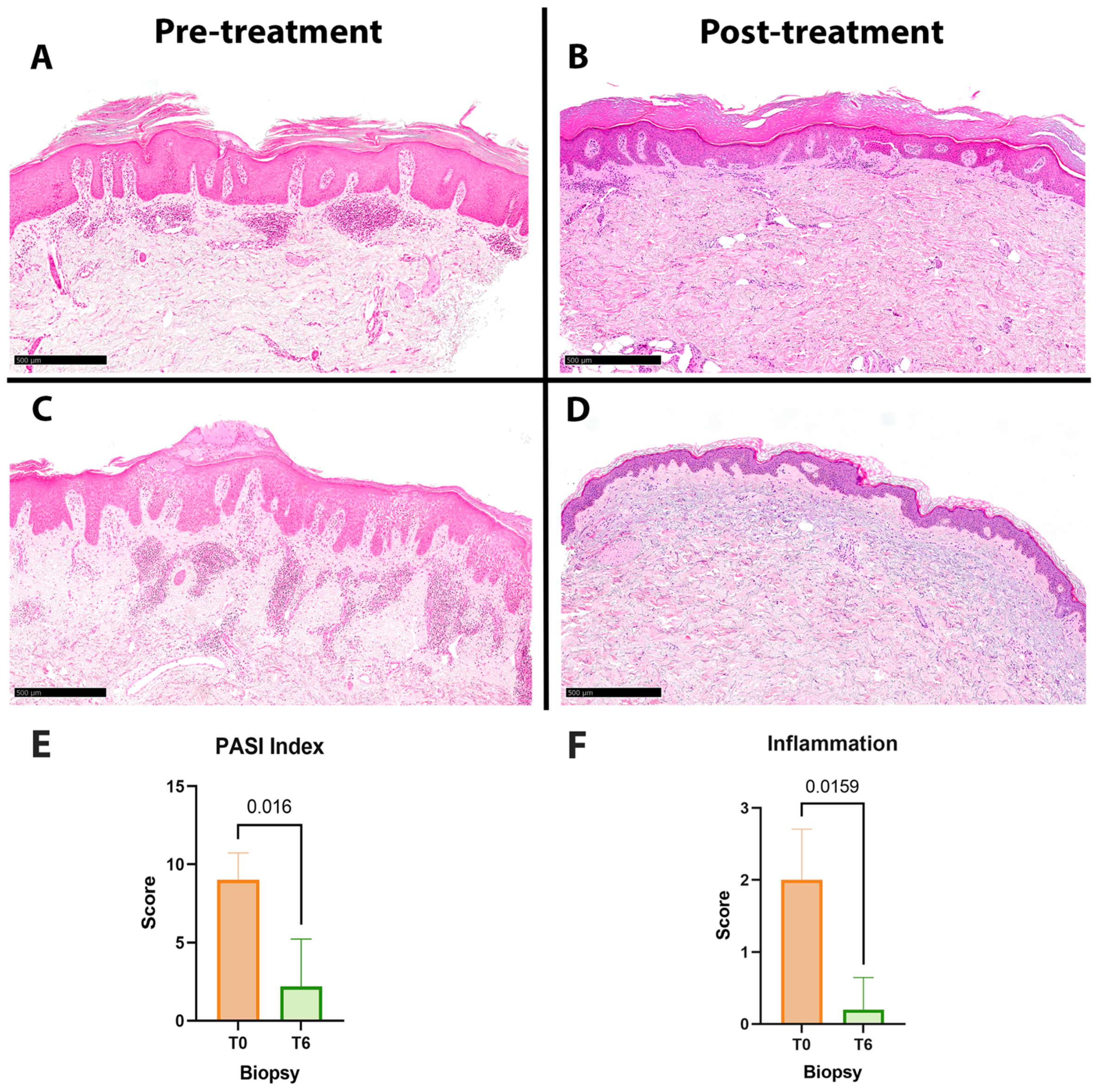

| Age | Sex | Involved Sites | T0 PASI | T6 PASI | T0 Inflammation Score | T6 Inflammation Score | |
|---|---|---|---|---|---|---|---|
| Patient 1 | 40 | F | Face, scalp | 6 | 0 | 1 | 0 |
| Patient 2 | 75 | M | Face, buttock, scalp, lower limbs | 10 | 0 | 2 | 0 |
| Patient 3 | 56 | M | Genitals, scalp, upper limbs, lower limbs | 10 | 5 | 2 | 1 |
| Patient 4 | 43 | F | Trunk, buttock, upper limbs | 10 | 0 | 3 | 0 |
| Patient 5 | 60 | M | Back, plantar | 9 | 6 | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, A.A.; Dapavo, P.; Mastorino, L.; Roccuzzo, G.; Wolff, S.; Ribero, S.; Cassoni, P.; Senetta, R.; Quaglino, P. Exploring Psoriasis Inflammatory Microenvironment by NanoString Technologies. J. Clin. Med. 2023, 12, 6820. https://doi.org/10.3390/jcm12216820
Ricci AA, Dapavo P, Mastorino L, Roccuzzo G, Wolff S, Ribero S, Cassoni P, Senetta R, Quaglino P. Exploring Psoriasis Inflammatory Microenvironment by NanoString Technologies. Journal of Clinical Medicine. 2023; 12(21):6820. https://doi.org/10.3390/jcm12216820
Chicago/Turabian StyleRicci, Alessia Andrea, Paolo Dapavo, Luca Mastorino, Gabriele Roccuzzo, Samanta Wolff, Simone Ribero, Paola Cassoni, Rebecca Senetta, and Pietro Quaglino. 2023. "Exploring Psoriasis Inflammatory Microenvironment by NanoString Technologies" Journal of Clinical Medicine 12, no. 21: 6820. https://doi.org/10.3390/jcm12216820





