CT Imaging Assessment of Pancreatic Adenocarcinoma Resectability after Neoadjuvant Therapy: Current Status and Perspective on the Use of Radiomics
Abstract
:1. Introduction
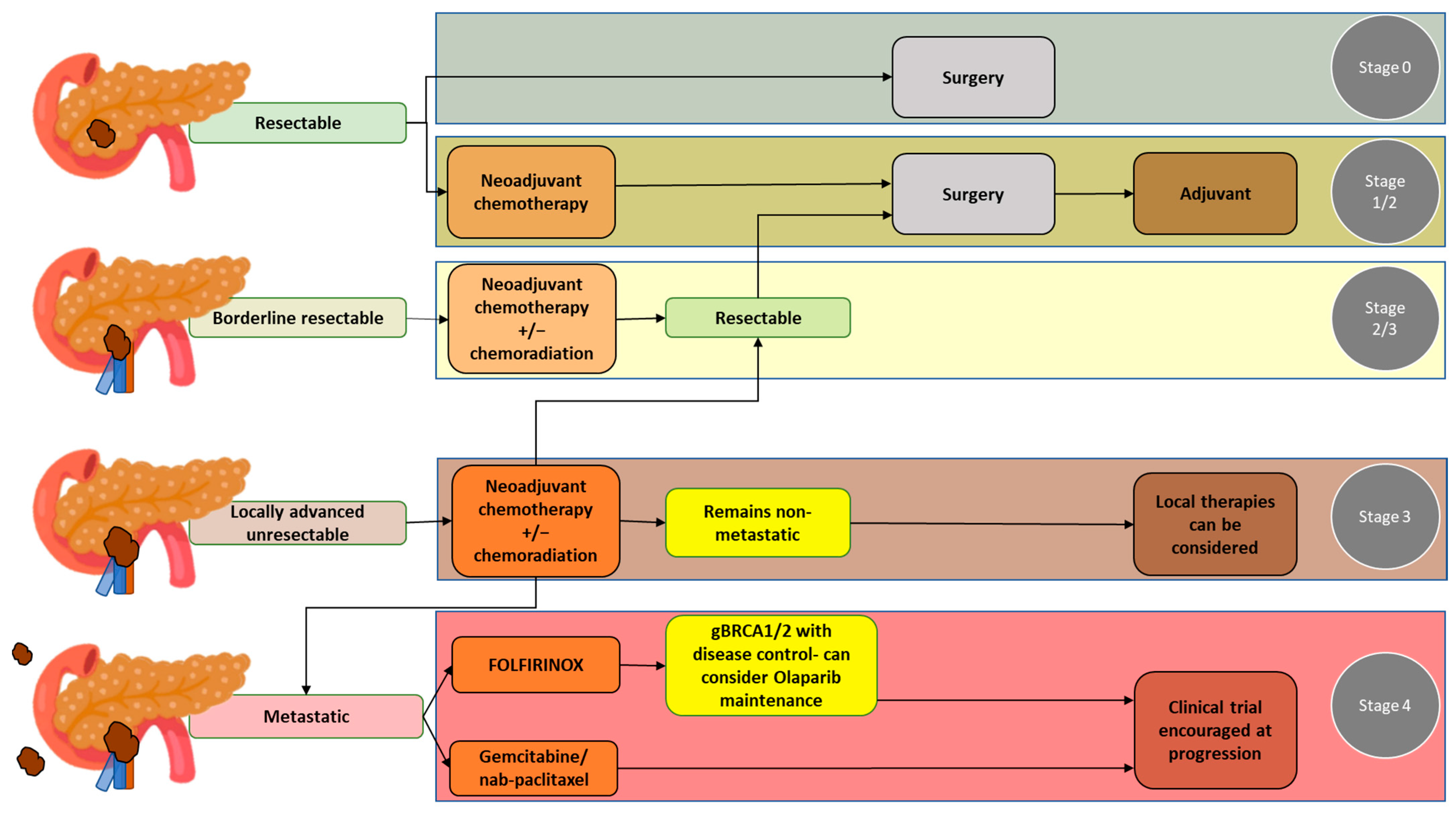
2. Overview of the Treatment of PDAC
2.1. Resectable PDAC
2.2. Borderline Resectable PDAC
2.3. Locally Advanced PDAC
2.4. Metastatic PDAC
3. Challenges to CT-Based Assessment of PDAC Treatment Response following Neoadjuvant Therapy
4. Literature Search Strategy
5. Semantic CT Imaging Features for the Assessment of PDAC Treatment Response after Neoadjuvant Therapy
6. Radiomics as a Valuable Tool to Aid in the Assessment of PDAC Treatment Response after Neoadjuvant Therapy
6.1. Pipeline of Studies Using Radiomics
- (a)
- Pre-execution: In this step, the research team determines the study design, the involvement of human subjects, the imaging modalities that will be used, and the clinically relevant endpoints that will be studied. This step aims to determine the feasibility of the study, including ensuring that sufficient high-quality data will be obtained.
- (b)
- Data curation: In this step, clinical data, imaging data, and other relevant metadata are collected and managed according to the needs of the study. Of note, image anonymization is a key aspect of data curation; proper image anonymization is not only crucial to maintaining patient/participant privacy, but it is also an essential characteristic of high-quality data.
- (c)
- Image segmentation: In this step, tumors, peritumoral or tumor subregions (also known as tumor habitats), 2D regions of interest, or 3D volumes of interest are segmented, whether manually, automatically, or semi-automatically. This step aims to provide a representative area or volume to be assessed.
- (d)
- Image pre-processing: This step involves the application of a filter, normalization, resampling, and/or thresholding, aiming to increase standardization and/or improve image quality.
- (e)
- Feature extraction: In this step, different classes of features are extracted from the segmented area or volume, including morphological features (i.e., visual characteristics and shape), first-order or histogram-based features (i.e., features describing the distribution of the pixel intensities within the segmented area), and second-order textural features (i.e., features capturing the spatial relationship between pixels intensities within the segmented area or volume).
- (f)
- Feature selection: In this step, from all the extracted features, those features that are stable, informative, and non-redundant are selected for model building.
- (g)
- Model building: Model building is a crucial step, in which a radiomic model is developed using the selected radiomic features to enable realistic and accurate diagnosis, prognosis, or response prediction. During this step, the comparison of the performance of the radiomic model against that of clinically used tools is helpful for determining the added value of the radiomic model.
- (h)
- Validation: This step involves assessing the performance and generalizability of the developed radiomic model by applying it to an independent dataset, ideally from a different institution. This step ensures model robustness and effectiveness before potential real-world implementation.
6.2. Studies Applying Radiomics in the Assessment of PDAC Treatment Response after Neoadjuvant Therapy
7. Current Recommendations for the Management of PDAC after Neoadjuvant Therapy
- -
- CT is currently not sufficiently accurate to predict R0 resection.
- -
- Imaging frequently underestimates resectability following neoadjuvant therapy.
- -
- Favorable imaging findings after neoadjuvant therapy include partial regression of tumor contact with peripancreatic vessels, a mild fat-stranding perivascular halo in place of solid tumor contact with a vessel, and reduction in tumor size according to RECIST 1.1 guidelines.
- -
- Surgery after neoadjuvant therapy should be considered even if imaging findings are unchanged from those at primary staging.
- -
- CT is more accurate for evaluating venous involvement than for arterial involvement after neoadjuvant therapy. Decreased venous stenosis or decreased contour deformation indicates improved venous involvement.
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef]
- National Cancer Institute. Cancer Stat Facts: Pancreatic Cancer; DCCPS, Surveillance Research Program; National Cancer Institute: Bethesda, MD, USA, 2023. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 17 August 2023).
- Barnes, C.A.; Chavez, M.I.; Tsai, S.; Aldakkak, M.; George, B.; Ritch, P.S.; Dua, K.; Clarke, C.N.; Tolat, P.; Hagen, C.; et al. Survival of patients with borderline resectable pancreatic cancer who received neoadjuvant therapy and surgery. Surgery 2019, 166, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France. Available online: https://gco.iarc.fr/today (accessed on 17 August 2023).
- Ferlay, J.; Partensky, C.; Bray, F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016, 55, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.B.; Rocha, F.G.; Alseidi, A.; Biehl, T.; Moonka, R.; Ryan, J.A.; Lin, B.; Picozzi, V.; Helton, S. Extended neoadjuvant chemotherapy for borderline resectable pancreatic cancer demonstrates promising postoperative outcomes and survival. Ann. Surg. Oncol. 2014, 21, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv. Nutr. 2016, 7, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Di Sebastiano, P.; Grottola, T.; di Mola, F.F. Borderline resectable pancreatic cancer and the role of neoadjuvant chemoradiotherapy. Updates Surg. 2016, 68, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Castells, A.; Ayuso, C.; Ayuso, J.R.; de Caralt, M.T.; Ginès, M.A.; Real, M.I.; Gilabert, R.; Quintó, L.; Trilla, A.; et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: Prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am. J. Gastroenterol. 2004, 99, 492–501. [Google Scholar] [CrossRef]
- Janssen, Q.P.; Buettner, S.; Suker, M.; Beumer, B.R.; Addeo, P.; Bachellier, P.; Bahary, N.; Bekaii-Saab, T.; Bali, M.A.; Besselink, M.G.; et al. Neoadjuvant FOLFIRINOX in Patients with Borderline Resectable Pancreatic Cancer: A Systematic Review and Patient-Level Meta-Analysis. J. Natl. Cancer Inst. 2019, 111, 782–794. [Google Scholar] [CrossRef]
- Kulkarni, N.M.; Mannelli, L.; Zins, M.; Bhosale, P.R.; Arif-Tiwari, H.; Brook, O.R.; Hecht, E.M.; Kastrinos, F.; Wang, Z.J.; Soloff, E.V.; et al. White paper on pancreatic ductal adenocarcinoma from society of abdominal radiology’s disease-focused panel for pancreatic ductal adenocarcinoma: Part II, update on imaging techniques and screening of pancreatic cancer in high-risk individuals. Abdom. Radiol. 2020, 45, 729–742. [Google Scholar] [CrossRef]
- Takahashi, S.; Ohno, I.; Ikeda, M.; Konishi, M.; Kobayashi, T.; Akimoto, T.; Kojima, M.; Morinaga, S.; Toyama, H.; Shimizu, Y.; et al. Neoadjuvant S-1 With Concurrent Radiotherapy Followed by Surgery for Borderline Resectable Pancreatic Cancer: A Phase II Open-label Multicenter Prospective Trial (JASPAC05). Ann. Surg. 2022, 276, e510–e517. [Google Scholar] [CrossRef] [PubMed]
- Tamm, E.P.; Loyer, E.M.; Faria, S.; Raut, C.P.; Evans, D.B.; Wolff, R.A.; Crane, C.H.; Dubrow, R.A.; Charnsangavej, C. Staging of pancreatic cancer with multidetector CT in the setting of preoperative chemoradiation therapy. Abdom. Imaging 2006, 31, 568–574. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Pancreatic Adenocarcinoma. Version 2. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 15 July 2023).
- Al-Hawary, M.M.; Francis, I.R.; Chari, S.T.; Fishman, E.K.; Hough, D.M.; Lu, D.S.; Macari, M.; Megibow, A.J.; Miller, F.H.; Mortele, K.J.; et al. Pancreatic ductal adenocarcinoma radiology reporting template: Consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014, 270, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Windsor, J.A.; Barreto, S.G. The concept of ‘borderline resectable’ pancreatic cancer: Limited foundations and limited future? J. Gastrointest. Oncol. 2017, 8, 189–193. [Google Scholar] [CrossRef]
- Balthazar, E.J. Pancreatitis associated with pancreatic carcinoma. Preoperative diagnosis: Role of CT imaging in detection and evaluation. Pancreatology 2005, 5, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Bassi, C.; Ghaneh, P.; Cunningham, D.; Goldstein, D.; Padbury, R.; Moore, M.J.; Gallinger, S.; Mariette, C.; et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. JAMA 2010, 304, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Castan, F.; Lopez, A.; Turpin, A.; Ben Abdelghani, M.; Wei, A.C.; Mitry, E.; Biagi, J.J.; Evesque, L.; Artru, P.; et al. Five-Year Outcomes of FOLFIRINOX vs Gemcitabine as Adjuvant Therapy for Pancreatic Cancer: A Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1571–1578. [Google Scholar] [CrossRef]
- Versteijne, E.; van Dam, J.L.; Suker, M.; Janssen, Q.P.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J. Clin. Oncol. 2022, 40, 1220–1230. [Google Scholar] [CrossRef]
- Dhir, M.; Malhotra, G.K.; Sohal, D.P.S.; Hein, N.A.; Smith, L.M.; O’Reilly, E.M.; Bahary, N.; Are, C. Neoadjuvant treatment of pancreatic adenocarcinoma: A systematic review and meta-analysis of 5520 patients. World J. Surg. Oncol. 2017, 15, 183. [Google Scholar] [CrossRef]
- Brown, Z.J.; Heh, V.; Labiner, H.E.; Brock, G.N.; Ejaz, A.; Dillhoff, M.; Tsung, A.; Pawlik, T.M.; Cloyd, J.M. Surgical resection rates after neoadjuvant therapy for localized pancreatic ductal adenocarcinoma: Meta-analysis. Br. J. Surg. 2022, 110, 34–42. [Google Scholar] [CrossRef]
- Yang, B.; Chen, K.; Liu, W.; Long, D.; Wang, Y.; Liu, X.; Ma, Y.; Tian, X.; Yang, Y. The benefits of neoadjuvant therapy for patients with resectable pancreatic cancer: An updated systematic review and meta-analysis. Clin. Exp. Med. 2023. [Google Scholar] [CrossRef]
- Ratnayake, B.; Savastyuk, A.Y.; Nayar, M.; Wilson, C.H.; Windsor, J.A.; Roberts, K.; French, J.J.; Pandanaboyana, S. Recurrence Patterns for Pancreatic Ductal Adenocarcinoma after Upfront Resection Versus Resection Following Neoadjuvant Therapy: A Comprehensive Meta-Analysis. J. Clin. Med. 2020, 9, 2132. [Google Scholar] [CrossRef]
- Lindemann, J.; du Toit, L.; Kotze, U.; Bernon, M.; Krige, J.; Jonas, E. Survival equivalence in patients treated for borderline resectable and unresectable locally advanced pancreatic ductal adenocarcinoma: A systematic review and network meta-analysis. HPB 2021, 23, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; de Hingh, I.; Homs, M.Y.V.; Intven, M.P.W.; Klaase, J.M.; van Santvoort, H.C.; de Vos-Geelen, J.; Wilmink, J.W.; van Tienhoven, G. Neoadjuvant Treatment for Resectable and Borderline Resectable Pancreatic Cancer: Chemotherapy or Chemoradiotherapy? Front. Oncol. 2021, 11, 744161. [Google Scholar] [CrossRef] [PubMed]
- Motoi, F.; Unno, M. Adjuvant and neoadjuvant treatment for pancreatic adenocarcinoma. Jpn. J. Clin. Oncol. 2020, 50, 483–489. [Google Scholar] [CrossRef]
- Ferrone, C.R.; Marchegiani, G.; Hong, T.S.; Ryan, D.P.; Deshpande, V.; McDonnell, E.I.; Sabbatino, F.; Santos, D.D.; Allen, J.N.; Blaszkowsky, L.S.; et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann. Surg. 2015, 261, 12–17. [Google Scholar] [CrossRef]
- Chen, X.; Oshima, K.; Schott, D.; Wu, H.; Hall, W.; Song, Y.; Tao, Y.; Li, D.; Zheng, C.; Knechtges, P.; et al. Assessment of treatment response during chemoradiation therapy for pancreatic cancer based on quantitative radiomic analysis of daily CTs: An exploratory study. PLoS ONE 2017, 12, e0178961. [Google Scholar] [CrossRef]
- Barreto, S.G.; Loveday, B.; Windsor, J.A.; Pandanaboyana, S. Detecting tumour response and predicting resectability after neoadjuvant therapy for borderline resectable and locally advanced pancreatic cancer. ANZ J. Surg. 2019, 89, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H.; Fleming, J.B.; Bhosale, P.; Varadhachary, G.; Lee, J.E.; Wolff, R.; Wang, H.; Abbruzzese, J.; Pisters, P.W.; Vauthey, J.N.; et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012, 118, 5749–5756. [Google Scholar] [CrossRef]
- Kim, Y.E.; Park, M.S.; Hong, H.S.; Kang, C.M.; Choi, J.Y.; Lim, J.S.; Lee, W.J.; Kim, M.J.; Kim, K.W. Effects of neoadjuvant combined chemotherapy and radiation therapy on the CT evaluation of resectability and staging in patients with pancreatic head cancer. Radiology 2009, 250, 758–765. [Google Scholar] [CrossRef]
- Cassinotto, C.; Cortade, J.; Belleannée, G.; Lapuyade, B.; Terrebonne, E.; Vendrely, V.; Laurent, C.; Sa-Cunha, A. An evaluation of the accuracy of CT when determining resectability of pancreatic head adenocarcinoma after neoadjuvant treatment. Eur. J. Radiol. 2013, 82, 589–593. [Google Scholar] [CrossRef]
- Wagner, M.; Antunes, C.; Pietrasz, D.; Cassinotto, C.; Zappa, M.; Sa Cunha, A.; Lucidarme, O.; Bachet, J.B. CT evaluation after neoadjuvant FOLFIRINOX chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur. Radiol. 2017, 27, 3104–3116. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.M.; Zaid, M.; Chaudhury, B.; Elganainy, D.; Lee, Y.; Wilke, C.T.; Cloyd, J.; Wang, H.; Maitra, A.; Wolff, R.A.; et al. Imaging-based biomarkers: Changes in the tumor interface of pancreatic ductal adenocarcinoma on computed tomography scans indicate response to cytotoxic therapy. Cancer 2018, 124, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, G.; Todaro, V.; Boninsegna, E.; Negrelli, R.; Sureka, B.; Bonamini, D.; Salvia, R.; Manfredi, R.; Pozzi Mucelli, R.; Bassi, C. Surgery after FOLFIRINOX treatment for locally advanced and borderline resectable pancreatic cancer: Increase in tumour attenuation on CT correlates with R0 resection. Eur. Radiol. 2018, 28, 4265–4273. [Google Scholar] [CrossRef]
- Kim, B.R.; Kim, J.H.; Ahn, S.J.; Joo, I.; Choi, S.Y.; Park, S.J.; Han, J.K. CT prediction of resectability and prognosis in patients with pancreatic ductal adenocarcinoma after neoadjuvant treatment using image findings and texture analysis. Eur. Radiol. 2019, 29, 362–372. [Google Scholar] [CrossRef]
- Wei, D.; Zaid, M.M.; Katz, M.H.; Prakash, L.R.; Kim, M.; Tzeng, C.D.; Lee, J.E.; Agrawal, A.; Rashid, A.; Wang, H.; et al. Clinicopathological correlation of radiologic measurement of post-therapy tumor size and tumor volume for pancreatic ductal adenocarcinoma. Pancreatology 2021, 21, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Perri, G.; Prakash, L.; Wang, H.; Bhosale, P.; Varadhachary, G.R.; Wolff, R.; Fogelman, D.; Overman, M.; Pant, S.; Javle, M.; et al. Radiographic and Serologic Predictors of Pathologic Major Response to Preoperative Therapy for Pancreatic Cancer. Ann. Surg. 2021, 273, 806–813. [Google Scholar] [CrossRef]
- Jang, J.K.; Byun, J.H.; Kang, J.H.; Son, J.H.; Kim, J.H.; Lee, S.S.; Kim, H.J.; Yoo, C.; Kim, K.P.; Hong, S.M.; et al. CT-determined resectability of borderline resectable and unresectable pancreatic adenocarcinoma following FOLFIRINOX therapy. Eur. Radiol. 2021, 31, 813–823. [Google Scholar] [CrossRef]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef]
- Attiyeh, M.A.; Chakraborty, J.; Doussot, A.; Langdon-Embry, L.; Mainarich, S.; Gönen, M.; Balachandran, V.P.; D’Angelica, M.I.; DeMatteo, R.P.; Jarnagin, W.R.; et al. Survival Prediction in Pancreatic Ductal Adenocarcinoma by Quantitative Computed Tomography Image Analysis. Ann. Surg. Oncol. 2018, 25, 1034–1042. [Google Scholar] [CrossRef]
- Khalvati, F.; Zhang, Y.; Baig, S.; Lobo-Mueller, E.M.; Karanicolas, P.; Gallinger, S.; Haider, M.A. Prognostic Value of CT Radiomic Features in Resectable Pancreatic Ductal Adenocarcinoma. Sci. Rep. 2019, 9, 5449. [Google Scholar] [CrossRef]
- Shur, J.D.; Doran, S.J.; Kumar, S.; Ap Dafydd, D.; Downey, K.; O’Connor, J.P.B.; Papanikolaou, N.; Messiou, C.; Koh, D.M.; Orton, M.R. Radiomics in Oncology: A Practical Guide. Radiographics 2021, 41, 1717–1732. [Google Scholar] [CrossRef] [PubMed]
- Horvat, N.; Miranda, J.; El Homsi, M.; Peoples, J.J.; Long, N.M.; Simpson, A.L.; Do, R.K.G. A primer on texture analysis in abdominal radiology. Abdom. Radiol. 2021, 47, 2972–2985. [Google Scholar] [CrossRef] [PubMed]
- Ciaravino, V.; Cardobi, N.; De Robertis, R.; Capelli, P.; Melisi, D.; Simionato, F.; Marchegiani, G.; Salvia, R.; D’Onofrio, M. CT Texture Analysis of Ductal Adenocarcinoma Downstaged After Chemotherapy. Anticancer Res. 2018, 38, 4889–4895. [Google Scholar] [CrossRef] [PubMed]
- Nasief, H.; Hall, W.; Zheng, C.; Tsai, S.; Wang, L.; Erickson, B.; Li, X.A. Improving Treatment Response Prediction for Chemoradiation Therapy of Pancreatic Cancer Using a Combination of Delta-Radiomics and the Clinical Biomarker CA19-9. Front. Oncol. 2019, 9, 1464. [Google Scholar] [CrossRef]
- Borhani, A.A.; Dewan, R.; Furlan, A.; Seiser, N.; Zureikat, A.H.; Singhi, A.D.; Boone, B.; Bahary, N.; Hogg, M.E.; Lotze, M.; et al. Assessment of Response to Neoadjuvant Therapy Using CT Texture Analysis in Patients with Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma. AJR Am. J. Roentgenol. 2020, 214, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Rigiroli, F.; Hoye, J.; Lerebours, R.; Lafata, K.J.; Li, C.; Meyer, M.; Lyu, P.; Ding, Y.; Schwartz, F.R.; Mettu, N.B.; et al. CT Radiomic Features of Superior Mesenteric Artery Involvement in Pancreatic Ductal Adenocarcinoma: A Pilot Study. Radiology 2021, 301, 610–622. [Google Scholar] [CrossRef]
- Soloff, E.V.; Al-Hawary, M.M.; Desser, T.S.; Fishman, E.K.; Minter, R.M.; Zins, M. Imaging Assessment of Pancreatic Cancer Resectability After Neoadjuvant Therapy: AJR Expert Panel Narrative Review. AJR Am. J. Roentgenol. 2022, 218, 570–581. [Google Scholar] [CrossRef]

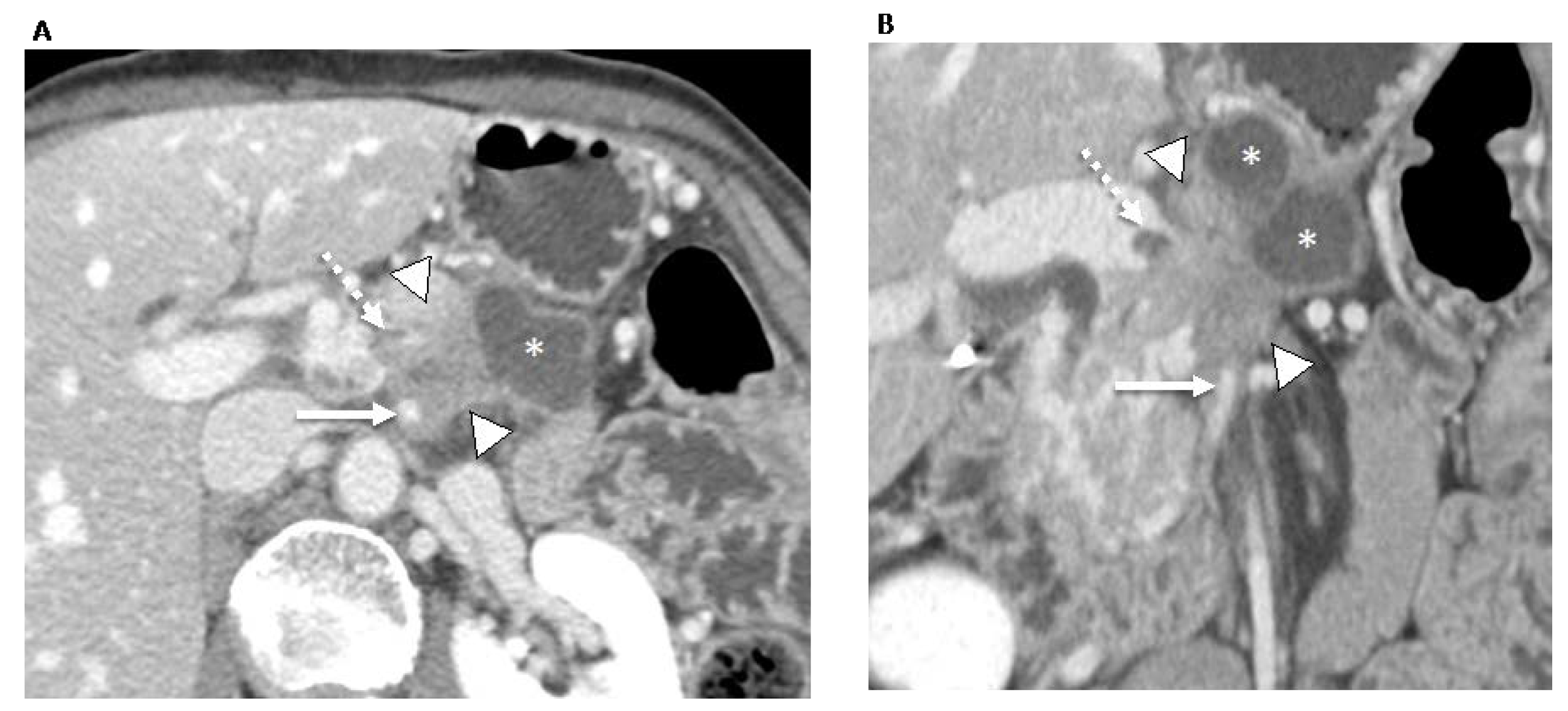
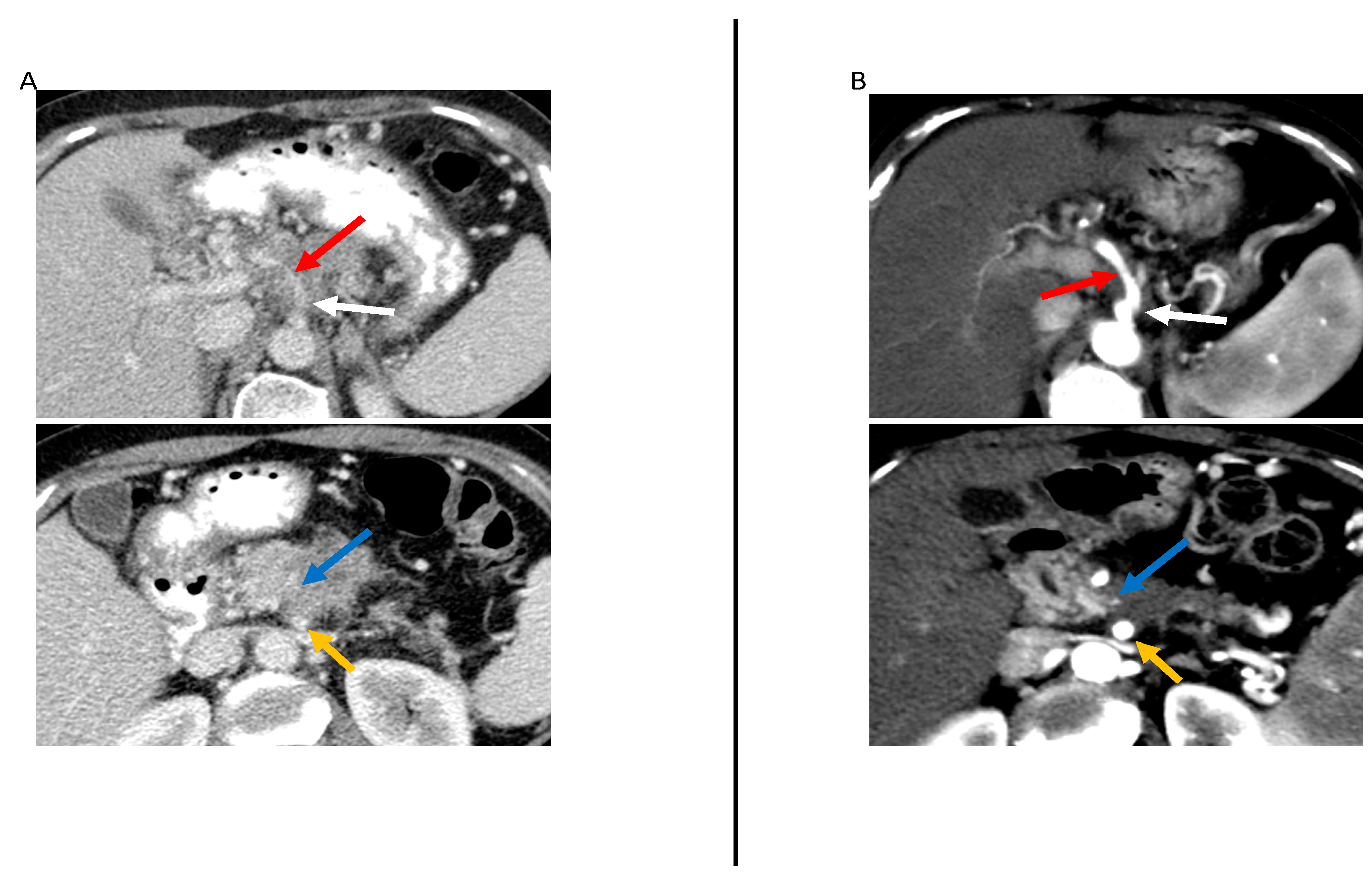

| Resectability Status | Arterial | Venous |
|---|---|---|
| Resectable | No tumor contact |
|
| Borderline Resectable | CA & branches:
| SMV or PV:
|
| Locally Advanced |
|
|
| Author, Year (Country) | n | Imaging Criteria | Type of Neoadjuvant Therapy | No. and Type of Readers | Main Results |
|---|---|---|---|---|---|
| Tamm et al. [13], 2006 (U.S.) | 55 | -Resectability based on NCCN criteria | -CRT | 3 radiologists | -Resectability as determined qualitatively by three radiologists had an accuracy of 87–95%. |
| Kim et al. [32], 2009 (South Korea) | 38 | -Institutional CT criteria ** | -CRT | 2 radiologists | -Presurgical CT interpreted based on institutional CT criteria had an accuracy of 83% (10/12), sensitivity of 91% (10/11), specificity of 0% (0/1), PPV of 91% (10/11), and NPV of 0% (0/1) for predicting resectability. |
| Katz et al. [31], 2012 (U.S.) | 129 | -Changes in tumor size or stage using RECIST 1.1 criteria | -Chemo -CRT -EBRT | 1 GI radiologists | -Response according to RECIST was not associated with OS (p = 0.78) |
| Cassinotto et al. [33], 2014 (France) | 47 | -Tumor diameter -Tumor attenuation -Tumor vascular contact | -CRT | 2 GI radiologists | -Partial regression of tumor contact with the SMV/portal vein had a PPV of 100% (10/10) for R0 resection. Partial regression of tumor contact with any peripancreatic vascular axis had a PPV of 91% (20/22) for R0 resection. |
| Wagner et al. [34], 2017 (France and Portugal) | 36 | -Morphologic criteria: Tumor size, large axial axis, small axial axis. height and product of the three axes, attenuation, and response according to RECIST criteria -Vascular involvement -NCCN classification | -Chemo -CRT | 2 radiologists | -Only the large axis and the product of the three axes were significantly associated with R0 resection. |
| Amer et al. [35], 2018 (U.S.) | 326 | -Response according to RECIST 1.1 criteria -Tumor/pancreas interface response developed by the authors | -Chemo -CRT | 3 radiologists | -Type I vs. Type II response at the interface was significantly associated with fewer viable cells after neoadjuvant therapy and was more likely to achieve major pathologic response (p = 0.01); Type I response also showed improved DFS and OS. |
| Marchegiani et al. [36], 2018 (Italy) | 59 | -Tumor attenuation -Longest tumor dimension -Response according to RECIST criteria -Resectability status according to the Americas Hepato-Pancreato-Biliary Association (AHPBA) guidelines | -Chemo -CRT | 2 radiologists | -Only an increase in mean tumor attenuation in the arterial and venous phases following neoadjuvant therapy was significantly associated with R0 resection (p < 0.001 and 0.001 for the arterial and venous phases, respectively). |
| Kim et al. [37], 2019 (South Korea) | 45 | -Resectability (not indicated if specific criteria were used) | -Chemo -CRT | 2 radiologists | -CT had 51–69% to predict R0 resection. |
| Wei et al. [38], 2021 (U.S.) | 343 | -Longest tumor diameter -Radiological tumor stage according to American Joint Committee on Cancer (AJCC) criteria -Tumor volume | -Chemo -CRT | 2 radiologists | -Longest tumor diameter tends to understage ypT -Radiological tumor stage and tumor volume post neoadjuvant therapy were correlated with ypT stage, tumor response grades, distance of superior mesenteric artery margin, and tumor recurrence/metastasis. |
| Perri et al. [39], 2021 (U.S.) | 290 | -Response according to RECIST 1.1 -Other changes in tumor size and anatomic extent | -Chemo -CRT | 1 surgeon | -RECIST partial response and reduction in tumor volume were significantly associated with major pathologic response (p < 0.01 for both) |
| Jang et al. [40], 2021 (South Korea) | 64 | -NCCN resectability criteria including extent of soft tissue contacting arteries and veins, depth of soft tissue contacting arteries and veins, contrast enhancement of the tumor, and of soft tissue surrounding arteries and veins, and tumor size. | -Chemo | 2 GI radiologists | -Only low contrast enhancement of the soft tissue contacting the artery (£ 46.4 HU) was significantly associated with R0 resection (adjusted odds ratio = 7.4; p = 0.01). |
| Author, Year (Country) | No. of Patients | Type of Neoadjuvant Therapy | Semantic Imaging Features or Laboratory Features Used for Comparison or Combination with Radiomic Features | Segmentation | Feature Extraction Software | Main Results |
|---|---|---|---|---|---|---|
| Chen et al. [29], 2017 (U.S.) | 20 | -CRT | None | Manual, ROIs containing the pancreas head | In-house MATLAB | -Changes in mean histograms of CT number (MCTN), standard deviation (SD), skewness, and kurtosis were associated with good vs. poor pathologic response (p = 0.046, 0.058, 0.042, and 0.12, respectively). |
| Ciaravino et al. [46], 2018 (Italy) | 31 | -Chemo -CRT | None | Manual, ROIs containing the tumor from primary staging and restaging CT | MaZda | -Of the texture features that were investigated, only kurtosis was significantly different between primary staging and restaging CT (p = 0.0046) and was indicative of tumor downstaging. |
| Kim et al. [37], 2019 (South Korea) | 45 | -Chemo -CRT | Resectability status based on NCCN criteria, CA 19-9 | Manual, ROIs containing the tumor from primary staging and restaging CT | MISSTA | -CA 19-9 nor any of the texture features at primary staging were significantly associated with R0 resection. -However, several subtracted texture values (i.e., between primary staging and restaging) were significantly associated with R0 resection, including lower subtracted value of surface area (HR 1.077, p = 0.011), higher subtracted values of GLCM IDM (HR 0.000, p = 0.005) and GLCM contrast (HR 0.982, p = 0.012). -Also, the higher subtracted value of entropy (HR 0.159, p = 0.005) and lower subtracted value of GLCM entropy (HR 10.235, p = 0.036) were associated with improved overall survival. |
| Nasief et al. [47], 2020 (U.S.) | 24 (672 CT) | -CRT | CA 19-9 | Manual, ROIs containing the tumor | IBEX | -The C-index for the prediction of pathologic response was 0.69 for CA 19.9 alone, which improved to 0.87 for the combination of CA 19-9 + delta radiomic features. -Decrease in CA19-9 levels and delta radiomic features were also significantly associated with survival (p = 0.031 and 0.001, respectively). |
| Borhani et al. [48], 2020 (U.S.) | 39 | -Chemo -CRT | None | Manual, ROIs containing the tumor from primary staging and restaging CT | TedRAD | -Higher mean perfusion parameter values at primary staging had higher odds of a favorable pathologic response (OR = 1.06; 95% CI, 1.002–1.12). -The Cox model containing three texture features was significantly associated with disease-free survival (p = 0.001). |
| Rigiroli et al. [49], 2021 (U.S.) | 194 | -Chemo -CRT | Resectability status based on NCCN criteria | Semi-automatic, 3D VOIs containing the tumor and perivascular tissue surrounding the SMA | Python | -The model containing five perivessel and tumor radiomic features had an AUC of 0.71 to determine tumor involvement of the SMA, whereas resectability status based on NCCN criteria had an AUC of 0.54. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khasawneh, H.; Ferreira Dalla Pria, H.R.; Miranda, J.; Nevin, R.; Chhabra, S.; Hamdan, D.; Chakraborty, J.; Biachi de Castria, T.; Horvat, N. CT Imaging Assessment of Pancreatic Adenocarcinoma Resectability after Neoadjuvant Therapy: Current Status and Perspective on the Use of Radiomics. J. Clin. Med. 2023, 12, 6821. https://doi.org/10.3390/jcm12216821
Khasawneh H, Ferreira Dalla Pria HR, Miranda J, Nevin R, Chhabra S, Hamdan D, Chakraborty J, Biachi de Castria T, Horvat N. CT Imaging Assessment of Pancreatic Adenocarcinoma Resectability after Neoadjuvant Therapy: Current Status and Perspective on the Use of Radiomics. Journal of Clinical Medicine. 2023; 12(21):6821. https://doi.org/10.3390/jcm12216821
Chicago/Turabian StyleKhasawneh, Hala, Hanna Rafaela Ferreira Dalla Pria, Joao Miranda, Rachel Nevin, Shalini Chhabra, Dina Hamdan, Jayasree Chakraborty, Tiago Biachi de Castria, and Natally Horvat. 2023. "CT Imaging Assessment of Pancreatic Adenocarcinoma Resectability after Neoadjuvant Therapy: Current Status and Perspective on the Use of Radiomics" Journal of Clinical Medicine 12, no. 21: 6821. https://doi.org/10.3390/jcm12216821





