Pre-Procedural Assessment of the Femoral Access Route for Transcatheter Aortic Valve Implantation: Comparison of a Non-Contrast Time-of-Flight Magnetic Resonance Angiography Protocol with Contrast-Enhanced Dual-Source Computed Tomography Angiography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CTA Data Acquisition
2.3. MRA Data Acquisition
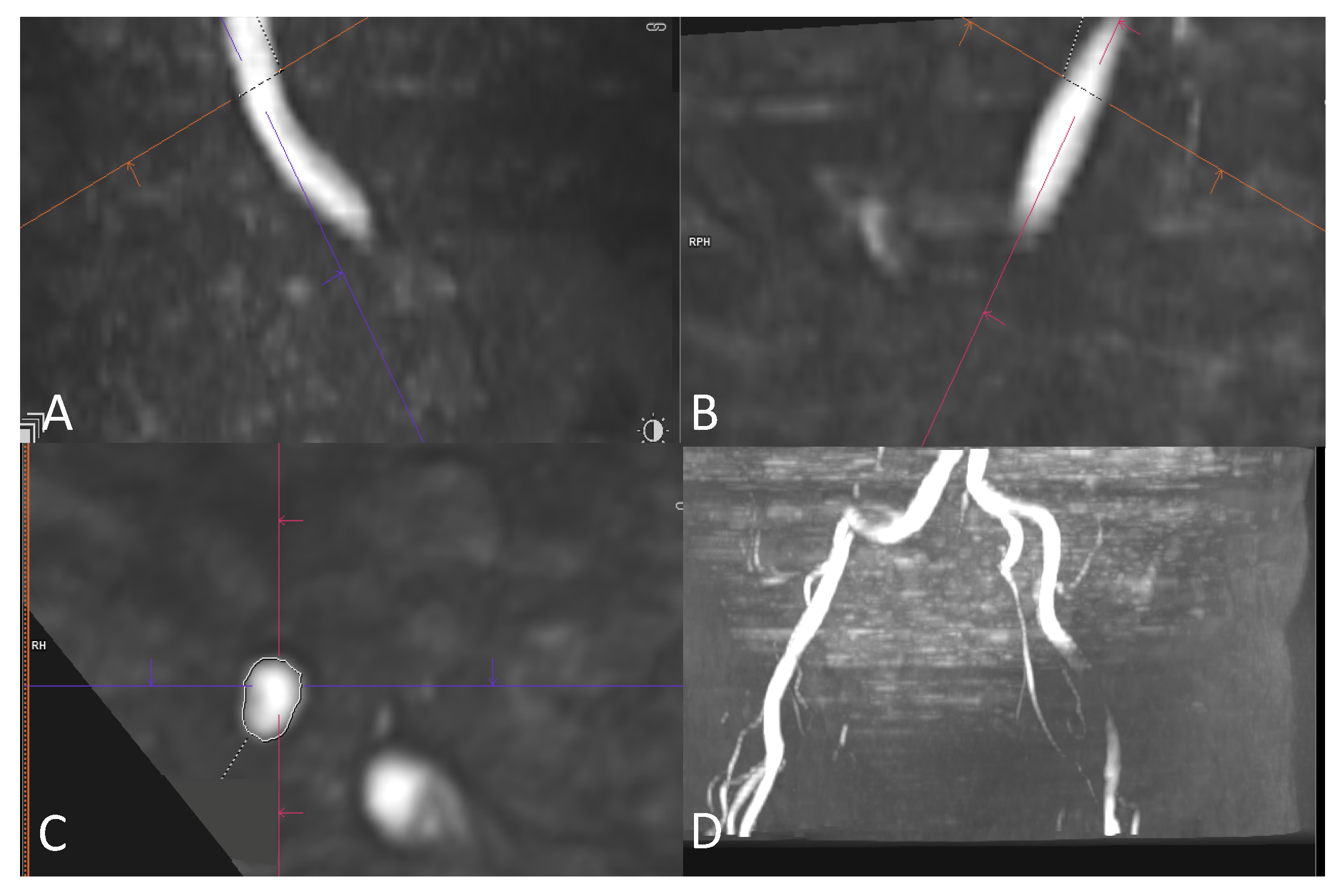
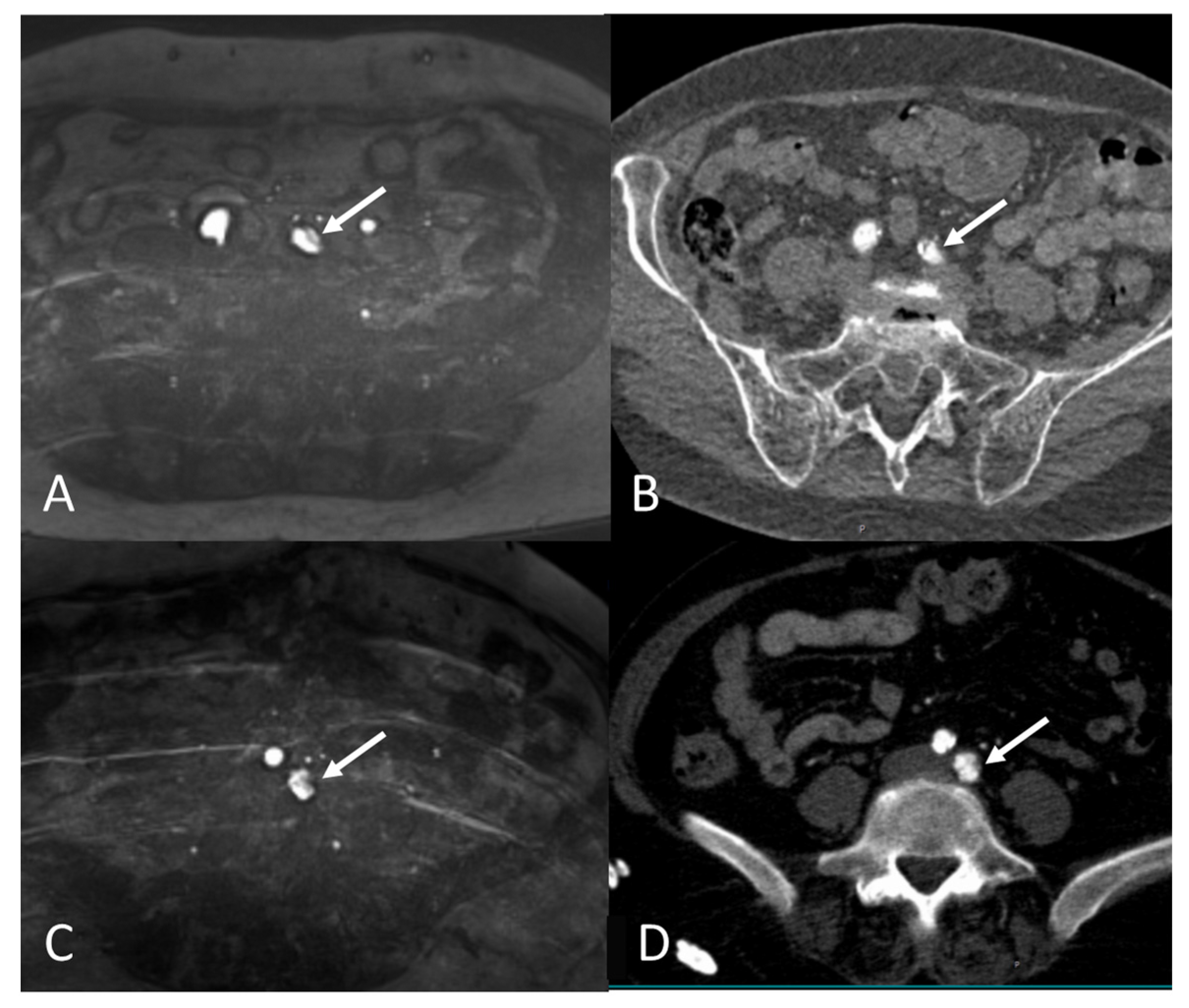
2.4. Image Review Methods
2.5. Assessment of the Access Route
2.6. Image Quality Assessment of MRA
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. MRA Image Quality
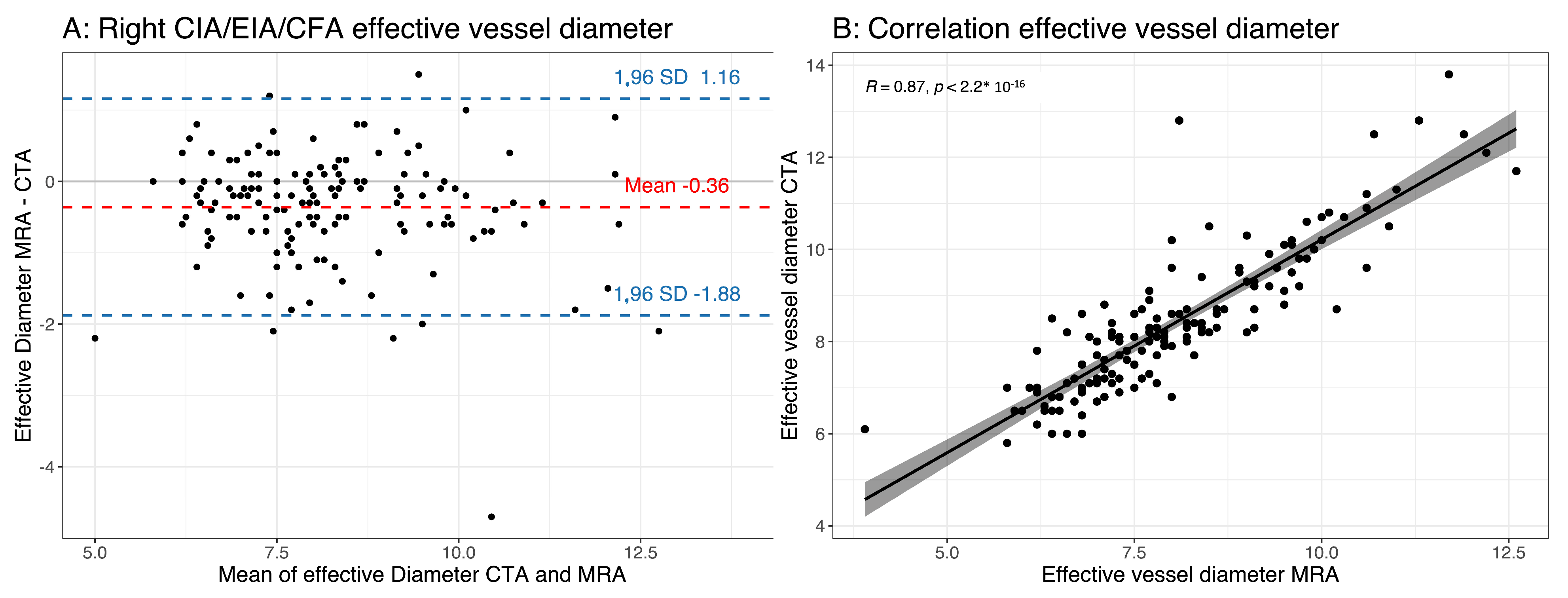
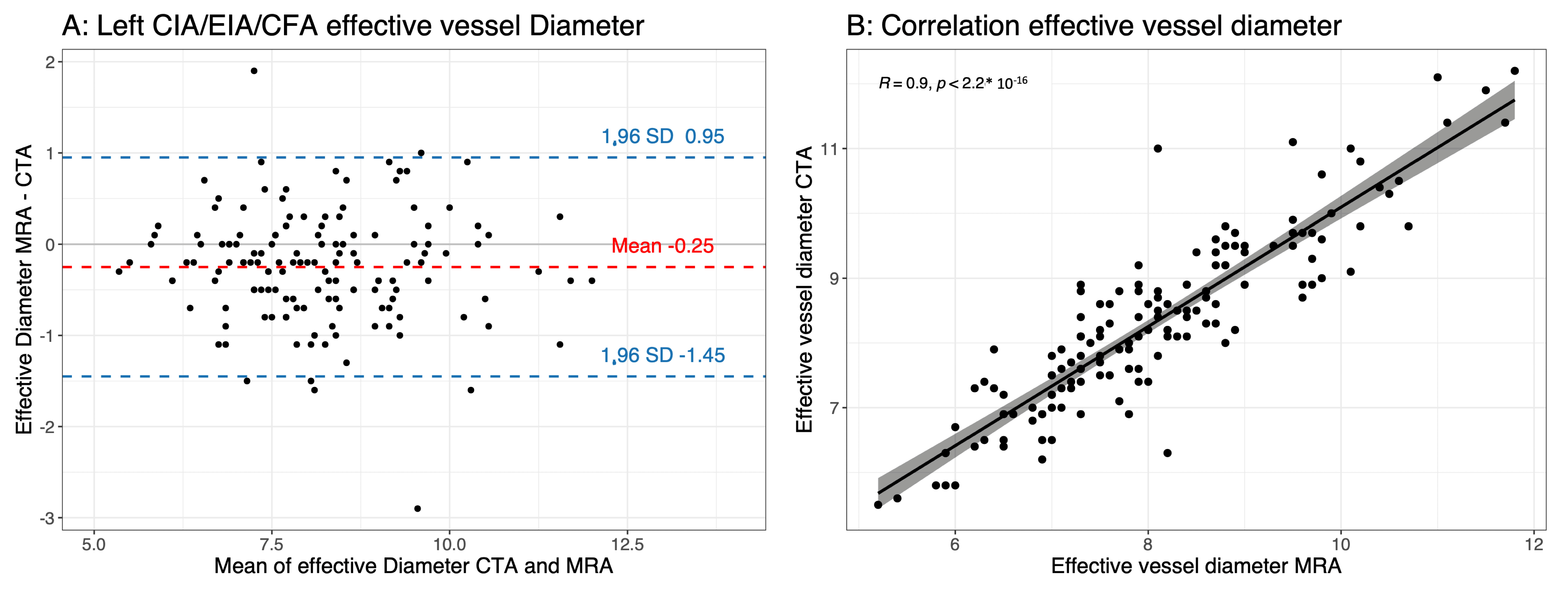
3.3. Effective Vessel Diameter
3.4. Feasibility of Transfemoral Access
4. Discussion
- The non-contrast MRA protocol was reliable for an assessment of the femoral access route prior to the TAVI in comparison with the gold-standard CTA for most of the patients.
- The non-contrast TOF-MRA underestimated the minimal vessel diameter compared with the CTA, but the absolute differences were small and did not affect the evaluation of the feasibility of the transfemoral access.
4.1. Comparison with Different Non-Contrast MRA Techniques
4.2. Feasibility of Transfemoral Access Using MRA and CTA
4.3. Contrast-Induced Acute Kidney Injury
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef]
- Blanke, P.; Schoepf, U.J.; Leipsic, J.A. CT in Transcatheter Aortic Valve Replacement. Radiology 2013, 269, 650–669. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Rudzinski, P.N.; Leipsic, J.A.; Schoepf, U.J.; Dudek, D.; Schwarz, F.; Andreas, M.; Zlahoda-Huzior, A.; Thilo, C.; Renker, M.; Burt, J.R.; et al. CT in Transcatheter-delivered Treatment of Valvular Heart Disease. Radiology 2022, 304, 4–17. [Google Scholar] [CrossRef]
- Thourani, V.H.; Keeling, W.B.; Sarin, E.L.; Guyton, R.A.; Kilgo, P.D.; Dara, A.B.; Puskas, J.D.; Chen, E.P.; Cooper, W.A.; Vega, J.D.; et al. Impact of Preoperative Renal Dysfunction on Long-Term Survival for Patients Undergoing Aortic Valve Replacement. Ann. Thorac. Surg. 2011, 91, 1798–1807. [Google Scholar] [CrossRef]
- Sinning, J.-M.; Ghanem, A.; Steinhäuser, H.; Adenauer, V.; Hammerstingl, C.; Nickenig, G.; Werner, N. Renal Function as Predictor of Mortality in Patients After Percutaneous Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Interv. 2010, 3, 1141–1149. [Google Scholar] [CrossRef]
- Julien, H.M.; Stebbins, A.; Vemulapalli, S.; Nathan, A.S.; Eneanya, N.D.; Groeneveld, P.; Fiorilli, P.N.; Herrmann, H.C.; Szeto, W.Y.; Desai, N.D.; et al. Incidence, Predictors, and Outcomes of Acute Kidney Injury in Patients Undergoing Transcatheter Aortic Valve Replacement: Insights From the Society of Thoracic Surgeons/American College of Cardiology National Cardiovascular Data Registry-Transcatheter Val. Circ. Cardiovasc. Interv. 2021, 14, E010032. [Google Scholar] [CrossRef]
- Ruile, P.; Blanke, P.; Krauss, T.; Dorfs, S.; Jung, B.; Jander, N.; Leipsic, J.; Langer, M.; Neumann, F.-J.; Pache, G. Pre-procedural assessment of aortic annulus dimensions for transcatheter aortic valve replacement: Comparison of a non-contrast 3D MRA protocol with contrast-enhanced cardiac dual-source CT angiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 458–466. [Google Scholar] [CrossRef]
- Ram, P.; Mezue, K.; Pressman, G.; Rangaswami, J. Acute kidney injury post–transcatheter aortic valve replacement. Clin. Cardiol. 2017, 40, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Edelman, R.R.; Koktzoglou, I. Noncontrast MR angiography: An update. J. Magn. Reson. Imaging 2019, 49, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, A.U.; Koktzoglou, I.; Edelman, R.R.; Gilkeson, R.; Mihai, G.; Shin, T.; Rajagopalan, S. Noncontrast Magnetic Resonance Angiography for the Diagnosis of Peripheral Vascular Disease. Circ. Cardiovasc. Imaging 2019, 12, e008844. [Google Scholar] [CrossRef] [PubMed]
- Pamminger, M.; Klug, G.; Kranewitter, C.; Reindl, M.; Reinstadler, S.J.; Henninger, B.; Tiller, C.; Holzknecht, M.; Kremser, C.; Bauer, A.; et al. Non-contrast MRI protocol for TAVI guidance: Quiescent-interval single-shot angiography in comparison with contrast-enhanced CT. Eur. Radiol. 2020, 30, 4847–4856. [Google Scholar] [CrossRef]
- Cannaò, P.M.; Muscogiuri, G.; Schoepf, U.J.; De Cecco, C.N.; Suranyi, P.; Lesslie, V.W.; Piccini, D.; Giri, S.; Varga-Szemes, A. Technical Feasibility of a Combined Noncontrast Magnetic Resonance Protocol for Preoperative Transcatheter Aortic Valve Replacement Evaluation. J. Thorac. Imaging 2018, 33, 60–67. [Google Scholar] [CrossRef]
- Andersson, C.; Jørgensen, M.E.; Martinsson, A.; Hansen, P.W.; Gustav Smith, J.; Jensen, P.F.; Gislason, G.H.; Køber, L.; Torp-Pedersen, C. Noncardiac Surgery in Patients With Aortic Stenosis: A Contemporary Study on Outcomes in a Matched Sample From the Danish Health Care System. Clin. Cardiol. 2014, 37, 680–686. [Google Scholar] [CrossRef]
- Prado, G.F.A.; Garzon, S.; Mariani, J.; Caixeta, A.; Almeida, B.O.; Ramalho, F.O.; Marcelo, M.L.; Fischer, C.H.; Szarf, G.; Ishikawa, W.; et al. Zero-contrast imaging for the assessment of transcatheter aortic valve implantation in candidates with renal dysfunction. Ren. Fail. 2023, 45, 2224888. [Google Scholar] [CrossRef]
- Jochheim, D.; Schneider, V.S.; Schwarz, F.; Kupatt, C.; Lange, P.; Reiser, M.; Massberg, S.; Gutiérrez-Chico, J.L.; Mehilli, J.; Becker, H.C. Contrast-induced acute kidney injury after computed tomography prior to transcatheter aortic valve implantation. Clin. Radiol. 2014, 69, 1034–1038. [Google Scholar] [CrossRef]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A.; Bagga, A.; Bakkaloglu, A.; Bonventre, J.V.; et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef]
- Davenport, M.S.; Perazella, M.A.; Yee, J.; Dillman, J.R.; Fine, D.; McDonald, R.J.; Rodby, R.A.; Wang, C.L.; Weinreb, J.C. Use of intravenous iodinated contrast media in patients with kidney disease: Consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology 2020, 294, 660–668. [Google Scholar] [CrossRef]
- Freire, A.F.D.; Nicz, P.F.G.; Ribeiro, H.B.; Filippini, F.B.; Accorsi, T.D.; Liberato, G.; Nomura, C.H.; Cassar, R.d.S.; Vieira, M.L.C.; Mathias, W.; et al. Non-contrast transcatheter aortic valve implantation for patients with aortic stenosis and chronic kidney disease: A pilot study. Front. Cardiovasc. Med. 2023, 10, 1175600. [Google Scholar] [CrossRef] [PubMed]
- Klug, G.; Reinstadler, S.; Troger, F.; Holzknecht, M.; Reindl, M.; Tiller, C.; Lechner, I.; Fink, P.; Pamminger, M.; Kremser, C.; et al. Cardiac magnetic resonance imaging versus computed tomography to guide transcatheter aortic valve replacement: Study protocol for a randomized trial (TAVR-CMR). Trials 2022, 23, 726. [Google Scholar] [CrossRef] [PubMed]
| Age (years) | 82.69 ± 5.69 |
| Female, n (%) | 31/51 (60.8%) |
| Body mass index (kg/m2) | 27.0 ± 4.93 |
| Body surface area (m2) | 1.82 ± 0.22 |
| Creatinine (mg/dL) | 1.13 ± 0.52 |
| eGFR (mL/min/1.73 m2) | 54.2 ± 20.8 |
| hs-cTnT (ng/L) | 0.08 ± 0.18 |
| Echo, LVEF (%) | 52.5 ± 13.6 |
| Contrast agent for CTA (mL) | 58.5 ± 10.5 |
| Atrial fibrillation, n (%) | 22/51 (43.1%) |
| Average scan time (min) | 7.6 ± 1.6 |
| Median MRA image quality | 5 (IQR 4–5) |
| MRA with sufficient image quality (≥3) | |
| Right CIA/EIA/CFA | 47/51 (92%) |
| Left CIA/EIA/CFA | 46/51 (90%) |
| Both sides simultaneously | 44/51 (86.3%) |
| MRA | CTA | p-Value (t-Test) | Pearson Correlation Coefficient | p-Value (Pearson Correlation) | Bland–Altman Analysis | |
|---|---|---|---|---|---|---|
| Diameter of right CIA/EIA/CFA | 8.04 ± 1.46 | 8.37 ± 1.54 | <0.0001 | 0.87 | <0.0001 | −0.36 ± 0.77 |
| Diameter of left CIA/EIA/CFA | 8.07 ± 1.32 | 8.28 ± 1.34 | <0.0001 | 0.9 | <0.0001 | −0.25 ± 0.61 |
| Diameter of right common iliac artery | 9.08 ± 1.6 | 9.61 ± 1.68 | <0.0001 | 0.86 | <0.0001 | −0.56 ± 0.88 |
| Diameter of right external iliac artery | 7.37 ± 1 | 7.68 ± 0.98 | 0.002 | 0.79 | <0.0001 | −0.3 ± 0.64 |
| Diameter of right common femoral artery | 7.64 ± 1.09 | 7.83 ± 1.04 | 0.047 | 0.75 | <0.0001 | −0.22 ± 0.75 |
| Diameter of left common iliac artery | 8.85 ± 1.27 | 9.07 ± 1.43 | 0.035 | 0.85 | <0.0001 | −0.23 ± 0.76 |
| Diameter of left external iliac artery | 7.57 ± 1.09 | 7.86 ± 1.08 | 0.0002 | 0.88 | <0.0001 | −0.31 ± 0.54 |
| Diameter of left common femoral artery | 7.75 ± 1.24 | 7.93 ± 1.16 | 0.0066 | 0.92 | <0.0001 | −0.2 ± 0.49 |
| ICC (95% CI) | |
|---|---|
| Right iliofemoral axis CIA/EIA/CFA minimal effective diameter | 0.867 (0.819–0.902) |
| Left iliofemoral axis CIA/EIA/CFA minimal effective diameter | 0.929 (0.902–0.948) |
| MRA | CTA | Agreement | Kappa Value | p-Value | |
|---|---|---|---|---|---|
| Right CIA/EIA/CFA | 46/47 | 51/51 | 97.9% | - | - |
| Left CIA/EIA/CFA | 44/46 | 49/51 | 95.7% | 0.477 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brado, J.; Breitbart, P.; Hein, M.; Pache, G.; Schmitt, R.; Hein, J.; Apweiler, M.; Soschynski, M.; Schlett, C.; Bamberg, F.; et al. Pre-Procedural Assessment of the Femoral Access Route for Transcatheter Aortic Valve Implantation: Comparison of a Non-Contrast Time-of-Flight Magnetic Resonance Angiography Protocol with Contrast-Enhanced Dual-Source Computed Tomography Angiography. J. Clin. Med. 2023, 12, 6824. https://doi.org/10.3390/jcm12216824
Brado J, Breitbart P, Hein M, Pache G, Schmitt R, Hein J, Apweiler M, Soschynski M, Schlett C, Bamberg F, et al. Pre-Procedural Assessment of the Femoral Access Route for Transcatheter Aortic Valve Implantation: Comparison of a Non-Contrast Time-of-Flight Magnetic Resonance Angiography Protocol with Contrast-Enhanced Dual-Source Computed Tomography Angiography. Journal of Clinical Medicine. 2023; 12(21):6824. https://doi.org/10.3390/jcm12216824
Chicago/Turabian StyleBrado, Johannes, Philipp Breitbart, Manuel Hein, Gregor Pache, Ramona Schmitt, Jonas Hein, Matthias Apweiler, Martin Soschynski, Christopher Schlett, Fabian Bamberg, and et al. 2023. "Pre-Procedural Assessment of the Femoral Access Route for Transcatheter Aortic Valve Implantation: Comparison of a Non-Contrast Time-of-Flight Magnetic Resonance Angiography Protocol with Contrast-Enhanced Dual-Source Computed Tomography Angiography" Journal of Clinical Medicine 12, no. 21: 6824. https://doi.org/10.3390/jcm12216824
APA StyleBrado, J., Breitbart, P., Hein, M., Pache, G., Schmitt, R., Hein, J., Apweiler, M., Soschynski, M., Schlett, C., Bamberg, F., Neumann, F.-J., Westermann, D., Krauss, T., & Ruile, P. (2023). Pre-Procedural Assessment of the Femoral Access Route for Transcatheter Aortic Valve Implantation: Comparison of a Non-Contrast Time-of-Flight Magnetic Resonance Angiography Protocol with Contrast-Enhanced Dual-Source Computed Tomography Angiography. Journal of Clinical Medicine, 12(21), 6824. https://doi.org/10.3390/jcm12216824






