Injury of the Vestibulocerebellar Tract and Signs of Ataxia in Patients with Cerebellar Stroke
Abstract
:1. Introduction
2. Material and Methods
2.1. Subjects
2.2. Clinical Evaluation
2.3. Diffusion Tensor Image Tractography
2.4. Probabilistic Fiber Tracking
3. Results
3.1. Clinical Evaluation
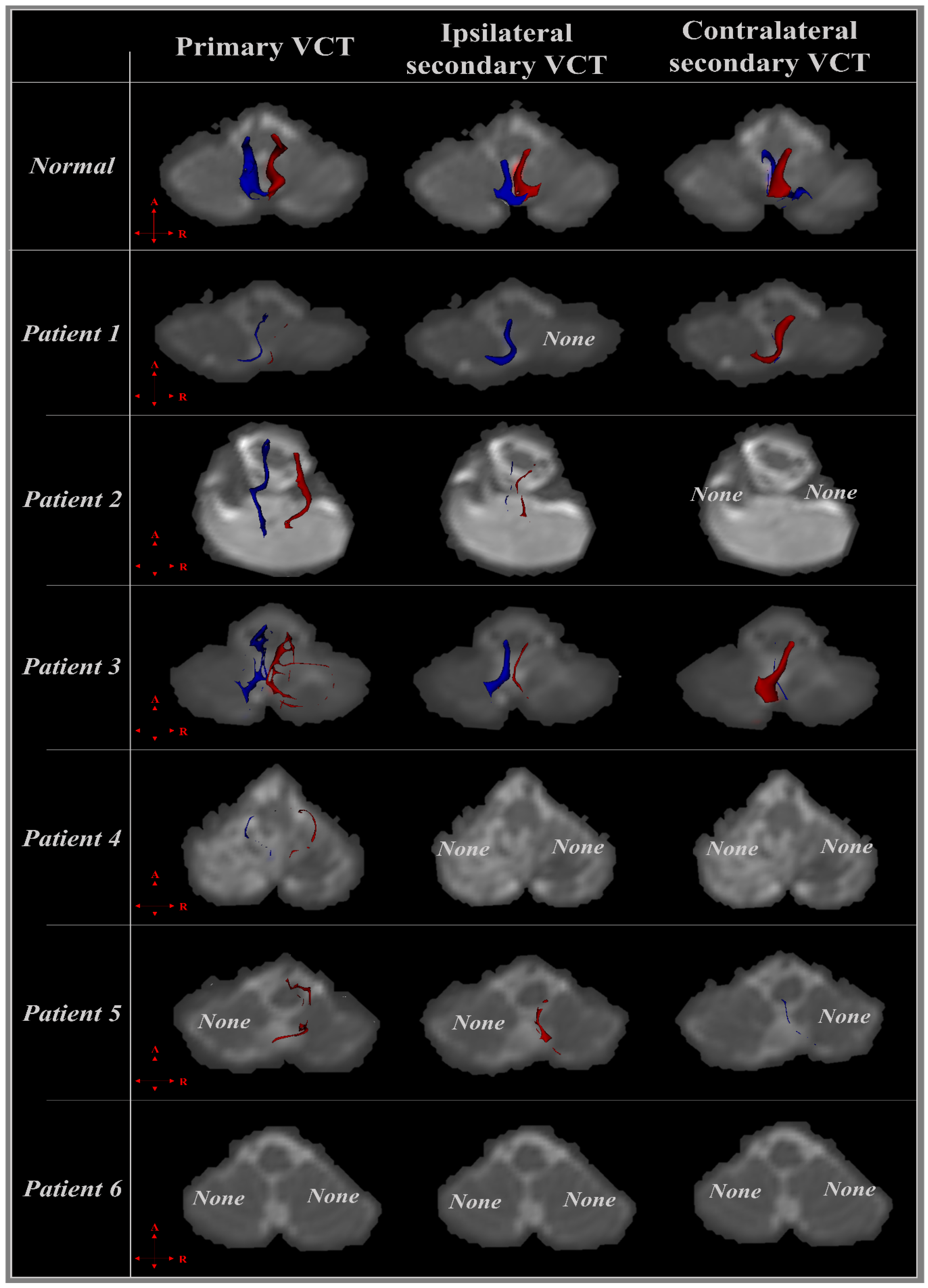
3.2. Diffusion Tensor Imaging
3.2.1. Patient 1
3.2.2. Patient 2
3.2.3. Patient 3
3.2.4. Patient 4
3.2.5. Patient 5
3.2.6. Patient 6
| Patient | Sex/Age (Year) | Onset Duration | Diagnosis | Lesion on MRI | Cerebellar Symptoms | Purdue Pegboard (Score) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ataxia | Nystagmus | Dysarthria | Dysphagia | Diplopia | Dominant | Non-Dominant | |||||
| 1 | M/82 | 29 days | S-ICH | Rt. | + | − | − | − | − | 5 | 6 |
| 2 | M/31 | 65 days | S-ICH | both | + | − | + | − | − | 8 | 7 |
| 3 | F/64 | 12 days | S-ICH | both | + | − | − | − | − | 7 | 8 |
| 4 | M/67 | 44 days | Infarction | both | − | − | + | − | − | 2 | 1 |
| 5 | M/77 | 17 days | Infarction | both | + | − | − | − | − | 10 | 8 |
| 6 | M/50 | 16 days | Infarction | both | + | − | − | − | − | 5 | 2 |
| Patient | CST | Primary VCT | Ipsilateral Secondary VCT | Contralateral Secondary VCT | |
|---|---|---|---|---|---|
| 1 | R | + | + | − | + |
| L | + | + | + | + | |
| 2 | R | + | + | − | − |
| L | + | + | − | − | |
| 3 | R | + | + | + | − |
| L | + | − | − | + | |
| 4 | R | + | + | + | − |
| L | + | + | + | − | |
| 5 | R | + | + | + | + |
| L | + | + | + | + | |
| 6 | R | + | − | − | − |
| L | + | − | − | − | |
| Injury rate (%) | 0.00 | 25.00 | 50.00 | 58.33 | |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vachha, B.A.; Massoud, T.F.; Huang, S.Y. Anatomy of the Cerebral Cortex, Lobes, and Cerebellum. Neuroimaging Clin. N. Am. 2022, 32, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, H.J. The basal ganglia and motor control. Neural Plast. 2003, 10, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Robles, C.M.; Anderson, B.; Dukelow, S.P.; Striemer, C.L. Assessment and recovery of visually guided reaching deficits following cerebellar stroke. Neuropsychologia 2023, 188, 108662. [Google Scholar] [CrossRef] [PubMed]
- Ng, Z.X.; Yang, W.R.; Seet, E.; Koh, K.M.; Teo, K.J.; Low, S.W.; Chou, N.; Yeo, T.T.; Venketasubramanian, N. Cerebellar strokes: A clinical outcome review of 79 cases. Singapore Med. J. 2015, 56, 145–149. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, B.; Yao, X.; Zhang, X.; He, D.; Cai, L.; Xu, Y.; Li, Q.; Wan, Z. Cerebellar infarction caused by vertebral artery dissection: A case report. Medicine 2023, 102, e34033. [Google Scholar] [CrossRef]
- Bruning, T.; Beyrich, T.; Al-Khaled, M. Wide symptom variability of cerebellar infarction and comparison with pons infarction. eNeurologicalSci 2023, 31, 100459. [Google Scholar] [CrossRef]
- Marquer, A.; Barbieri, G.; Perennou, D. The assessment and treatment of postural disorders in cerebellar ataxia: A systematic review. Ann. Phys. Rehabil. Med. 2014, 57, 67–78. [Google Scholar] [CrossRef]
- Sullivan, R.; Kaiyrzhanov, R.; Houlden, H. Cerebellar ataxia, neuropathy, vestibular areflexia syndrome: Genetic and clinical insights. Curr. Opin. Neurol. 2021, 34, 556–564. [Google Scholar] [CrossRef]
- Sullivan, R.; Yau, W.Y.; O’Connor, E.; Houlden, H. Spinocerebellar ataxia: An update. J. Neurol. 2019, 266, 533–544. [Google Scholar] [CrossRef]
- Akbar, U.; Ashizawa, T. Ataxia. Neurol. Clin. 2015, 33, 225–248. [Google Scholar] [CrossRef] [PubMed]
- Miyai, I.; Ito, M.; Hattori, N.; Mihara, M.; Hatakenaka, M.; Yagura, H.; Sobue, G.; Nishizawa, M.; Trialists, C. Cerebellar ataxia rehabilitation trial in degenerative cerebellar diseases. Neurorehabil. Neural Repair. 2012, 26, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Fujii, A.; Tokuda, A.; Yoneda, M.; Kuriyama, M. A case showing Wallerian degeneration of the bilateral middle cerebellar peduncles on MRI followed by the right pontine infarction. Rinsho Shinkeigaku 2004, 44, 105–107. [Google Scholar]
- Jang, S.H.; Chang, C.H.; Jung, Y.J.; Kwon, H.G. Severe ataxia due to injuries of neural tract detected by diffusion tensor tractography in a patient with pontine hemorrhage: A case report. Medicine 2016, 95, e5590. [Google Scholar] [CrossRef]
- Jang, S.H.; Kim, T.H.; Lee, H.D. The effect of walnut rolling training on hand function and corticospinal tract. Ann. Transl. Med. 2019, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Irie, K.; Iseki, H.; Okamoto, K.; Nishimura, S.; Kagechika, K. Introduction of the Purdue Pegboard Test for fine assessment of severity of cervical myelopathy before and after surgery. J. Phys. Ther. Sci. 2020, 32, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Gallus, J.; Mathiowetz, V. Test-retest reliability of the Purdue Pegboard for persons with multiple sclerosis. Am. J. Occup. Ther. 2003, 57, 108–111. [Google Scholar] [CrossRef]
- Barbuto, S.; Mackenzie, S.; Kuo, S.H.; Kitago, T.; Stein, J. Measurements of Hand Function in Degenerative Cerebellar Disease: A Case-Control Pilot Study. Am. J. Phys. Med. Rehabil. 2020, 99, 795–800. [Google Scholar] [CrossRef]
- Küper, M.; Brandauer, B.; Thürling, M.; Schoch, B.; Gizewski, E.R.; Timmann, D.; Hermsdörfer, J. Impaired prehension is associated with lesions of the superior and inferior hand representation within the human cerebellum. J. Neurophysiol. 2011, 105, 2018–2029. [Google Scholar] [CrossRef]
- Kuper, M.; Hermsdorfer, J.; Brandauer, B.; Thurling, M.; Schoch, B.; Theysohn, N.; Timmann, D. Lesions of the dentate and interposed nuclei are associated with impaired prehension in cerebellar patients. Neurosci. Lett. 2011, 499, 132–136. [Google Scholar] [CrossRef]
- Park, S.Y.; Yeo, S.S.; Jang, S.H.; Cho, I.H. Anatomical Location of the Vestibulocerebellar Tract in the Healthy Human Brain: A Diffusion Tensor Imaging Study. Brain Sci. 2021, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Chang, R. Anatomy of the vestibular system: A review. NeuroRehabilitation 2013, 32, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Jahn, K. The Aging Vestibular System: Dizziness and Imbalance in the Elderly. Adv. Otorhinolaryngol. 2019, 82, 143–149. [Google Scholar] [CrossRef]
- Cortese, A.; Curro, R.; Vegezzi, E.; Yau, W.Y.; Houlden, H.; Reilly, M.M. Cerebellar ataxia, neuropathy and vestibular areflexia syndrome (CANVAS): Genetic and clinical aspects. Pract. Neurol. 2022, 22, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Schniepp, R.; Mohwald, K.; Wuehr, M. Gait ataxia in humans: Vestibular and cerebellar control of dynamic stability. J. Neurol. 2017, 264, 87–92. [Google Scholar] [CrossRef]
- Yeo, S.S.; Park, S.Y.; Cho, I.H. Injury of the vestibulocerebellar tract in a patient with intracerebral hemorrhage: A case report. Neurosci. Lett. 2022, 783, 136723. [Google Scholar] [CrossRef]
- Chang, M.C.; Kwak, S.G.; Park, D. Prediction of the motor prognosis with diffusion tensor imaging in hemorrhagic stroke: A meta-analysis. J. Integr. Neurosci. 2021, 20, 1011–1017. [Google Scholar] [CrossRef]
- Wang, Q.; Yap, P.-T.; Wu, G.; Shen, D. Diffusion tensor image registration using hybrid connectivity and tensor features. Human. Brain Mapp. 2014, 35, 3529–3546. [Google Scholar] [CrossRef]
- Assaf, Y.; Pasternak, O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J. Mol. Neurosci. MN 2008, 34, 51–61. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Liu, D.; Li, G.; Guo, Z.; Fan, Y.; Niu, Y. Evaluations of diffusion tensor image registration based on fiber tractography. Biomed. Eng. Online 2017, 16, 9. [Google Scholar] [CrossRef]
- Bigelow, R.T.; Semenov, Y.R.; Trevino, C.; Ferrucci, L.; Resnick, S.M.; Simonsick, E.M.; Xue, Q.L.; Agrawal, Y. Association between Visuospatial Ability and Vestibular Function in the Baltimore Longitudinal Study of Aging. J. Am. Geriatr. Soc. 2015, 63, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.J.; Greenhalgh, R. Normative data for assessing the manual dexterity of visually handicapped adults in vocational rehabilitation. J. Occup. Psychol. 1987, 60, 73–80. [Google Scholar] [CrossRef]
- Wittich, W.; Nadon, C. The Purdue Pegboard test: Normative data for older adults with low vision. Disabil. Rehabil. Assist. Technol. 2017, 12, 272–279. [Google Scholar] [CrossRef]
- Galons, J.P. Diffusion weighted and diffusion tensor imaging: A clinical guide. J. Magn. Reson. Imaging 2017, 46, 1230–1231. [Google Scholar] [CrossRef] [PubMed]
- Takeshige, H.; Ueno, Y.; Kamagata, K.; Sasaki, F.; Yamashiro, K.; Tanaka, R.; Aoki, S.; Hattori, N. Pathways Linked to Internuclear Ophthalmoplegia on Diffusion-Tensor Imaging in a Case with Midbrain Infarction. J. Stroke Cerebrovasc. Dis. 2016, 25, 2575–2579. [Google Scholar] [CrossRef]
- Jang, H.S.; Kim, H.J.; Kim, H.D.; Kwon, G.H. The Vestibulocerebellar Tract in the Human Brain: A Diffusion Tensor Tractography Study. Curr. Med. Imaging 2018, 14, 617–620. [Google Scholar] [CrossRef]
- Barmack, N.H. Central vestibular system: Vestibular nuclei and posterior cerebellum. Brain Res. Bull. 2003, 60, 511–541. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.S.; Jang, S.H.; Park, G.Y.; Oh, S. Effects of injuries to descending motor pathways on restoration of gait in patients with pontine hemorrhage. J. Stroke Cerebrovasc. Dis. 2020, 29, 104857. [Google Scholar] [CrossRef] [PubMed]
- Marvin, K. Purdue Pegboard Test (PPT). Stroke Engine 2012. Available online: https://strokengine.ca/en/assessments/purdue-pegboard-test-ppt (accessed on 11 September 2023).
- Dietrichs, E. Clinical manifestation of focal cerebellar disease as related to the organization of neural pathways. Acta Neurol. Scand. Suppl. 2008, 188, 6–11. [Google Scholar] [CrossRef]
- Morton, S.M.; Bastian, A.J. Mechanisms of cerebellar gait ataxia. Cerebellum 2007, 6, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.S.; Jang, S.H.; Kwon, J.W. Central vestibular disorder due to ischemic injury on the parieto-insular vestibular cortex in patients with middle cerebral artery territory infarction: Observational study. Medicine 2017, 96, e9349. [Google Scholar] [CrossRef] [PubMed]
- Barmack, N.H.; Yakhnitsa, V. Topsy turvy: Functions of climbing and mossy fibers in the vestibulo-cerebellum. Neuroscientist 2011, 17, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Barmack, N.H. Vestibular Nuclei and Their Cerebellar Connections. In Essentials of Cerebellum and Cerebellar Disorders: A Primer For Graduate Students; Gruol, D.L., Koibuchi, N., Manto, M., Molinari, M., Schmahmann, J.D., Shen, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 69–78. [Google Scholar]
- Alahyane, N.; Fonteille, V.; Urquizar, C.; Salemme, R.; Nighoghossian, N.; Pelisson, D.; Tilikete, C. Separate neural substrates in the human cerebellum for sensory-motor adaptation of reactive and of scanning voluntary saccades. Cerebellum 2008, 7, 595–601. [Google Scholar] [CrossRef]
- Judge, J.; Stirling, J. Fine motor skill performance in left- and right-handers: Evidence of an advantage for left-handers. Laterality 2003, 8, 297–306. [Google Scholar] [CrossRef]
- Kuper, M.; Doring, K.; Spangenberg, C.; Konczak, J.; Gizewski, E.R.; Schoch, B.; Timmann, D. Location and restoration of function after cerebellar tumor removal-a longitudinal study of children and adolescents. Cerebellum 2013, 12, 48–58. [Google Scholar] [CrossRef]
- Riechers, R.G., 2nd; Ruff, R.L. Rehabilitation in the patient with mild traumatic brain injury. Continuum 2010, 16, 128–149. [Google Scholar] [CrossRef]
- Rajasekhar Sajja Srinivasa Siva, N. Anatomy of Cerebellum. In Spinocerebellar Ataxia; Patricia Bozzetto, A., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 1. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, S.-S.; Nam, S.-M.; Cho, I.-H. Injury of the Vestibulocerebellar Tract and Signs of Ataxia in Patients with Cerebellar Stroke. J. Clin. Med. 2023, 12, 6877. https://doi.org/10.3390/jcm12216877
Yeo S-S, Nam S-M, Cho I-H. Injury of the Vestibulocerebellar Tract and Signs of Ataxia in Patients with Cerebellar Stroke. Journal of Clinical Medicine. 2023; 12(21):6877. https://doi.org/10.3390/jcm12216877
Chicago/Turabian StyleYeo, Sang-Seok, Seung-Min Nam, and In-Hee Cho. 2023. "Injury of the Vestibulocerebellar Tract and Signs of Ataxia in Patients with Cerebellar Stroke" Journal of Clinical Medicine 12, no. 21: 6877. https://doi.org/10.3390/jcm12216877





