Optically Guided High-Frequency Ultrasound to Differentiate High-Risk Basal Cell Carcinoma Subtypes: A Single-Centre Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
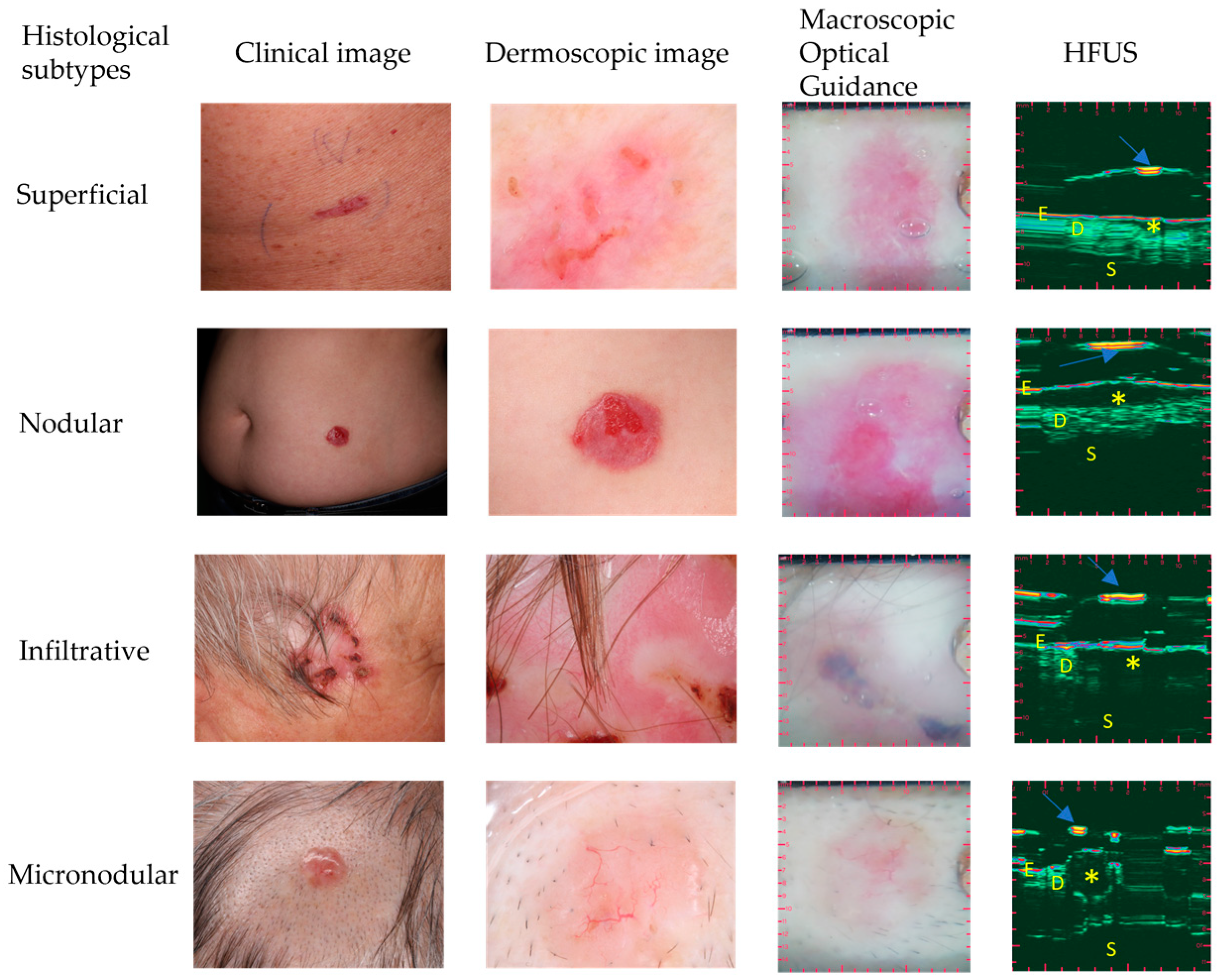
2.2. Optically Guided High-Frequency Ultrasound Imaging
2.3. The Evaluation of Macroscopic Clinical and Dermoscopic Images
3. Results
3.1. Patient Data
3.2. Statistical Analysis
3.3. OG-HFUS Imaging
3.4. BCC Risk Categorization Algorithm
3.5. OG-HFUS Compared to Clinical and Dermoscopic Image Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lear, W.; Dahlke, E.; Murray, C.A. Basal cell carcinoma: Review of epidemiology, pathogenesis, and associated risk factors. J. Cutan. Med. Surg. 2007, 11, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Gilbody, J.S.; Aitken, J.; Green, A. What causes basal cell carcinoma to be the commonest cancer? Aust. J. Public Health 1994, 18, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Ting, P.T.; Kasper, R.; Arlette, J.P. Metastatic basal cell carcinoma: Report of two cases and literature review. J. Cutan. Med. Surg. 2005, 9, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.; Walton, S.; Keczkes, K. Extensive and fatal basal cell carcinoma: A report of three cases. Br. J. Dermatol. 1992, 127, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.I.; Chen, E.H.; Ratner, D. Basal-cell carcinoma. N. Engl. J. Med. 2005, 353, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Marchac, D.; Papadopoulos, O.; Duport, G. Curative and aesthetic results of surgical treatment of 138 basal-cell carcinomas. J. Dermatol. Surg. Oncol. 1982, 8, 379–387. [Google Scholar] [CrossRef]
- Hoorens, I.; Vossaert, K.; Ongenae, K.; Brochez, L. Is early detection of basal cell carcinoma worthwhile? Systematic review based on the WHO criteria for screening. Br. J. Dermatol. 2016, 174, 1258–1265. [Google Scholar] [CrossRef]
- Bichakjian, C.K.; Olencki, T.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Blitzblau, R.; Bowen, G.M.; Contreras, C.M.; Daniels, G.A.; Decker, R.; et al. Merkel cell carcinoma, version 1.2018, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 742–774. [Google Scholar] [CrossRef]
- Saldanha, G.; Fletcher, A.; Slater, D. Basal cell carcinoma: A dermatopathological and molecular biological update. Br. J. Dermatol. 2003, 148, 195–202. [Google Scholar] [CrossRef]
- Coldiron, B.; Storrs, P. Why appropriate use criteria for Mohs micrographic surgery? J. Am. Acad. Dermatol. 2012, 67, 551. [Google Scholar] [CrossRef]
- Hendrix, J.D.; Parlette, H.L. Micronodular basal cell carcinoma: A deceptive histologic subtype with frequent clinically undetected tumor extension. Arch. Dermatol. 1996, 132, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Akay, B.N.; Saral, S.; Heper, A.O.; Erdem, C.; Rosendahl, C. Basosquamous carcinoma: Dermoscopic clues to diagnosis. J. Dermatol. 2017, 44, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X. Textbook of Dermatologic Ultrasound; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Del Marmol, V.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef] [PubMed]
- Berson, M.; Gregoire, J.M.; Gens, F.; Rateau, J.; Jamet, F.; Vaillant, L.; Tranquart, F.; Pourcelot, L. High frequency (20 MHz) ultrasonic devices: Advantages and applications. Eur. J. Ultrasound. 1999, 10, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Luz, F.B.; Ferron, C.; Cardoso, G.P. Surgical treatment of basal cell carcinoma: An algorithm based on the literature. An. Bras. Dermatol. 2015, 90, 377–383. [Google Scholar] [CrossRef]
- Cairnduff, F.; Stringer, M.; Hudson, E.; Ash, D.; Brown, S. Superficial photodynamic therapy with topical 5-aminolaevulinic acid for superficial primary and secondary skin cancer. Br. J. Dermatol. 1994, 69, 605–608. [Google Scholar] [CrossRef]
- Morton, C.; Szeimies, R.M.; Sidoroff, A.; Braathen, L. European guidelines for topical photodynamic therapy part 1: Treatment delivery and current indications–actinic keratoses, Bowen’s disease, basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 536–544. [Google Scholar] [CrossRef]
- Andrews, M.D. Cryosurgery for common skin conditions. Am. Fam. Physician 2004, 69, 2365–2372. [Google Scholar]
- Avril, M.F.; Auperin, A.; Margulis, A.; Gerbaulet, A.; Duvillard, P.; Benhamou, E.; Guillaume, J.C.; Chalon, R.; Petit, J.Y.; Sancho-Garnier, H.; et al. Basal cell carcinoma of the face: Surgery or radiotherapy? Results of a randomized study. Br. J. Cancer 1997, 76, 100–106. [Google Scholar] [CrossRef]
- Hall, V.; Leppard, B.J.; McGill, J.; Kesseler, M.; White, J.; Goodwin, P. Treatment of basal-cell carcinoma: Comparison of radiotherapy and cryotherapy. Clin. Radiol. 1986, 37, 33–34. [Google Scholar] [CrossRef]
- Marzuka, A.G.; Book, S. Basal cell carcinoma: Pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J. Biol. Med. 2015, 88, 167–179. [Google Scholar] [PubMed]
- Álvarez-Salafranca, M.; Ara, M.; Zaballos, P. Dermoscopy in Basal Cell Carcinoma: An Updated Review. Actas Dermo-Sifiliográficas Engl. Ed. 2021, 112, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.D.; Desai, A.D.; Horowitz, D.C.; Kartono, F.; Wahl, T. The use of high-frequency ultrasound in the evaluation of superficial and nodular basal cell carcinomas. Dermatol. Surg. 2007, 33, 1220–1227. [Google Scholar] [PubMed]
- Pierce, M.C.; Strasswimmer, J.; Hyle Park, B.; Cense, B.; De Boer, J.F. Birefringence measurements in human skin using polarization-sensitive optical coherence tomography. J. Biomed. Opt. 2004, 9, 287–291. [Google Scholar] [CrossRef]
- González, S.; Tannous, Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J. Am. Acad. Dermatol. 2002, 47, 869–874. [Google Scholar] [CrossRef]
- Vestergaard, M.; Macaskill, P.; Holt, P.; Menzies, S. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: A meta-analysis of studies performed in a clinical setting. Br. J. Dermatol. 2008, 159, 669–676. [Google Scholar] [CrossRef]
- Argenziano, G.; Fabbrocini, G.; Carli, P.; De Giorgi, V.; Sammarco, E.; Delfino, M. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions: Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch. Dermatol. 1998, 134, 1563–1570. [Google Scholar] [CrossRef]
- Menzies, S.W.; Westerhoff, K.; Rabinovitz, H.; Kopf, A.W.; McCarthy, W.H.; Katz, B. Surface microscopy of pigmented basal cell carcinoma. Arch. Dermatol. 2000, 136, 1012–1016. [Google Scholar] [CrossRef]
- Braun, R.P.; Rabinovitz, H.S.; Oliviero, M.; Kopf, A.W.; Saurat, J.-H. Dermoscopy of pigmented skin lesions. J. Am. Acad. Dermatol. 2005, 52, 109–121. [Google Scholar] [CrossRef]
- Nelson, J.S.; Kelly, K.M.; Zhao, Y.; Chen, Z. Imaging blood flow in human port-wine stain in situ and in real time using optical Doppler tomography. Arch. Dermatol. 2001, 137, 741–744. [Google Scholar]
- De Boer, J.F.; Milner, T.E. Review of polarization sensitive optical coherence tomography and Stokes vector determination. J. Biomed. Opt. 2002, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.V.; Von Braunmühl, T.; Berking, C.; Sattler, E.; Ulrich, M.; Reinhold, U.; Kurzen, H.; Dirschka, T.; Kellner, C.; Schuh, S.; et al. Optical coherence tomography of basal cell carcinoma: Influence of location, subtype, observer variability and image quality on diagnostic performance. Br. J. Dermatol. 2018, 178, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Suppa, M.; Fontaine, M.; Dejonckheere, G.; Cinotti, E.; Yélamos, O.; Diet, G.; Tognetti, L.; Miyamoto, M.; Orte Cano, C.; Perez-Anker, J.; et al. Line-field confocal optical coherence tomography of basal cell carcinoma: A descriptive study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Halip, I.A.; Vâţă, D.; Statescu, L.; Salahoru, P.; Patraşcu, A.I.; Temelie Olinici, D.; Tarcau, B.; Popescu, I.A.; Mocanu, M.; Constantin, A.M.; et al. Assessment of basal cell carcinoma using dermoscopy and high frequency ultrasound examination. Diagnostics 2022, 12, 735. [Google Scholar] [CrossRef]
- Bobadilla, F.; Wortsman, X.; Munoz, C.; Segovia, L.; Espinoza, M.; Jemec, G.B. Pre-surgical high resolution ultrasound of facial basal cell carcinoma: Correlation with histology. Cancer Imaging 2008, 8, 163. [Google Scholar] [CrossRef]
- Network NCC. Practice Guidelines in Oncology–v. 1.2005. Pancreatic Adenocarcinoma [online]. Available online: http://www.nccn.org (accessed on 29 November 2005).
- Kim, D.P.; Kus, K.J.; Ruiz, E. Basal cell carcinoma review. Hematol. Oncol. Clin. N. Am. 2019, 33, 13–24. [Google Scholar] [CrossRef]
- Xu, H.; Guo, L.; Wang, Q. Diagnostic Ultrasound in Dermatology; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Zhang, L.W.; Shen, X.; Fu, L.X.; Meng, H.M.; Lu, Y.H.; Chen, T.; Xu, R.H. Dermoscopy, reflectance confocal microscopy, and high-frequency ultrasound for the noninvasive diagnosis of morphea-form basal cell carcinoma. Skin. Res. Technol. 2022, 28, 766. [Google Scholar] [CrossRef]
- Csány, G.; Gergely, L.H.; Kiss, N.; Szalai, K.; Lőrincz, K.; Strobel, L.; Csabai, D.; Hegedüs, I.; Marosán-Vilimszky, P.; Füzesi, K.; et al. Preliminary Clinical Experience with a Novel Optical-Ultrasound Imaging Device on Various Skin Lesions. Diagnostics 2022, 12, 204. [Google Scholar] [CrossRef]
- Hernandez-Ibanez, C.; Blazquez-Sanchez, N.; Aguilar-Bernier, M.; Fúnez-Liébana, R.; Rivas-Ruiz, F.; de Troya-Martin, M. Usefulness of high-frequency ultrasound in the classification of histologic subtypes of primary basal cell carcinoma. Actas Dermo-Sifiliográficas Engl. Ed. 2017, 108, 42–51. [Google Scholar] [CrossRef]
- Hernández-Ibáñez, C.; Aguilar-Bernier, M.; Fúnez-Liébana, R.; Del Boz, J.; Blázquez, N.; de Troya, M. The usefulness of high-resolution ultrasound in detecting invasive disease in recurrent basal cell carcinoma after nonsurgical treatment. Actas Dermo-Sifiliográficas Engl. Ed. 2014, 105, 935–939. [Google Scholar] [CrossRef]
- Laverde-Saad, A.; Simard, A.; Nassim, D.; Jfri, A.; Alajmi, A.; O’Brien, E.; Wortsman, X. Performance of ultrasound for identifying morphological characteristics and thickness of cutaneous basal cell carcinoma: A systematic review. Dermatology 2022, 238, 692–710. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X.; Vergara, P.; Castro, A.; Saavedra, D.; Bobadilla, F.; Sazunic, I.; Zemelman, V.; Wortsman, J. Ultrasound as predictor of histologic subtypes linked to recurrence in basal cell carcinoma of the skin. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Zhu, A.Q.; Wang, Q.; Li, X.L.; Yan, J.N.; Li, M.X.; Jin, F.S.; Chen, S.T.; Guo, L.H.; Xu, H.X. Value of high-frequency ultrasound for differentiating invasive basal cell carcinoma from non-invasive types. Ultrasound Med. Biol. 2021, 47, 2910–2920. [Google Scholar] [CrossRef] [PubMed]
- Khlebnikova, A.N.; Molochkov, V.A.; Selezneva, E.V.; Belova, L.A.; Bezugly, A.; Molochkov, A.V. Ultrasonographic features of superficial and nodular basal cell carcinoma. Med. Ultrason. 2018, 20, 475–479. [Google Scholar] [CrossRef]
- Bens, G.; Binois, R.; Roussel, A.; Kerdraon, R.; Esteve, E. High-resolution ultrasonography for differential diagnosis between nodular basal carcinoma and sebaceous hyperplasia of the face: A pilot study. Annales de Dermatologie et de Venereologie 2015, 142, 646–652. [Google Scholar] [CrossRef]
- Siskou, S.; Pasquali, P.; Trakatelli, M. High Frequency Ultrasound of Basal Cell Carcinomas: Ultrasonographic Features and Histological Subtypes, a Retrospective Study of 100 Tumors. J. Clin. Med. 2023, 12, 3893. [Google Scholar] [CrossRef]
- Qin, J.; Wang, J.; Zhu, Q.; Liu, J.; Gao, Y.; Wang, Y.; Jin, H. Usefulness of high-frequency ultrasound in differentiating basal cell carcinoma from common benign pigmented skin tumors. Skin. Res. Technol. 2021, 27, 766–773. [Google Scholar] [CrossRef]
- Alfageme, F.; Salgüero, I.; Nájera, L.; Suarez, M.L.; Roustan, G. Increased Marginal Stiffness Differentiates Infiltrative from Noninfiltrative Cutaneous Basal Cell Carcinomas in the Facial Area: A Prospective Study. J. Ultrasound Med. 2019, 38, 1841–1845. [Google Scholar] [CrossRef]
- Wang, S.Q.; Liu, J.; Zhu, Q.L.; Zhao, C.Y.; Qu, T.; Li, F.; Wortsman, X.; Jin, H.Z. High-frequency ultrasound features of basal cell carcinoma and its association with histological recurrence risk. Chin. Med. J. 2019, 132, 2021–2026. [Google Scholar] [CrossRef]
- Nassiri-Kashani, M.; Sadr, B.; Fanian, F.; Kamyab, K.; Noormohammadpour, P.; Shahshahani, M.M.; Zartab, H.; Naghizadeh, M.M.; Sarraf-Yazdy, M.; Firooz, A. Pre-operative assessment of basal cell carcinoma dimensions using high frequency ultrasonography and its correlation with histopathology. Skin. Res. Technol. 2013, 19, e132–e138. [Google Scholar] [CrossRef]
- Campanella, G.; Navarrete-Dechent, C.; Liopyris, K.; Monnier, J.; Aleissa, S.; Minhas, B.; Scope, A.; Longo, C.; Guitera, P.; Pellacani, G.; et al. Deep learning for basal cell carcinoma detection for reflectance confocal microscopy. J. Investig. Dermatol. 2022, 142, 97–103. [Google Scholar] [CrossRef] [PubMed]


| Low-Risk HST | High-Risk HST | ||||
|---|---|---|---|---|---|
| Characteristic | Subgroup | Nodular | Superficial | Micronodular | Infiltrative |
| Shape | Regular (oval) | 31 | 0 | 2 | 1 |
| Regular (ribbon-like) | 1 | 12 | 1 | 0 | |
| Irregular | 3 | 0 | 2 | 10 | |
| Margin | Well-defined | 33 | 12 | 1 | 1 |
| Ill-defined | 2 | 0 | 4 | 10 | |
| Internal echoes | Homogenous | 25 | 10 | 3 | 1 |
| Non-homogenous | 10 | 2 | 2 | 10 | |
| Hyperechoic fields | Yes | 1 | 0 | 0 | 0 |
| No | 34 | 12 | 5 | 11 | |
| Posterior echoes | Yes (acoustic) | 5 | 0 | 0 | 0 |
| Yes (shadow) | 1 | 0 | 0 | 1 | |
| No | 29 | 12 | 5 | 10 | |
| Depth | Epidermis/dermis | 33 | 12 | 4 | 4 |
| Subcutis | 2 | 0 | 1 | 7 | |
| BCC Histological Subtype Classifier Algorithm Based on Optically Guided High-Frequency Ultrasound Characteristics. 3 or More Points: High-Risk; Below 3 Points: Low-Risk | ||
|---|---|---|
| Shape | Irregular shape: +3 point | Regular shape: 0 point |
| Margin | Ill-defined: +3 point | Well-defined: 0 point |
| Internal echoes | Non-homogenous: +2 point | Homogenous: 0 point |
| Depth | Hypodermis: +1 point | Epidermis/dermis: 0 point |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozsányi, S.; Boostani, M.; Farkas, K.; Hamilton-Meikle, P.; Varga, N.N.; Szabó, B.; Vasanits, F.; Kuroli, E.; Meznerics, F.A.; Lőrincz, K.; et al. Optically Guided High-Frequency Ultrasound to Differentiate High-Risk Basal Cell Carcinoma Subtypes: A Single-Centre Prospective Study. J. Clin. Med. 2023, 12, 6910. https://doi.org/10.3390/jcm12216910
Bozsányi S, Boostani M, Farkas K, Hamilton-Meikle P, Varga NN, Szabó B, Vasanits F, Kuroli E, Meznerics FA, Lőrincz K, et al. Optically Guided High-Frequency Ultrasound to Differentiate High-Risk Basal Cell Carcinoma Subtypes: A Single-Centre Prospective Study. Journal of Clinical Medicine. 2023; 12(21):6910. https://doi.org/10.3390/jcm12216910
Chicago/Turabian StyleBozsányi, Szabolcs, Mehdi Boostani, Klára Farkas, Phyllida Hamilton-Meikle, Noémi Nóra Varga, Boglárka Szabó, Flóra Vasanits, Enikő Kuroli, Fanni Adél Meznerics, Kende Lőrincz, and et al. 2023. "Optically Guided High-Frequency Ultrasound to Differentiate High-Risk Basal Cell Carcinoma Subtypes: A Single-Centre Prospective Study" Journal of Clinical Medicine 12, no. 21: 6910. https://doi.org/10.3390/jcm12216910
APA StyleBozsányi, S., Boostani, M., Farkas, K., Hamilton-Meikle, P., Varga, N. N., Szabó, B., Vasanits, F., Kuroli, E., Meznerics, F. A., Lőrincz, K., Holló, P., Bánvölgyi, A., Wikonkál, N. M., Paragh, G., & Kiss, N. (2023). Optically Guided High-Frequency Ultrasound to Differentiate High-Risk Basal Cell Carcinoma Subtypes: A Single-Centre Prospective Study. Journal of Clinical Medicine, 12(21), 6910. https://doi.org/10.3390/jcm12216910









