Impact of Vein of Marshall Ethanol Infusion Combined with Anatomical Ablation for the Treatment of Persistent Atrial Fibrillation: A Long-Term Follow-Up Based on Implantable Loop Recorders
Abstract
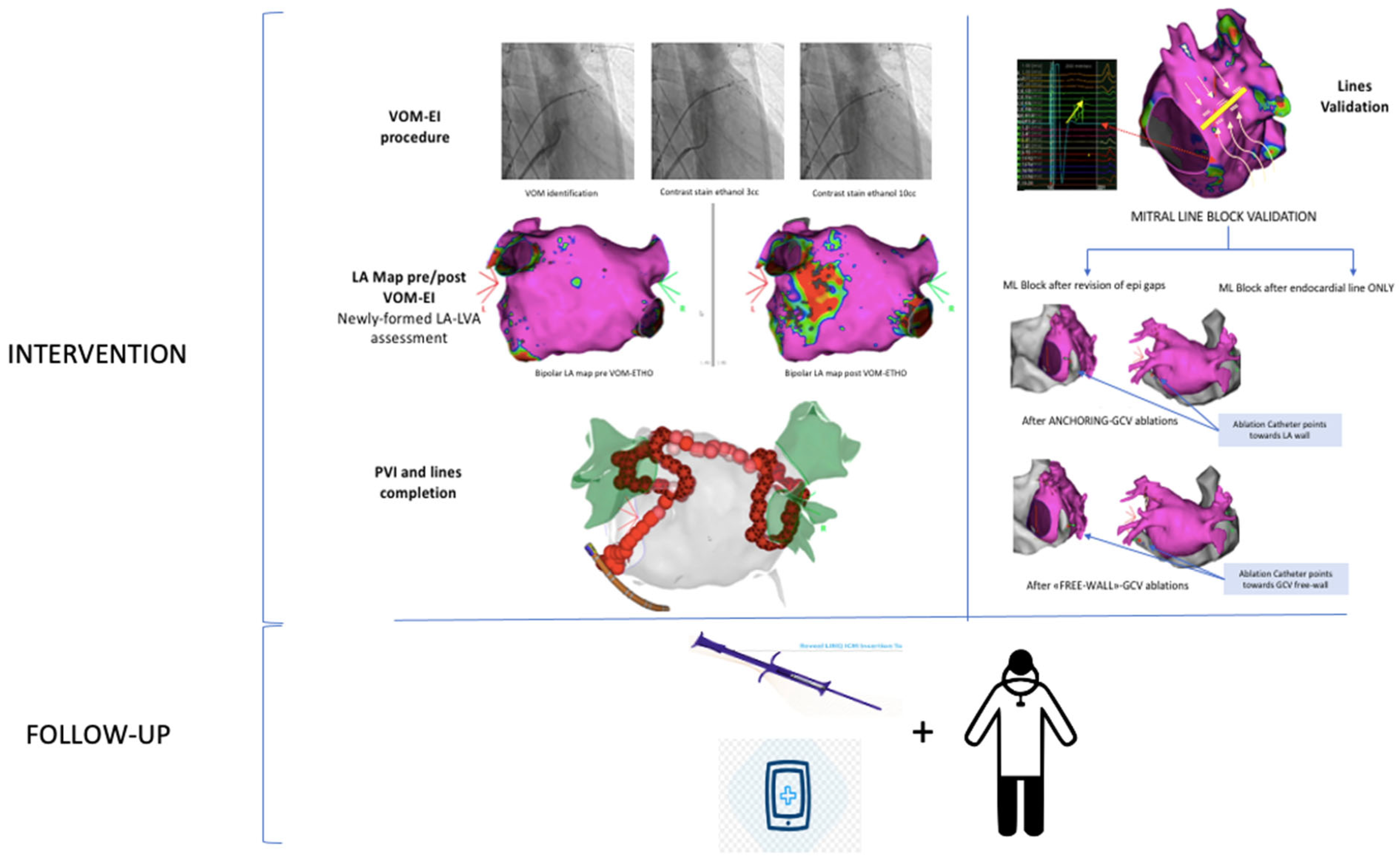
:1. Introduction
2. Methods
2.1. Patient Selection
2.2. Voltage Mapping
2.3. Vein of Marshall Ethanol Infusion
2.4. Catheter Ablation
2.5. Follow-up
2.6. Statistical Analysis
3. Results
3.1. Population
3.2. Vein of Marshall Ethanol Infusion
3.3. LA Mapping
3.4. Ablation
3.5. Follow-up
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.-H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. J. Arrhythmia 2017, 33, 369–409. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Marrouche, N.F.; Natale, A. Pulmonary vein antrum isolation: Intracardiac echocardiography-guided technique. J. Cardiovasc. Electrophysiol. 2004, 15, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Arentz, T.; Weber, R.; Bürkle, G.; Herrera, C.; Blum, T.; Stockinger, J.; Minners, J.; Neumann, F.J.; Kalusche, D. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation 2007, 115, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Katritsis, D.; Merchant, F.M.; Mela, T.; Singh, J.P.; Heist, E.K.; Armoundas, A.A. Catheter ablation of atrial fibrillation: The search for substrate-driven end points. J. Am. Coll. Cardiol. 2010, 55, 2293–2298. [Google Scholar] [CrossRef]
- Cappato, R.; Calkins, H.; Chen, S.A.; Davies, W.; Iesaka, Y.; Kalman, J.; Kim, Y.-H.; Klein, G.; Natale, A.; Packer, D.; et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2010, 3, 32–38. [Google Scholar] [CrossRef]
- Bhargava, M.; Di Biase, L.; Mohanty, P.; Prasad, S.; Martin, D.O.; Williams-Andrews, M.; Wazni, O.M.; Burkhardt, J.D.; Cummings, J.E.; Khaykin, Y.; et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: Results from a multicenter study. Heart Rhythm 2009, 6, 1403–1412. [Google Scholar] [CrossRef]
- Elayi, C.S.; Verma, A.; Di Biase, L.; Ching, C.K.; Patel, D.; Barrett, C.; Martin, D.; Rong, B.; Fahmy, T.S.; Khaykin, Y.; et al. Ablation for longstanding permanent atrial fibrillation: Results from a randomized study comparing three different strategies. Heart Rhythm 2008, 5, 1658–1664. [Google Scholar] [CrossRef]
- Verma, A.; Jiang, C.-Y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to Catheter Ablation for Persistent Atrial Fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef]
- Lee, S.-H.; Tai, C.-T.; Hsieh, M.-H.; Tsao, H.-M.; Lin, Y.-J.; Chang, S.-L.; Huang, J.-L.; Lee, K.-T.; Chen, Y.-J.; Cheng, J.-J.; et al. Predictors of non-pulmonary vein ectopic beats initiating paroxysmal atrial fibrillation. J. Am. Coll. Cardiol. 2005, 46, 1054–1059. [Google Scholar] [CrossRef]
- Vlachos, K.; Denis, A.; Takigawa, M.; Kitamura, T.; Martin, C.A.; Frontera, A.; Martin, R.; Bazoukis, G.; Bourier, F.; Cheniti, G.; et al. The role of Marshall bundle epicardial connections in atrial tachycardias after atrial fi-brillation ablation. Heart Rhythm 2019, 9, 1341–1347. [Google Scholar] [CrossRef]
- Ulphani, J.S.; Arora, R.; Cain, J.H.; Villuendas, R.; Shen, S.; Gordon, D.; Inderyas, F.; Harvey, L.A.; Morris, A.; Goldberger, J.J.; et al. The ligament of Marshall as a parasympathetic conduit. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Baez-Escudero, J.; Takehiko, K.; Amish, D. Ethanol infusion in the vein of Marshall leads to parasympathetic denervation of the human left Atrium. J. Am. Coll. Cardiol. 2014, 63, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.T.; Lai, A.C.; Hwang, C.; Fan, L.-T.; Karagueuzian, H.S.; Chen, P.-S.; Fishbein, M.C. The ligament of Marshall: A structural analysis in human hearts with implications for atrial arrhythmias. J. Am. Coll. Cardiol. 2000, 36, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Kamanu, S.; Tan, A.Y.; Peter, C.T.; Hwang, C.; Chen, P. Vein of Marshall Activity During Sustained Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2006, 17, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Pambrun, T.; Vlachos, K.; Goujeau, C.; André, C.; Krisai, P.; Ramirez, F.D.; Kamakura, T.; Takagi, T.; Nakatani, Y.; et al. Impact of vein of Marshall ethanol infusion on mitral isthmus block efficacy and durability. Circ. Arrhythmia Electrophysiol. 2020, 13, e008884. [Google Scholar] [CrossRef]
- Valderrábano, M.; Peterson, L.E.; Swarup, V.; Schurmann, P.A.; Makkar, A.; Doshi, R.N.; DeLurgio, D.; Athill, C.A.; Ellenbogen, K.A.; Natale, A.; et al. Effect of Catheter Ablation with Vein of Marshall Ethanol Infusion vs Catheter Ablation Alone on Persistent Atrial Fibrillation The VENUS Randomized Clinical Trial. J. Am. Med. Assoc. 2020, 324, 1620–1628. [Google Scholar] [CrossRef]
- Derval, N.; Duchateau, J.; Denis, A.; Ramirez, F.D.; Mahida, S.; André, C.; Krisai, P.; Nakatani, Y.; Kitamura, T.; Takigawa, M.; et al. Marshall bundle elimination, Pulmonary vein isolation, and Line completion for anatomical ablation of persistent atrial fibrillation (Marshall-PLAN): Prospective, single-center study. Heart Rhythm 2020, 18, 529–537. [Google Scholar] [CrossRef]
- Sanders, P.; Pürerfellner, H.; Pokushalov, E.; Sarkar, S.; Di Bacco, M.; Maus, B.; Dekker, L.R. Performance of a 9 new atrial fibrillation detection algorithm in a miniaturized insertable cardiac monitor: Results from the 10 Reveal LINQ Usability Study. Heart Rhythm 2016, 13, 1425–1430. [Google Scholar] [CrossRef]
- Parameswaran, R.; Al-Kaisey, A.M.; Kalman, J.M. Catheter ablation for atrial fibrillation: Current indications and evolving technologies. Nat. Rev. Cardiol. 2021, 18, 210–225. [Google Scholar] [CrossRef]
- Lee, J.M.; Shim, J.; Park, J.; Yu, H.T.; Kim, T.-H.; Park, J.-K.; Uhm, J.-S.; Kim, J.-B.; Joung, B.; Lee, M.-H.; et al. The Electrical Isolation of the Left Atrial Posterior Wall in Catheter Ablation of Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2019, 5, 1253–1261, Correction in JACC Clin. Electrophysiol. 2023, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajah, A.; Kadhim, K.; Lau, D.H.; Emami, M.; Linz, D.; Khokhar, K.; Munawar, D.A.; Mishima, R.; Malik, V.; O’shea, C.; et al. Feasibility, Safety, and Efficacy of Posterior Wall Isolation During Atrial Fibrillation Ablation: A Systematic Review and Meta-Analysis. Circ. Arrhythmia Electrophysiol. 2019, 12, e007005. [Google Scholar] [CrossRef]
- Gillis, K.; O’Neill, L.; Wielandts, J.Y.; Hilfiker, G.; Almorad, A.; Lycke, M.; El Haddad, M.; le Polain de Waroux, J.B.; Tavernier, R.; Duytschaever, M.; et al. Vein of Marshall Ethanol Infusion as First Step for Mitral Isthmus Linear Ablation. JACC Clin. Electrophysiol. 2022, 8, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Mhanna, M.; Beran, A.; Al-Abdouh, A.; Sajdeya, O.; Altujjar, M.; Alom, M.; Abumoawad, A.M.; Elzanaty, A.M.; Chacko, P.; Eltahawy, E.A. Adjunctive Vein of Marshall Ethanol Infusion During Atrial Fibrillation Ablation: A Systematic Review and Meta-Analysis. J. Atr. Fibrillation 2021, 14, 20200492. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Lo, L.W.; Lin, Y.J.; Lin, C.; Chang, S.; Chung, F.; Chao, T.; Hu, Y.; Tuan, T.; Liao, J.; et al. Long-term efficacy and safety of adjunctive ethanol infusion into the vein of Marshall during catheter ablation for nonparoxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2019, 30, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, M.; Vlachos, K.; A Martin, C.; Bourier, F.; Denis, A.; Kitamura, T.; Cheniti, G.; Lam, A.; Martin, R.; Frontera, A.; et al. Acute and mid-term outcome of ethanol infusion of vein of Marshall for the treatment of perimitral flutter. Europace 2020, 22, 1252–1260. [Google Scholar] [CrossRef]
- Vilcant, V.; Kousa, O.; Hai, O. Implantable Loop Recorder; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Andrade, J.G.; Champagne, J.; Dubuc, M.; Deyell, M.W.; Verma, A.; Macle, L.; Leong-Sit, P.; Novak, P.; Badra-Verdu, M.; Sapp, J.; et al. Cryoballoon or Radiofrequency Ablation for Atrial Fibrillation Assessed by Continuous Monitoring: A Randomized Clinical Trial. Circulation 2019, 140, 1779–1788. [Google Scholar] [CrossRef]
- Lai, Y.; Liu, X.; Sang, C.; Long, D.; Li, M.; Ge, W.; Liu, X.; Lu, Z.; Guo, Q.; Jiang, C.; et al. Effectiveness of ethanol infusion into the vein of Marshall combined with a fixed anatomical ablation strategy (the “upgraded 2C3L” approach) for catheter ablation of persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2021, 32, 1849–1856. [Google Scholar] [CrossRef]

| Patients (n = 31) | |
| Age (yr) | 66 ± 8 |
| Male sex, n (%) | 71 |
| Structural cardiac disease, n (%) Ischaemic Valvular heart disease Hypertrophic cardiomyopathy Dilated cardiomyopaty AF-induced cardiomyopathy Previous open-chest surgery | 13 (58.1) 6 (19.4) 2 (3.2) 2 (6.5) 1 (2.1) 6 (19.4) 2 (6.5) |
| Hypertension (%) | 71 |
| CHA2DS2-VASc score (mean) | 2.48 ± 1.48 |
| Antiarrhythmic Drugs n (%) Amiodarone Ic drugs Sotalol | 30 (96.8) 16 (51.6) 11 (35.5) 3 (9.7) |
| AF Characteristics | |
| Maximum duration (mo) | 8.13 ± 4.37 |
| Long-standing AF, n (%) | 15 (48.4) |
| AT/AF at the start of the procedure, n (%) | 12 (38.7) |
| Echocardiographic Parameters | |
| Left atrial volume (mL/m2) | 32 ± 5.00 |
| Telediastolic ventricular diameter (mm) | 50 ± 3.63 |
| Left ventricular ejection fraction (%) | 56 ± 11.22 |
| Patients (n = 31) | |
| VOM Ethanol Infusion | |
| VOM-EI time (min) | 21.29 ± 10.07 |
| VOM-EI fluoroscopy time (min) | 10.13 ± 3.91 |
| Ethanol infused (cc) | 10.71 ± 2.00 (8–16) |
| Complications Overall, n (%) VOM dissection VOM perforation Pericardial effusion Tamponade | 5 (16.1) 1 (3.2) 3 (9.7) 2 (6.5) 0 |
| Incomplete ethanol delivery | 4 (12.9) |
| Acute pericarditis | 2 (6.5) |
| Ethanol infusion in wrong veins | 0 |
| Stroke | 0 |
| Vascular complications | 0 |
| Left Atrium Voltage Analysis | |
| Presence of left atrium low-voltage areas, n (%) | 13 (41.9) |
| Basal LA-LVA (cm2) | 2.30 ± 3.45 (0–14) |
| Newly formed LVA post-VOM-EI (cm2) | 8.22 ± 4.40 (0–16.50) |
| Ablation | |
| PVI completion/validation, n (%) | 31 (100) |
| Roofline block, n (%) | 28 (90.3) |
| Mitral line block, n (%) Endocardial only RF anchoring—GCV RF free wall—GCV | 30 (96.8) 17 (54.8) 8 (25.8) 5 (16.1) |
| Cavotricuspid isthmus block, n (%) | 31 (100) |
| Anatomical lesion set completed, n (%) | 27 (87.1) |
| Time to mitral line block (min) | 10.77 ± 8.72 (2–36) |
| Mean duration (months) | 12 ± 7 |
| AT/AF recurrences, n (%) | 4/27 (87%) |
| AAD discontinuation, n (%) | 23/31 (74%) |
| Delayed pericarditis, n | 0 |
| Delayed tamponade, n | 0 |
| (n = 4) | |
| Type of Recurrence | |
| Recurrence as AT, n (%) | 1 (25%) |
| Recurrence as AF, n (%) | 3 (75%) |
| Lesion Set | |
| Completed lesion set, n (%) | 2 (50%) |
| Roofline block, n (%) | 1 (25%) |
| Mitral line block, n (%) | 1 (25%) |
| Suboptimal VOM-EI, n (%) | 3 (75%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesti, M.; Luca, F.; Panchetti, L.; Garibaldi, S.; Startari, U.; Mirizzi, G.; Landra, F.; Giannoni, A.; Piacenti, M.; Rossi, A. Impact of Vein of Marshall Ethanol Infusion Combined with Anatomical Ablation for the Treatment of Persistent Atrial Fibrillation: A Long-Term Follow-Up Based on Implantable Loop Recorders. J. Clin. Med. 2023, 12, 6916. https://doi.org/10.3390/jcm12216916
Nesti M, Luca F, Panchetti L, Garibaldi S, Startari U, Mirizzi G, Landra F, Giannoni A, Piacenti M, Rossi A. Impact of Vein of Marshall Ethanol Infusion Combined with Anatomical Ablation for the Treatment of Persistent Atrial Fibrillation: A Long-Term Follow-Up Based on Implantable Loop Recorders. Journal of Clinical Medicine. 2023; 12(21):6916. https://doi.org/10.3390/jcm12216916
Chicago/Turabian StyleNesti, Martina, Fabiana Luca, Luca Panchetti, Silvia Garibaldi, Umberto Startari, Gianluca Mirizzi, Federico Landra, Alberto Giannoni, Marcello Piacenti, and Andrea Rossi. 2023. "Impact of Vein of Marshall Ethanol Infusion Combined with Anatomical Ablation for the Treatment of Persistent Atrial Fibrillation: A Long-Term Follow-Up Based on Implantable Loop Recorders" Journal of Clinical Medicine 12, no. 21: 6916. https://doi.org/10.3390/jcm12216916






