Animal Bite Injuries to the Face: A Retrospective Evaluation of 111 Cases
Abstract
:1. Introduction
2. Patients and Methods
3. Results
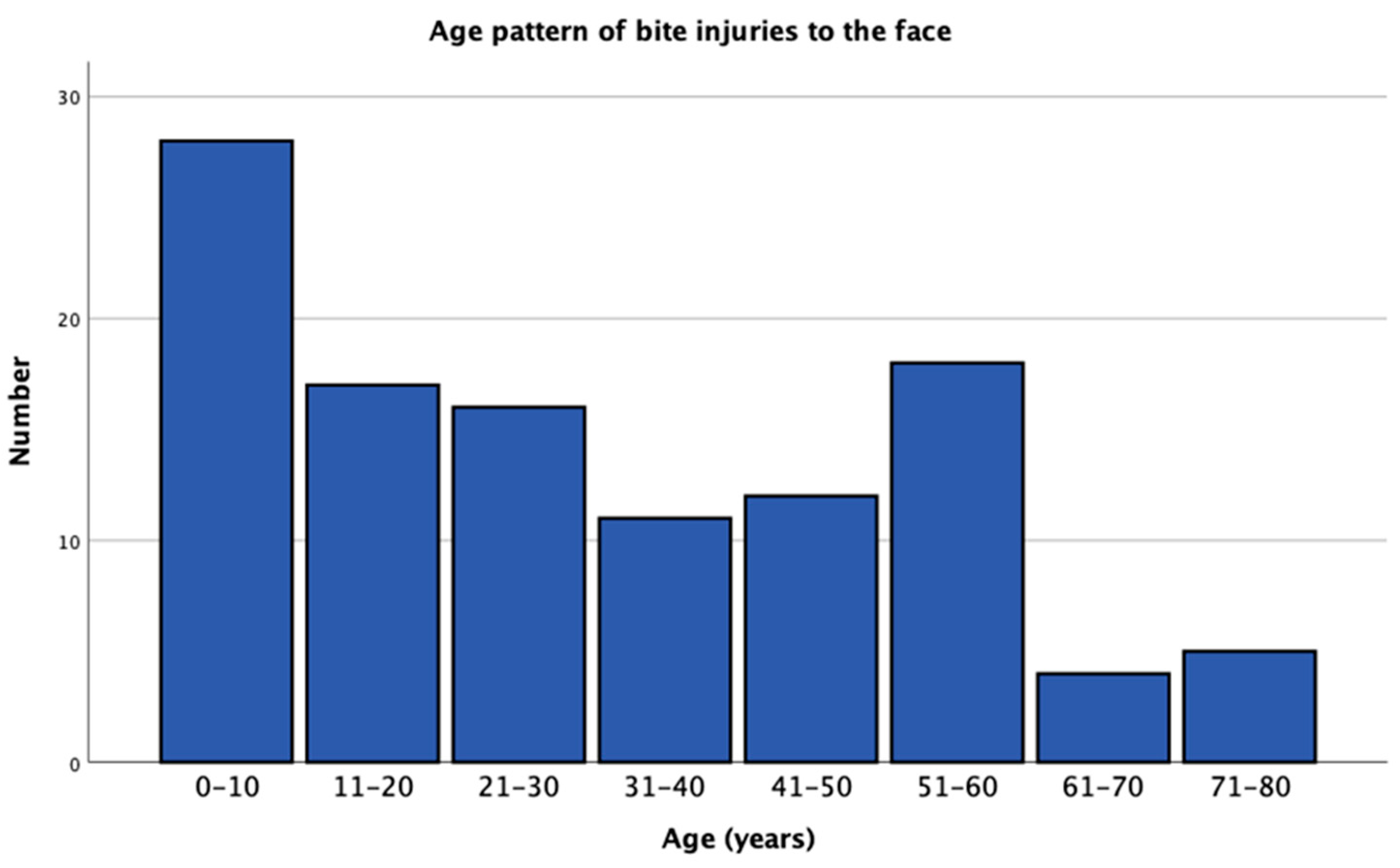
3.1. Epidemiology
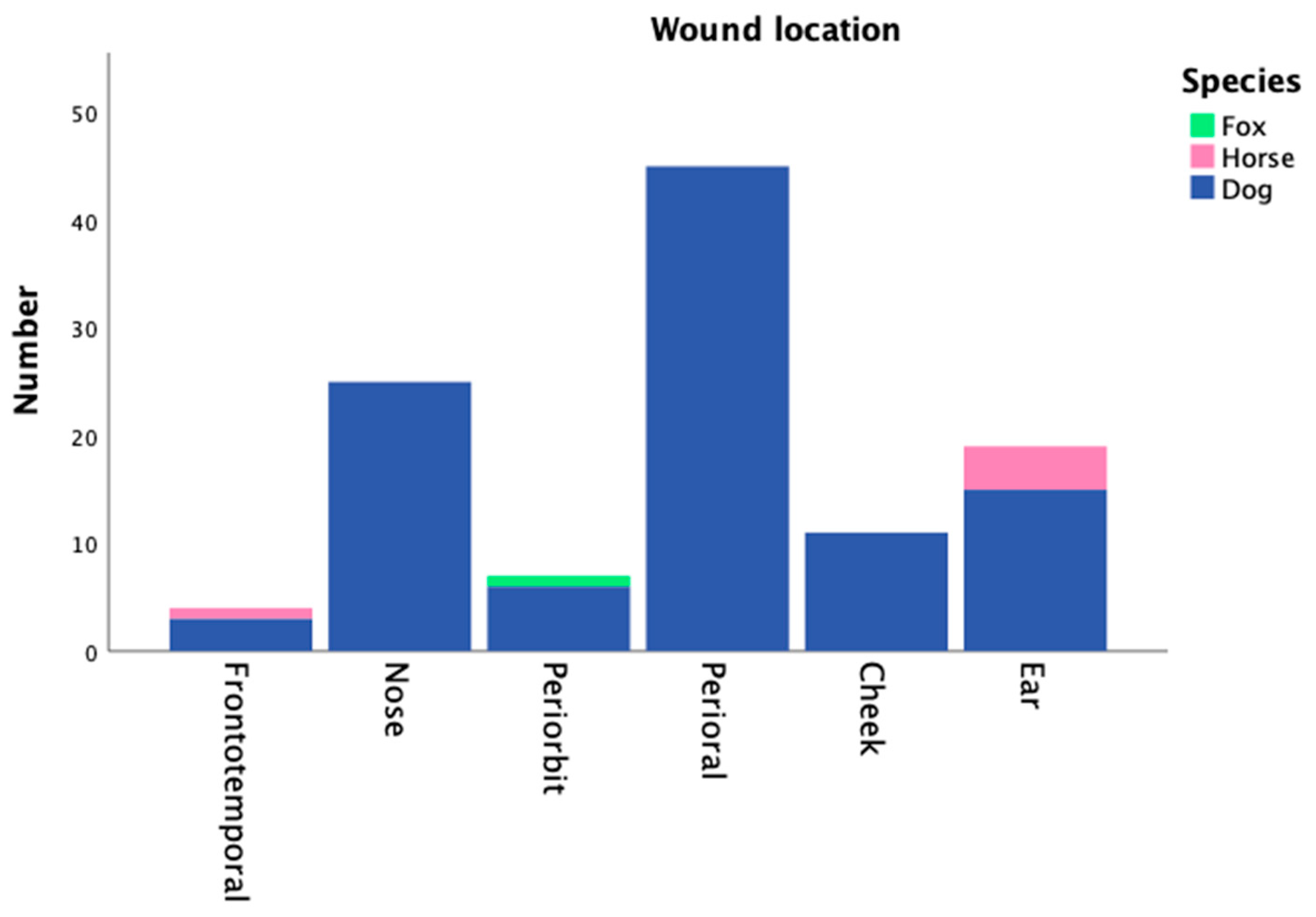
3.2. Wound Characteristics
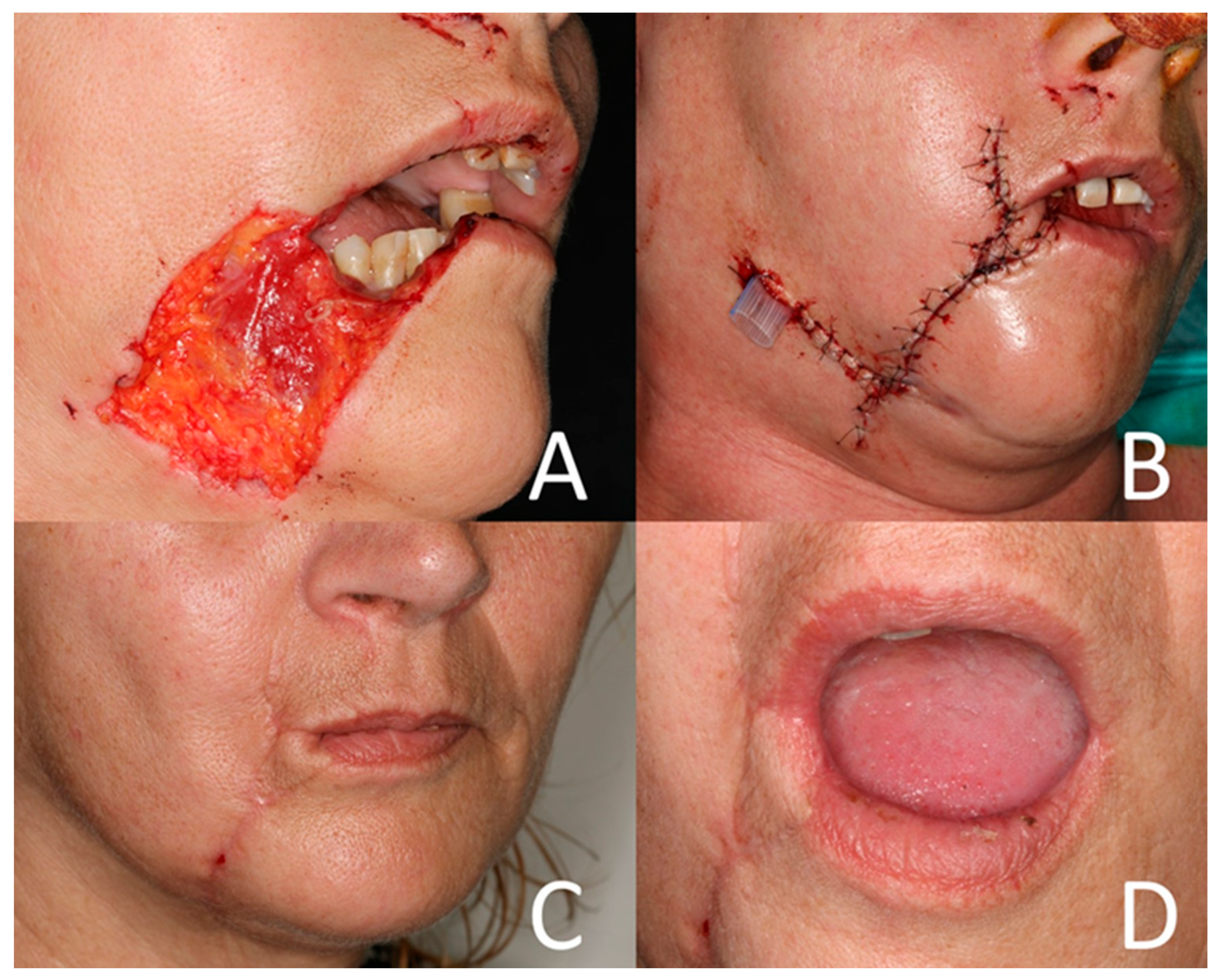
3.3. Surgical Treatment
3.4. Antibiotic Treatment
3.5. Wound Infections
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rothe, K.; Tsokos, M.; Handrick, W. Animal and Human Bite Wounds. Dtsch. Arztebl. Int. 2015, 112, 433–442; quiz 443. [Google Scholar] [CrossRef]
- Herbert, I.; Bünger, B. Hundebissverletzungen im Kopf-Hals-Bereich. Laryngol. Rhinol. Otol. 1986, 65, 92–95. [Google Scholar] [CrossRef]
- Boenning, D.A.; Fleisher, G.R.; Campos, J.M. Dog bites in children: Epidemiology, microbiology, and penicillin prophylactic therapy. Am. J. Emerg. Med. 1983, 1, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Griego, R.D.; Rosen, T.; Orengo, I.F.; Wolf, J.E. Dog, cat, and human bites: A review. J. Am. Acad. Dermatol. 1995, 33, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Kesting, M.R.; Hölzle, F.; Pox, C.; Thurmüller, P.; Wolff, K.-D. Animal bite injuries to the head: 132 cases. Br. J. Oral Maxillofac. Surg. 2006, 44, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Wolff, K.D. Management of animal bite injuries of the face: Experience with 94 patients. J. Oral Maxillofac. Surg. 1998, 56, 838–843; discussion 843-844. [Google Scholar] [CrossRef]
- Méndez Gallart, R.; Gómez Tellado, M.; Somoza Argibay, I.; Liras Muñoz, J.; Pais Piñeiro, E.; Vela Nieto, D. Mordeduras de perro. Análisis de 654 casos en 10 años. An. Esp. Pediatr. 2002, 56, 425–429. [Google Scholar] [CrossRef]
- Wu, P.S.; Beres, A.; Tashjian, D.B.; Moriarty, K.P. Primary repair of facial dog bite injuries in children. Pediatr. Emerg. Care 2011, 27, 801–803. [Google Scholar] [CrossRef]
- Stefanopoulos, P.K.; Tarantzopoulou, A.D. Facial bite wounds: Management update. Int. J. Oral Maxillofac. Surg. 2005, 34, 464–472. [Google Scholar] [CrossRef]
- Lackmann, G.M.; Draf, W.; Isselstein, G.; Töllner, U. Surgical treatment of facial dog bite injuries in children. J. Craniomaxillofac. Surg. 1992, 20, 81–86. [Google Scholar] [CrossRef]
- Talan, D.A.; Citron, D.M.; Abrahamian, F.M.; Moran, G.J.; Goldstein, E.J. Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N. Engl. J. Med. 1999, 340, 85–92. [Google Scholar] [CrossRef]
- Ferreira, S.; Ayres Quaresma, L.E.; Timóteo, C.A.; da Silva Fabris, A.L.; Faverani, L.P.; Francisconi, G.B.; Souza, F.A.; Júnior, I.R.G. The primary closure approach of dog bite injuries of the nose. J. Craniofac. Surg. 2014, 25, e216–e218. [Google Scholar] [CrossRef]
- Aydin, O.; Aydin Goker, E.T.; Arslan, Z.A.; Sert, H.M.; Teksam, O. Clinical features and management of animal bites in an emergency department: A single-center experience. Postgrad. Med. 2023, 135, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Mattice, T.; Schnaith, A.; Ortega, H.W.; Segura, B.; Kaila, R.; Amoni, I.; Shanley, R.; Louie, J.P. A Pediatric Level III Trauma Center Experience with Dog Bite Injuries. Clin. Pediatr. 2023, 99228231200097. [Google Scholar] [CrossRef] [PubMed]
- Becerra, C.M.C.; Hodge, D.O.; Bradley, E.A. Incidence and Characteristics of Facial and Ophthalmic Injuries from Domestic Mammal Bites: Parts of the data in the manuscript were presented at the American Academy of Ophthalmology Annual Meeting, 2022. Am. J. Ophthalmol. 2023, 252, 164–169. [Google Scholar] [CrossRef]
- Palacio, J.; León-Artozqui, M.; Pastor-Villalba, E.; Carrera-Martín, F.; García-Belenguer, S. Incidence of and risk factors for cat bites: A first step in prevention and treatment of feline aggression. J. Feline Med. Surg. 2007, 9, 188–195. [Google Scholar] [CrossRef]
- Selvi, F.; Stanbouly, D.; Stanbouly, R.; Baron, M.; Francois, K.; Halsey, J.; Marx, R.E.; Chuang, S.-K. Early Childhood (0 to 5 years) Presents the Greatest Risk for Facial Dog Bites. J. Oral Maxillofac. Surg. 2022, 80, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Stanbouly, D.; Stewart, S.J.; Harris, J.A.; Chuang, S.-K. Risk factors associated with infection in patients sustaining dog bites to the face. Oral Maxillofac. Surg. 2023, 27, 305–311. [Google Scholar] [CrossRef]
- Hwang, M.; Engelstad, M.; Chandra, S.R. Management of Soft Tissue Injuries in Children—A Comprehensive Review. Oral Maxillofac. Surg. Clin. N. Am. 2023, 35, 619–629. [Google Scholar] [CrossRef]
- Ali, S.S.; Ali, S.S. Dog bite injuries to the face: A narrative review of the literature. World J. Otorhinolaryngol. Head Neck Surg. 2022, 8, 239–244. [Google Scholar] [CrossRef]
- Chen, T.; Karim, M.; Grace, Z.T.; Magdich, A.R.; Carniol, E.C.; Benson, B.E.; Svider, P.F. Surgical management of facial dog bite trauma: A contemporary perspective and review. World J. Otorhinolaryngol. Head Neck Surg. 2023, 9, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Lisong, H.; Lianfu, W.; Jinhong, Y.; Haibin, Z. Clinical effect analysis of using medical glue versus conventional suturing to treat dog bite in children’s maxillofacial region after negative pressure sealing drainage: A randomized trial. Medicine 2023, 102, e34837. [Google Scholar] [CrossRef] [PubMed]
- Paschos, N.K.; Makris, E.A.; Gantsos, A.; Georgoulis, A.D. Primary closure versus non-closure of dog bite wounds. A randomised controlled trial. Injury 2014, 45, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-Q.; Yang, X.; Wang, X.-T.; Ji, A.-P.; Bai, J. Risk Factors for Infection of Sutured Maxillofacial Soft Tissue Injuries. Surg. Infect. 2022, 23, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Bracaglia, R.; D’Ettorre, M.; Gentileschi, S.; Tambasco, D. Was the surgeon a satisfactory informant? How to minimize room for claims. Aesthet. Surg. J. 2014, 34, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Tabaka, M.E.; Quinn, J.V.; Kohn, M.A.; Polevoi, S.K. Predictors of infection from dog bite wounds: Which patients may benefit from prophylactic antibiotics? Emerg. Med. J. 2015, 32, 860–863. [Google Scholar] [CrossRef]
- Monroy, A.; Behar, P.; Nagy, M.; Poje, C.; Pizzuto, M.; Brodsky, L. Head and neck dog bites in children. Otolaryngol. Head Neck Surg. 2009, 140, 354–357. [Google Scholar] [CrossRef]
- Cummings, P. Antibiotics to prevent infection in patients with dog bite wounds: A meta-analysis of randomized trials. Ann. Emerg. Med. 1994, 23, 535–540. [Google Scholar] [CrossRef]
- Greene, C.E. Greene’s Infectious Diseases of the Dog and Cat, 5th ed.; Elsevier: St. Louis, MO, USA, 2023; ISBN 9780323509336. [Google Scholar]
- Morgan, M.; Palmer, J. Dog bites. BMJ 2007, 334, 413–417. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Everett, E.D.; Dellinger, P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, E.L.; Montoya, J.G.; et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin. Infect. Dis. 2005, 41, 1373–1406. [Google Scholar] [CrossRef]
- Evans, J.; Hanoodi, M.; Wittler, M. StatPearls: Amoxicillin Clavulanate; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Goldstein, E.J. Current concepts on animal bites: Bacteriology and therapy. Curr. Clin. Top. Infect. Dis. 1999, 19, 99–111. [Google Scholar] [PubMed]
- Fielding, P.; Messahel, S. Guideline review—Human and animal bites: Antimicrobial prescribing. Arch. Dis. Child. Educ. Pract. Ed. 2022, 107, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Soper, D.E. Clindamycin. Obstet. Gynecol. Clin. N. Am. 1992, 19, 483–496. [Google Scholar] [CrossRef]
- Cefuroxime axetil: A new oral cephalosporin. DTB 1989, 27, 5–7. [CrossRef]
- WHO. Rabies vaccines: WHO position paper, April 2018—Recommendations. Vaccine 2018, 36, 5500–5503. [Google Scholar] [CrossRef]
- Robert Koch-Insitut. RKI Ratgeber Tollwut. Epid Bull. 2022, 3–11. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_Tollwut.html (accessed on 29 September 2022).
- Ellis, R.; Ellis, C. Dog and cat bites. Am. Fam. Physician 2014, 90, 239–243. [Google Scholar]
| Stage | Clinical Features |
|---|---|
| I | Superficial injury not involving muscle |
| II | Deep injury involving muscle |
| III | Deep injury involving muscle with loss of tissue |
| IVa | Deep injury involving muscle with loss of tissue and injury to vessels or nerves |
| IVb | The above, and bone involvement |
| Total | 111 |
| Mean Age | 30.30 ± 21.50 years |
| Sex | |
| Female | 59 (53.2%) |
| Male | 52 (46.8%) |
| Species | |
| Dog | 105 (94.6%) |
| Horse | 5 (4.5%) |
| Fox | 1 (0.9%) |
| Animal familiar | |
| Yes | 71 (63.9%) |
| No | 40 (36.0%) |
| Treatment delay | |
| Treatment within 6 h | 100 (90.1%) |
| Treatment after 6 h or more | 11 (9.9%) |
| Immunisation status animal | |
| Complete | 20 (18.0%) |
| Unknown | 91 (82.0%) |
| Main Location Wound | |
| Frontotemporal | 4 (3.6%) |
| Nose | 25 (22.5%) |
| Periorbit | 7 (6.3%) |
| Perioral | 45 (40.5%) |
| Cheek | 11 (9.9%) |
| Ear | 19 (17.1%) |
| Oral perforation | |
| Yes | 14 (12.6%) |
| No | 97 (87.4%) |
| Lackmann classification | |
| I | 30 (27.0%) |
| II | 41 (36.9%) |
| III | 37 (33.3%) |
| IVa | 1 (0.9%) |
| IVb | 2 (1.8%) |
| Tissue defect | |
| Yes | 40 (36.0%) |
| No | 71 (64.0%) |
| Fracture | |
| Yes | 2 (1.8%) |
| No | 109 (98.2%) |
| Anaesthesia | |
| Local | 69 (62.2%) |
| General | 42 (37.8%) |
| Surgical treatment | |
| Wound-cleansing alone | 6 (5.4%) |
| Debridement and primary closure | 83 (74.8%) |
| Local flap reconstruction | 5 (4.5%) |
| Avascular graft reconstruction | 12 (10.8%) |
| Microsurgical replantation or free flap reconstruction | 5 (4.5%) |
| Wound drain | |
| Yes | 21 (18.9%) |
| No | 90 (81.1%) |
| Lacrimal duct reconstruction | |
| Yes | 2 (1.8%) |
| No | 109 (98.2%) |
| Scar correction required | |
| Required | 8 (7.2%) |
| Not required | 103 (92.8%) |
| After primary closure | 2/83 (2.4%) |
| After other surgical treatment | 6/28 (21.4%) |
| Antibiotic Prophylaxis | |
| No | 9 (8.1%) |
| Yes | 102 (91.9%) |
| Type of antibiotics | |
| Amoxycillin with clavulanic acid | 69 (62.1%) |
| Clindaymcin | 15 (13.5%) |
| Cefuroxim | 12 (10.8%) |
| Cefazolin | 4 (3.6%) |
| Penicilline | 1 (0.9%) |
| Ciprofloxacin | 1 (0.9%) |
| Metronidazol (additional) | 3 (2.7%) |
| Wound Infection | ||
| Yes | 9 (8.1%) | |
| No | 102 (91.9%) | |
| Cases of infection/Wound location | p = 0.003 | |
| Nose | 1/25 (4.0%) | |
| Periorbit | 1/7 (14.3%) | |
| Perioral | 1/45 (2.3%) | |
| Cheek | 4/11 (36.4%) | |
| Ear | 1/19 (5.2%) | |
| Frontotemporal | 1/4 (25%) | |
| Scar correction required | p = 0.001 | |
| After wound infection | 3/9 (33.3%) | |
| Without wound infection | 5/102 (4.9%) | |
| Infections in | p = 0.887 | |
| Wounds with oral perforation | 1/14 (7.1%) | |
| Wounds without oral perforation | 8/97 (8.2%) | |
| Infections in | p = 0.461 | |
| Defect wounds | 4/37 (10.8%) | |
| Wounds without tissue defect | 5/74 (6.8%) | |
| Infections in | p = 0.212 | |
| Wounds with drain | 3/20 (15.0%) | |
| Wounds without drain | 6/91 (6.6%) | |
| Infections in | p = 0.900 | |
| Wounds treated within 6 h | 8/100 (8.0%) | |
| Wounds treated after 6 h | 1/11 (9.1%) | |
| Infections in wounds treated by | p = 0.048 | |
| Cleansing alone | 1/6 (16.7%) | |
| Primary closure | 4/83 (5.1%) | |
| Local flap reconstruction | 2/5 (40%) | |
| Reconstruction with avascular graft | 1/12 (8.3%) | |
| Microsurgical replantation or free flap reconstruction | 1/5 (20%) | |
| Infections in wounds treated by | p = 0.029 | |
| Primary closure | 4/83 (4.8%) | |
| Other treatment | 5/28 (17.9%) | |
| Infections/Lackmann Classification | p = 0.750 | |
| I | 1/29 (3.4%) | |
| II | 5/42 (11.9%) | |
| III | 3/37 (8.1%) | |
| IVa | 0/1 (0%) | |
| IVb | 0/2 (0%) | |
| Infections in patients with | p = 0.197 | |
| antibiotic prophylaxis | 7/104 (6.7%) | |
| without antibiotic prophylaxis | 2/7(28.6%) | |
| Infection in patients | p = 0.982 | |
| age under 10 years | 2/25 (8.0%) | |
| age 10 or older | 7/86 (8.1%) | |
| Infection/Antibiotic prophylaxis | p = 0.407 | |
| None | 2/9 (22.2%) | |
| Amoxycillin with clavulanic acid | 5/69 (7.2%) | |
| Clindaymcin | 0/15 (0%) | |
| Cefuroxim | 1/12 (0%) | |
| Cefazolin | 1/4 (25%) | |
| Penicilline | 0/1 (0%) | |
| Ciprofloxacin | 0/1 (0%) | |
| Metronidazol (additional) | 0/3 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurer, M.; Schlipköter, C.; Gottsauner, M.; Waiss, W.; Meier, J.K.; Fiedler, M.; Schuderer, J.G.; Taxis, J.; Reichert, T.E.; Ettl, T. Animal Bite Injuries to the Face: A Retrospective Evaluation of 111 Cases. J. Clin. Med. 2023, 12, 6942. https://doi.org/10.3390/jcm12216942
Maurer M, Schlipköter C, Gottsauner M, Waiss W, Meier JK, Fiedler M, Schuderer JG, Taxis J, Reichert TE, Ettl T. Animal Bite Injuries to the Face: A Retrospective Evaluation of 111 Cases. Journal of Clinical Medicine. 2023; 12(21):6942. https://doi.org/10.3390/jcm12216942
Chicago/Turabian StyleMaurer, Michael, Cornelius Schlipköter, Maximilian Gottsauner, Waltraud Waiss, Johannes K. Meier, Mathias Fiedler, Johannes G. Schuderer, Juergen Taxis, Torsten E. Reichert, and Tobias Ettl. 2023. "Animal Bite Injuries to the Face: A Retrospective Evaluation of 111 Cases" Journal of Clinical Medicine 12, no. 21: 6942. https://doi.org/10.3390/jcm12216942
APA StyleMaurer, M., Schlipköter, C., Gottsauner, M., Waiss, W., Meier, J. K., Fiedler, M., Schuderer, J. G., Taxis, J., Reichert, T. E., & Ettl, T. (2023). Animal Bite Injuries to the Face: A Retrospective Evaluation of 111 Cases. Journal of Clinical Medicine, 12(21), 6942. https://doi.org/10.3390/jcm12216942







