Neurogenic Bowel Dysfunction in Patients with Spinal Cord Injury and Multiple Sclerosis—An Updated and Simplified Treatment Algorithm
Abstract
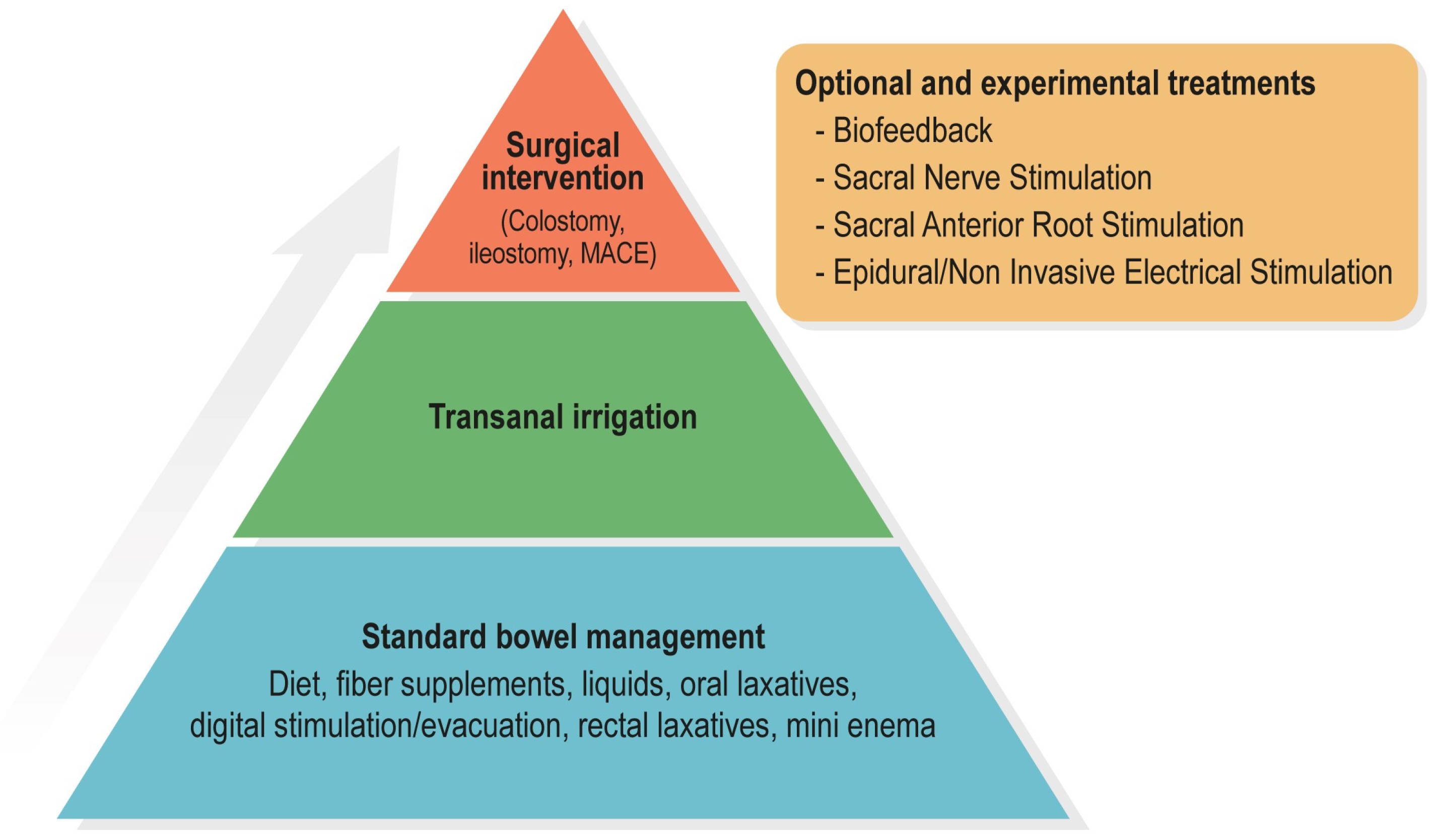
:1. Introduction
2. Methods
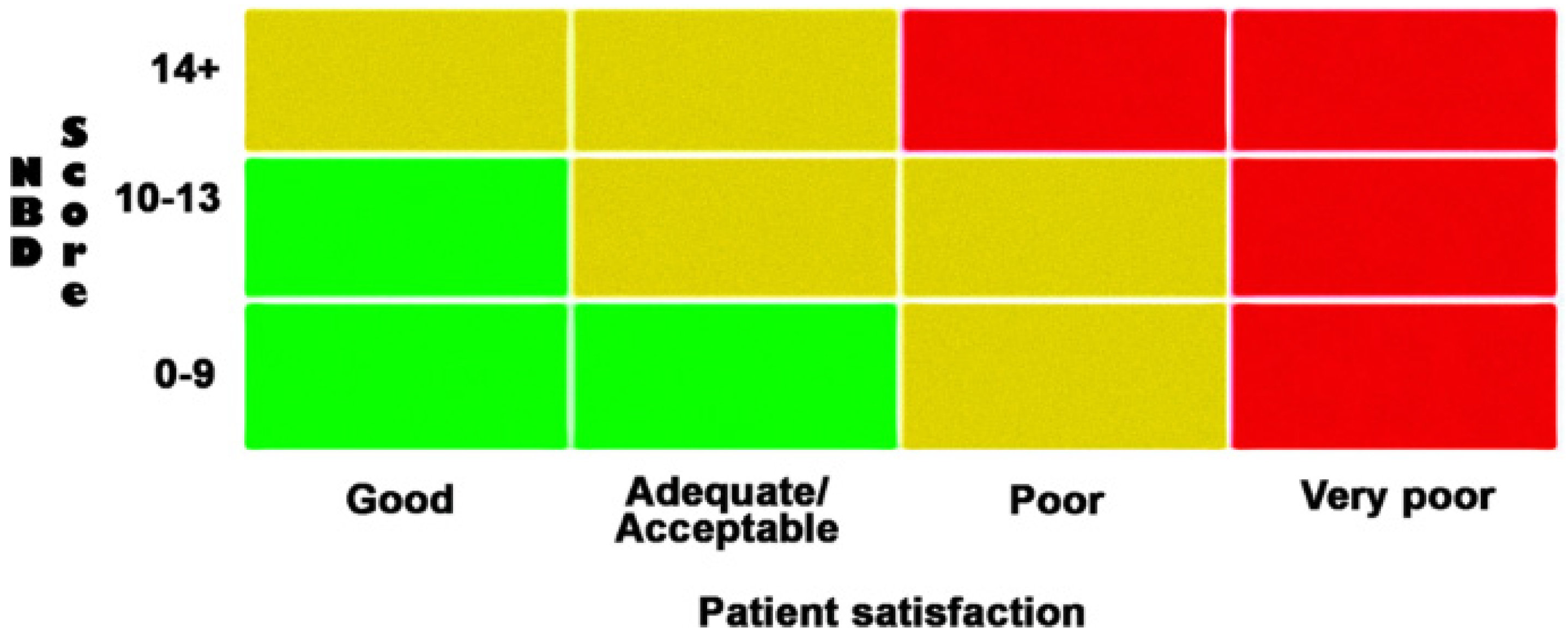
3. Assessment of NBD and Treatment Evaluation
4. Standard Bowel Management
4.1. Diet, Fibers, and Fluids
4.2. Timing
4.3. Oral Laxatives
4.3.1. Bulking Laxatives
4.3.2. Osmotic Laxatives
4.3.3. Stimulant Laxatives
4.3.4. Prokinetics
4.4. Digital Anorectal Stimulation/Evacuation
4.5. Rectal Laxatives
4.5.1. Rectal Suppositories
4.5.2. Mini Enema
5. Transanal Irrigation
6. Surgical Interventions
6.1. Stoma
6.2. The Malone Antegrade Continence Enema (MACE)
7. Experimental Treatments
7.1. Biofeedback (BF)
7.2. Sacral Nerve Stimulation (SNS) and Sacral Anterior Root Stimulation (SARS)
7.3. Epidural or Noninvasive (Transcutaneous) Electrical Stimulation of Spinal Cord
7.4. More Neuromodulations
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christensen, P.; Bazzocchi, G.; Coggrave, M.; Abel, R.; Hultling, C.; Krogh, K.; Media, S.; Laurberg, S. A randomized, controlled trial of transanal irrigation versus conservative bowel management in spinal cord-injured patients. Gastroenterology 2006, 131, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Consortium Spinal Cord Medicine CPG (Ed.) Management of Neurogenic Bowel Dysfunction in Adults after Spinal Cord Injury: Clinical Practice Guideline for Health Care Providers. In Clinical Practice Guidelines: Spinal Cord Medicine; American Spinal Injury Association: Richmond, VA, USA, 2021. [Google Scholar]
- Preziosi, G.; Raptis, D.A.; Raeburn, A.; Thiruppathy, K.; Panicker, J.; Emmanuel, A. Gut dysfunction in patients with multiple sclerosis and the role of spinal cord involvement in the disease. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Miget, G.; Moutounaïck, M.; Kervinio, F.; Charlanes, A.; Chesnel, C.; Le Breton, F.; Amarenco, G. Transanal Irrigation for Neurogenic Bowel Dysfunction in Multiple Sclerosis: A Retrospective Study. J. Neurogastroenterol. Motil. 2022, 28, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Passananti, V.; Wilton, A.; Preziosi, G.; Storrie, J.B.; Emmanuel, A. Long-term efficacy and safety of transanal irrigation in multiple sclerosis. Neurogastroenterol. Motil. 2016, 28, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Coggrave, M.; Norton, C.; Wilson-Barnett, J. Management of neurogenic bowel dysfunction in the community after spinal cord injury: A postal survey in the United Kingdom. Spinal Cord 2009, 47, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.D. Targeting recovery: Priorities of the spinal cord-injured population. J. Neurotrauma 2004, 21, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Krogh, K.; Christensen, P.; Sabroe, S.; Laurberg, S. Neurogenic bowel dysfunction score. Spinal Cord 2006, 44, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Ozisler, Z.; Koklu, K.; Ozel, S.; Unsal-Delialioglu, S. Outcomes of bowel program in spinal cord injury patients with neurogenic bowel dysfunction. Neural Regen. Res. 2015, 10, 1153–1158. [Google Scholar] [CrossRef]
- Tate, D.G.; Wheeler, T.; Lane, G.I.; Forchheimer, M.; Anderson, K.D.; Biering-Sorensen, F.; Cameron, A.P.; Santacruz, B.G.; Jakeman, L.B.; Kennelly, M.J.; et al. Recommendations for evaluation of neurogenic bladder and bowel dysfunction after spinal cord injury and/or disease. J. Spinal Cord Med. 2020, 43, 141–164. [Google Scholar] [CrossRef]
- Del Popolo, G.; Mosiello, G.; Pilati, C.; Lamartina, M.; Battaglino, F.; Buffa, P.; Redaelli, T.; Lamberti, G.; Menarini, M.; Di Benedetto, P.; et al. Treatment of neurogenic bowel dysfunction using transanal irrigation: A multicenter Italian study. Spinal Cord 2008, 46, 517–522. [Google Scholar] [CrossRef]
- Dibley, L.; Coggrave, M.; McClurg, D.; Woodward, S.; Norton, C. “It’s just horrible”: A qualitative study of patients’ and carers’ experiences of bowel dysfunction in multiple sclerosis. J. Neurol. 2017, 264, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Stiens, S.A.; Bergman, S.B.; Goetz, L.L. Neurogenic bowel dysfunction after spinal cord injury: Clinical evaluation and rehabilitative management. Arch. Phys. Med. Rehabil. 1997, 78, S86–S102. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for Management of Neurogenic Bowel Dysfunction in Individuals with a Spinal Cord Injury and Other Central Neurological Conditions. Multidisciplinary Association of Spinal Cord Injured Professionals; 2012. Available online: https://www.mascip.co.uk/wp-content/uploads/2022/02/Feb-2021-MASCIP-Neurogenic-Bowel-Guidlines-text.pdf (accessed on 25 October 2023).
- Valles, M.; Mearin, F. Pathophysiology of bowel dysfunction in patients with motor incomplete spinal cord injury: Comparison with patients with motor complete spinal cord injury. Dis. Colon Rectum 2009, 52, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Johns, J.; Krogh, K.; Ethans, K.; Chi, J.; Querée, M.; Eng, J.J. Pharmacological Management of Neurogenic Bowel Dysfunction after Spinal Cord Injury and Multiple Sclerosis: A Systematic Review and Clinical Implications. J. Clin. Med. 2021, 10, 882. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.J.; Barros, T.; Biering-Sorensen, F.; Burns, S.P.; Donovan, W.H.; Graves, D.E.; Haak, M.; Hudson, L.M.; Priebe, M. International standards for neurological classification of spinal cord injury. J. Spinal Cord Med. 2003, 26, S50–S56. [Google Scholar] [CrossRef] [PubMed]
- Maynard, F.M., Jr.; Bracken, M.B.; Creasey, G.; Donovan, W.H.; Ducker, T.B.; Garber, S.L.; Marino, R.J.; Stover, S.L.; Tator, C.H.; Waters, R.L.; et al. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord 1997, 35, 266–274. [Google Scholar] [CrossRef]
- Preziosi, G.; Emmanuel, A. Neurogenic bowel dysfunction: Pathophysiology, clinical manifestations and treatment. Expert Rev. Gastroenterol. Hepatol. 2009, 3, 417–423. [Google Scholar] [CrossRef]
- Wecht, J.M.; Krassioukov, A.V.; Alexander, M.; Handrakis, J.P.; McKenna, S.L.; Kennelly, M.; Trbovich, M. International Standards to document Autonomic Function following SCI (ISAFSCI): Second Edition. Top. Spinal Cord Inj. Rehabil. 2021, 27, 23–49. [Google Scholar] [CrossRef]
- Alexander, M.S.; Biering-Sorensen, F.; Bodner, D.; Brackett, N.L.; Cardenas, D.; Charlifue, S.; Creasey, G.; Dietz, V.; Ditunno, J.; Donovan, W.; et al. International standards to document remaining autonomic function after spinal cord injury. Spinal Cord 2009, 47, 36–43. [Google Scholar] [CrossRef]
- Mosiello, G.; Safder, S.; Marshall, D.; Rolle, U.; Benninga, M.A. Neurogenic Bowel Dysfunction in Children and Adolescents. J. Clin. Med. 2021, 10, 1669. [Google Scholar] [CrossRef]
- Hakim, S.; Gaglani, T.; Cash, B.D. Neurogenic Bowel Dysfunction: The Impact of the Central Nervous System in Constipation and Fecal Incontinence. Gastroenterol. Clin. N. Am. 2022, 51, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, M.; Anand, P.; Korsten, M.A.; Gong, B. Targeting Ion Channels: An Important Therapeutic Implication in Gastrointestinal Dysmotility in Patients With Spinal Cord Injury. J. Neurogastroenterol. Motil. 2015, 21, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Malakooti, J.; Saksena, S.; Gill, R.K.; Dudeja, P.K. Transcriptional regulation of the intestinal luminal Na+ and Cl− transporters. Biochem. J. 2011, 435, 313–325. [Google Scholar] [CrossRef]
- Preziosi, G.; Gordon-Dixon, A.; Emmanuel, A. Neurogenic bowel dysfunction in patients with multiple sclerosis: Prevalence, impact, and management strategies. Degener. Neurol. Neuromuscul. Dis. 2018, 8, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Coggrave, M.; Norton, C.; Cody, J.D. Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane Database Syst. Rev. 2014, CD002115. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, A.V.; Krogh, K.; Bazzocchi, G.; Leroi, A.-M.; Bremers, A.; Leder, D.; van Kuppevelt, D.; Mosiello, G.; Vogel, M.; Perrouin-Verbe, B.; et al. Consensus review of best practice of transanal irrigation in adults. Spinal Cord 2013, 51, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, A.; Krogh, K.; Kirshblum, S.; Christensen, P.; Spinelli, M.; van Kuppevelt, D.; Abel, R.; Leder, D.; Santacruz, B.G.; Bain, K.; et al. Creation and validation of a new tool for the monitoring efficacy of neurogenic bowel dysfunction treatment on response: The MENTOR tool. Spinal Cord 2020, 58, 795–802. [Google Scholar] [CrossRef]
- Krogh, K.; Emmanuel, A.; Perrouin-Verbe, B.; Korsten, M.A.; Mulcahey, M.J.; Biering-Sorensen, F. International spinal cord injury bowel function basic data set (Version 2.0). Spinal Cord 2017, 55, 692–698. [Google Scholar] [CrossRef]
- Miget, G.; Tan, E.; Pericolini, M.; Chesnel, C.; Haddad, R.; Turmel, N.; Amarenco, G.; Hentzen, C. The Neurogenic Bowel Dysfunction score (NBD) is not suitable for patients with multiple sclerosis. Spinal Cord 2022, 60, 1130–1135. [Google Scholar] [CrossRef]
- Correa, G.I.; Rotter, K.P. Clinical evaluation and management of neurogenic bowel after spinal cord injury. Spinal Cord 2000, 38, 301–308. [Google Scholar] [CrossRef]
- Coggrave, M.J.; Norton, C. The need for manual evacuation and oral laxatives in the management of neurogenic bowel dysfunction after spinal cord injury: A randomized controlled trial of a stepwise protocol. Spinal Cord 2010, 48, 504–510. [Google Scholar] [CrossRef]
- Bernardi, M.; Fedullo, A.L.; Bernardi, E.; Munzi, D.; Peluso, I.; Myers, J.; Lista, F.R.; Sciarra, T. Diet in neurogenic bowel management: A viewpoint on spinal cord injury. World J. Gastroenterol. 2020, 26, 2479–2497. [Google Scholar] [CrossRef] [PubMed]
- Coggrave, M.; Burrows, D.; Durand, M.A. Progressive protocol in the bowel management of spinal cord injuries. Br. J. Nurs. 2006, 15, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- DasGupta, R.; Fowler, C.J. Bladder, bowel and sexual dysfunction in multiple sclerosis: Management strategies. Drugs 2003, 63, 153–166. [Google Scholar] [CrossRef] [PubMed]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Krogh, K.; Jensen, M.B.; Gandrup, P.; Laurberg, S.; Nilsson, J.; Kerstens, R.; De Pauw, M. Efficacy and tolerability of prucalopride in patients with constipation due to spinal cord injury. Scand. J. Gastroenterol. 2002, 37, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Daniali, M.; Nikfar, S.; Abdollahi, M. Evaluating naloxegol for the treatment of opioid-induced constipation. Expert. Opin. Pharmacother. 2020, 21, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.E.S.; Orr, M. Digital rectal stimulation as an intervention in persons with spinal cord injury and upper motor neuron neurogenic bowel. An evidenced-based systematic review of the literature. J. Spinal Cord Med. 2021, 44, 525–532. [Google Scholar] [CrossRef]
- Wincentak, J.; Xu, Y.; Rudden, L.; Kassam-Lallani, D.; Mullin, A.; Truong, C.; Krog, K.; Kingsnorth, S. Current State of Knowledge on Digital Rectal Stimulation in Individuals with Traumatic and Nontraumatic Spinal Cord Injury: A Scoping Review. Arch. Phys. Med. Rehabil. 2021, 102, 1816–1825. [Google Scholar] [CrossRef]
- House, J.G.; Stiens, S.A. Pharmacologically initiated defecation for persons with spinal cord injury: Effectiveness of three agents. Arch. Phys. Med. Rehabil. 1997, 78, 1062–1065. [Google Scholar] [CrossRef]
- Stiens, S.A.; Luttrel, W.; Binard, J.E. Polyethylene glycol versus vegetable oil based bisacodyl suppositories to initiate side-lying bowel care: A clinical trial in persons with spinal cord injury. Spinal Cord 1998, 36, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Amir, I.; Sharma, R.; Bauman, W.A.; Korsten, M.A. Bowel care for individuals with spinal cord injury: Comparison of four approaches. J. Spinal Cord Med. 1998, 21, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.L.; Galka, M.L. A comparison of the effectiveness of Therevac SB and bisacodyl suppositories in SCI patients’ bowel programs. Rehabil. Nurs. 1994, 19, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Harris, G. An overview of transanal irrigation devices: An update. Br. J. Nurs. 2022, 31, 612–618. [Google Scholar] [CrossRef]
- Yates, A. Transanal irrigation: Is it the magic intervention for bowel management in individuals with bowel dysfunction? Br. J. Nurs. 2020, 29, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M. A review of transanal irrigation in adults. Br. J. Nurs. 2017, 26, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.; Olsen, N.; Krogh, K.; Bacher, T.; Laurberg, S. Scintigraphic assessment of retrograde colonic washout in fecal incontinence and constipation. Dis. Colon Rectum 2003, 46, 68–76. [Google Scholar] [CrossRef]
- Faaborg, P.M.; Christensen, P.; Kvitsau, B.; Buntzen, S.; Laurberg, S.; Krogh, K. Long-term outcome and safety of transanal colonic irrigation for neurogenic bowel dysfunction. Spinal Cord 2009, 47, 545–549. [Google Scholar] [CrossRef]
- Tamvakeras, P.; Horrobin, C.; Chang, J.; Chapman, M. Long-Term Outcomes of Transanal Irrigation for Bowel Dysfunction. Cureus 2023, 15, e42507. [Google Scholar] [CrossRef]
- Fourtassi, M.; Charvier, K.; Hajjioui, A.; Have, L.; Rode, G. Transanal irrigation for bowel and anorectal management in spinal cord-injured patients. Prog. Urol. 2012, 22, 467–474. [Google Scholar] [CrossRef]
- Ascanelli, S.; Bombardini, C.; Chimisso, L.; Carcoforo, P.; Turroni, S.; D’amico, F.; Caniati, M.L.; Baldi, E.; Tugnoli, V.; Morotti, C.; et al. Trans-anal irrigation in patients with multiple sclerosis: Efficacy in treating disease-related bowel dysfunctions and impact on the gut microbiota: A monocentric prospective study. Mult. Scler. J. Exp. Transl. Clin. 2022, 8, 20552173221109771. [Google Scholar] [CrossRef] [PubMed]
- Preziosi, G.; Gosling, J.; Raeburn, A.; Storrie, J.; Panicker, J.; Emmanuel, A. Transanal irrigation for bowel symptoms in patients with multiple sclerosis. Dis. Colon Rectum 2012, 55, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Branagan, G.; Tromans, A.; Finnis, D. Effect of stoma formation on bowel care and quality of life in patients with spinal cord injury. Spinal Cord 2003, 41, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Rosito, O.; Nino-Murcia, M.; Wolfe, V.A.; Kiratli, B.J.; Perkash, I. The effects of colostomy on the quality of life in patients with spinal cord injury: A retrospective analysis. J. Spinal Cord Med. 2002, 25, 174–183. [Google Scholar] [CrossRef]
- van Ginkel, F.; Post, M.W.M.; Faber, W.X.M.; Meij, V.; Stolwijk-Swuste, J.M. Spinal cord injuries and bowel stomas: Timing and satisfaction with stoma formation and alterations in quality of life. Spinal Cord Ser. Cases 2021, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Waddell, O.; McCombie, A.; Frizelle, F. Colostomy and quality of life after spinal cord injury: Systematic review. BJS Open 2020, 4, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.R.; Shashidharan, M.; Borwell, B.; Tromans, A.M.; Finnis, D.; Grundy, D.J. The role of intestinal stoma in patients with spinal cord injury. Spinal Cord 1999, 37, 211–214. [Google Scholar] [CrossRef]
- Patton, V.; Lubowski, D.Z. Clinical outcome and efficacy of antegrade colonic enemas administered via an indwelling cecostomy catheter in adults with defecatory disorders. Dis. Colon Rectum 2015, 58, 457–462. [Google Scholar] [CrossRef]
- Uno, Y. Introducer method of percutaneous endoscopic cecostomy and antegrade continence enema by use of the Chait Trapdoor cecostomy catheter in patients with adult neurogenic bowel. Gastrointest. Endosc. 2006, 63, 666–673. [Google Scholar] [CrossRef]
- Brinas, P.; Zalay, N.; Philis, A.; Castel-Lacanal, E.; Barrieu, M.; Portier, G. Use of Malone antegrade continence enemas in neurologic bowel dysfunction. J. Visc. Surg. 2020, 157, 453–459. [Google Scholar] [CrossRef]
- Smith, P.H.; Decter, R.M. Antegrade continence enema procedure: Impact on quality of life in patients with spinal cord injury. Spinal Cord 2015, 53, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Teichman, J.M.; Zabihi, N.; Kraus, S.R.; Harris, J.M.; Barber, D.B. Long-term results for Malone antegrade continence enema for adults with neurogenic bowel disease. Urology 2003, 61, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Gulick, E.E. Neurogenic Bowel Dysfunction Over the Course of Multiple Sclerosis: A Review. Int. J. MS Care 2022, 24, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Mazor, Y.; Jones, M.; Andrews, A.; Kellow, J.E.; Malcolm, A. Anorectal biofeedback for neurogenic bowel dysfunction in incomplete spinal cord injury. Spinal Cord 2016, 54, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.M.; Kutzenberger, J.; Krogh, K.; Zepke, F.; Bodin, C.; Domurath, B.; Christensen, P. Sacral anterior root stimulation improves bowel function in subjects with spinal cord injury. Spinal Cord 2015, 53, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Mowatt, G.; Glazener, C.; Jarrett, M. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst. Rev. 2007, CD004464. [Google Scholar] [CrossRef]
- Holzer, B.; Rosen, H.R.; Novi, G.; Ausch, C.; Holbling, N.; Schiessel, R. Sacral nerve stimulation for neurogenic faecal incontinence. Br. J. Surg. 2007, 94, 749–753. [Google Scholar] [CrossRef]
- Kreydin, E.; Zhong, H.; Lavrov, I.; Edgerton, V.R.; Gad, P. The Effect of Non-invasive Spinal Cord Stimulation on Anorectal Function in Individuals With Spinal Cord Injury: A Case Series. Front. Neurosci. 2022, 16, 816106. [Google Scholar] [CrossRef]
- Pettigrew, R.I.; Heetderks, W.J.; Kelley, C.A.; Peng, G.C.Y.; Krosnick, S.H.; Jakeman, L.B.; Egan, K.D.; Marge, M. Epidural Spinal Stimulation to Improve Bladder, Bowel, and Sexual Function in Individuals with Spinal Cord Injuries: A Framework for Clinical Research. IEEE Trans. Biomed. Eng. 2017, 64, 253–262. [Google Scholar] [CrossRef]
- Tator, C.H.; Minassian, K.; Mushahwar, V.K. Spinal cord stimulation: Therapeutic benefits and movement generation after spinal cord injury. Handb. Clin. Neurol. 2012, 109, 283–296. [Google Scholar]
- Lin, V.W.; Nino-Murcia, M.; Frost, F.; Wolfe, V.; Hsiao, I.; Perkash, I. Functional magnetic stimulation of the colon in persons with spinal cord injury. Arch. Phys. Med. Rehabil. 2001, 82, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Parittotokkaporn, S.; Varghese, C.; O’Grady, G.; Svirskis, D.; Subramanian, S.; O’Carroll, S.J. Non-invasive neuromodulation for bowel, bladder and sexual restoration following spinal cord injury: A systematic review. Clin. Neurol. Neurosurg. 2020, 194, 105822. [Google Scholar] [CrossRef] [PubMed]
- Sanagapalli, S.; Neilan, L.; Lo, J.Y.T.; Anandan, L.; Liwanag, J.; Raeburn, A.; Athanasakos, E.; Zarate-Lopez, N.; Emmanuel, A. Efficacy of Percutaneous Posterior Tibial Nerve Stimulation for the Management of Fecal Incontinence in Multiple Sclerosis: A Pilot Study. Neuromodulation 2018, 21, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Awad, R.A. Neurogenic bowel dysfunction in patients with spinal cord injury, myelomeningocele, multiple sclerosis and Parkinson’s disease. World J. Gastroenterol. 2011, 17, 5035–5048. [Google Scholar] [CrossRef] [PubMed]
- Musco, S.; Bazzocchi, G.; Martellucci, J.; Amato, M.P.; Manassero, A.; Putignano, D.; Lopatriello, S.; Cafiero, D.; Paoloni, F.; Del Popolo, G. Treatments in neurogenic bowel dysfunctions: Evidence reviews and clinical recommendations in adults. Eur. J. Phys. Rehabil. Med. 2020, 56, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Kurze, I.; Geng, V.; Bothig, R. Guideline for the management of neurogenic bowel dysfunction in spinal cord injury/disease. Spinal Cord 2022, 60, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Skjaerbaek, C.; Knudsen, K.; Horsager, J.; Borghammer, P. Gastrointestinal Dysfunction in Parkinson’s Disease. J. Clin. Med. 2021, 10, 493. [Google Scholar] [CrossRef]
- Camilleri, M. Gastrointestinal motility disorders in neurologic disease. J. Clin. Investig. 2021, 131, e143771. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnuson, F.S.; Christensen, P.; Krassioukov, A.; Rodriguez, G.; Emmanuel, A.; Kirshblum, S.; Krogh, K. Neurogenic Bowel Dysfunction in Patients with Spinal Cord Injury and Multiple Sclerosis—An Updated and Simplified Treatment Algorithm. J. Clin. Med. 2023, 12, 6971. https://doi.org/10.3390/jcm12226971
Magnuson FS, Christensen P, Krassioukov A, Rodriguez G, Emmanuel A, Kirshblum S, Krogh K. Neurogenic Bowel Dysfunction in Patients with Spinal Cord Injury and Multiple Sclerosis—An Updated and Simplified Treatment Algorithm. Journal of Clinical Medicine. 2023; 12(22):6971. https://doi.org/10.3390/jcm12226971
Chicago/Turabian StyleMagnuson, Fredrika S., Peter Christensen, Andrei Krassioukov, Gianna Rodriguez, Anton Emmanuel, Steven Kirshblum, and Klaus Krogh. 2023. "Neurogenic Bowel Dysfunction in Patients with Spinal Cord Injury and Multiple Sclerosis—An Updated and Simplified Treatment Algorithm" Journal of Clinical Medicine 12, no. 22: 6971. https://doi.org/10.3390/jcm12226971
APA StyleMagnuson, F. S., Christensen, P., Krassioukov, A., Rodriguez, G., Emmanuel, A., Kirshblum, S., & Krogh, K. (2023). Neurogenic Bowel Dysfunction in Patients with Spinal Cord Injury and Multiple Sclerosis—An Updated and Simplified Treatment Algorithm. Journal of Clinical Medicine, 12(22), 6971. https://doi.org/10.3390/jcm12226971





