Accuracy of a Dual-Sensor Heat-Flux (DHF) Non-Invasive Core Temperature Sensor in Pediatric Patients Undergoing Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Ethics and Registration
2.3. Patients
2.4. Measurements
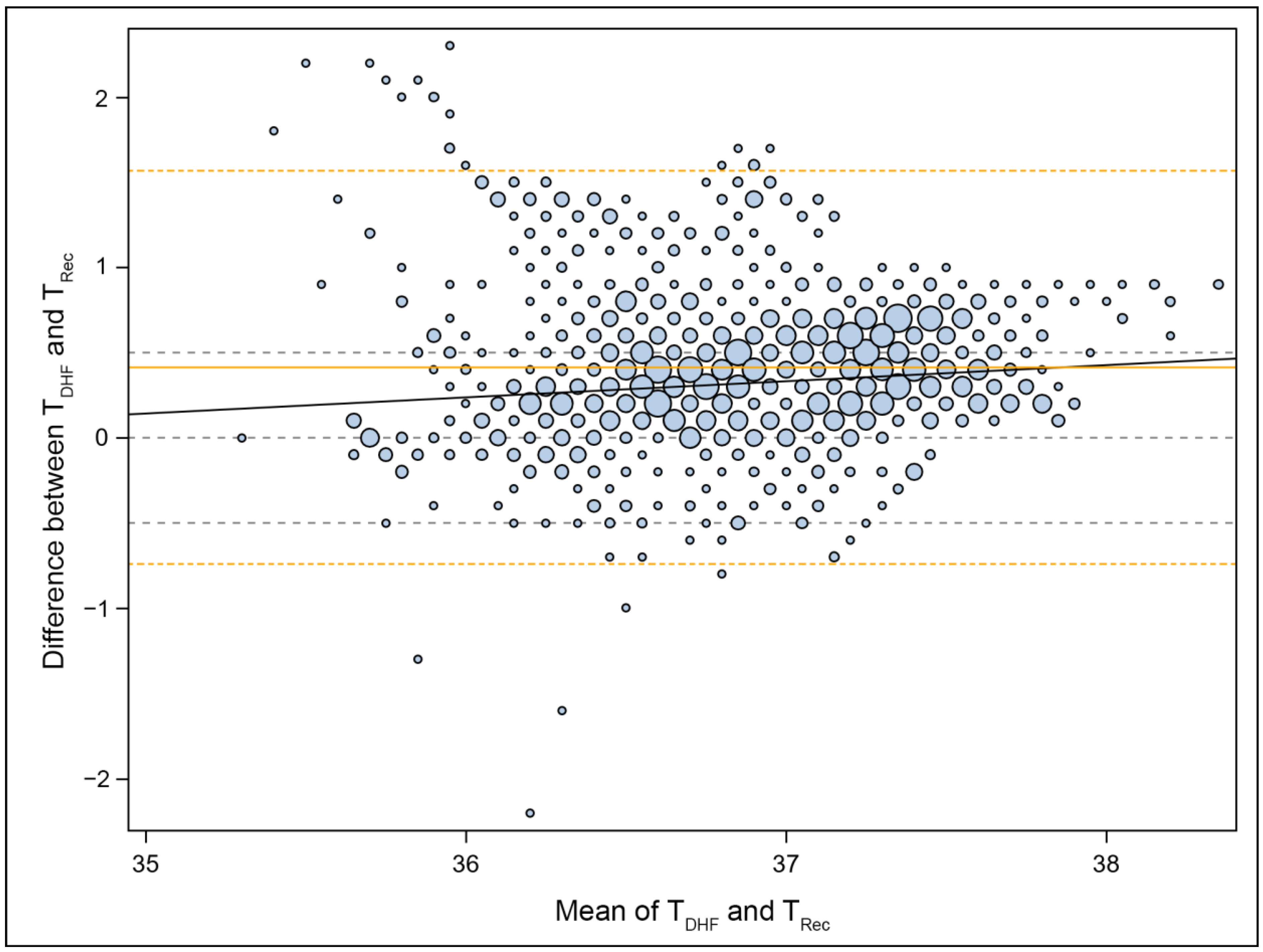
2.5. Statistical Methods
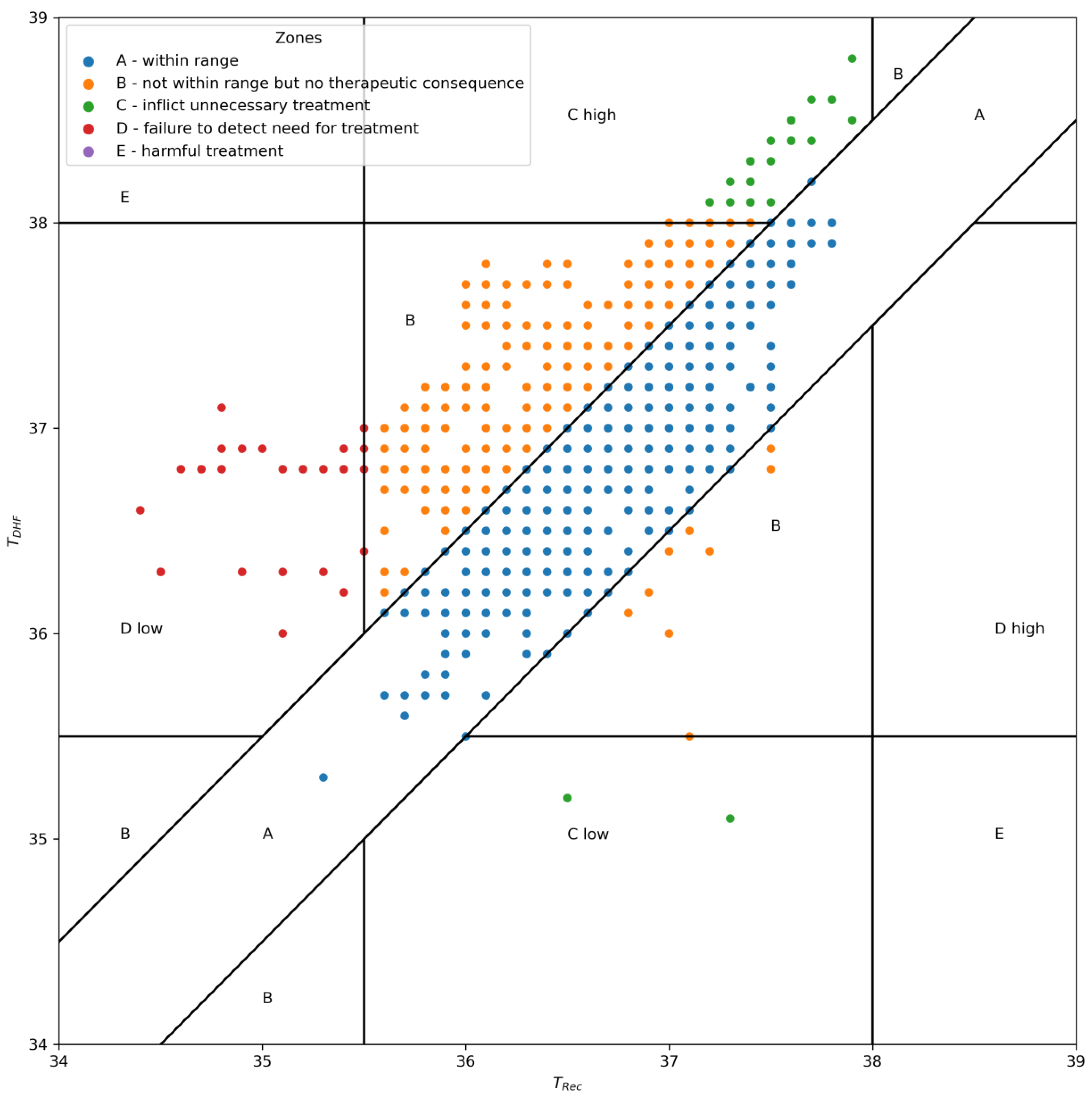
2.6. Outcome Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poveda, V.d.B.; Oliveira, R.A.; Galvão, C.M. Perioperative body temperature maintenance and occurrence of surgical site infection: A systematic review with meta-analysis. Am. J. Infect. Control 2020, 48, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Kiekkas, P.; Fligou, F.; Igoumenidis, M.; Stefanopoulos, N.; Konstantinou, E.; Karamouzos, V.; Aretha, D. Inadvertent hypothermia and mortality in critically ill adults: Systematic review and meta-analysis. Aust. Crit. Care Off. J. Confed. Aust. Crit. Care Nurses 2018, 31, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Niven, D.J.; Laupland, K.B. Pyrexia: Aetiology in the ICU. Crit. Care 2016, 20, 247. [Google Scholar] [CrossRef] [PubMed]
- Torossian, A. AWMF S3 Guidelines on Preventing Inadvertant Perioperative Hypothermia. Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin e.V. (DGAI). 2019. Available online: https://www.awmf.org/leitlinien/detail/ll/001-018.html (accessed on 2 August 2023).
- Radauceanu, D.S.; Dragnea, D.; Craig, J. NICE guidelines for inadvertent peri-operative hypothermia. Anaesthesia 2009, 64, 1381–1382. [Google Scholar] [CrossRef] [PubMed]
- Simbruner, G.; Weninger, W.; Popow, C.; Herholdt, W.J. Regional heat loss in newborn infants. Part II. Heat loss in newborns with various diseases—A method of assessing local metabolism and perfusion. S. Afr. Med. J. Suid-Afr. Tydskr. Vir. Geneeskd. 1985, 68, 945–948. [Google Scholar]
- Nemeth, M.; Miller, C.; Bräuer, A. Perioperative Hypothermia in Children. Int. J. Environ. Res. Public Health 2021, 18, 7541. [Google Scholar] [CrossRef]
- Görges, M.; Afshar, K.; West, N.; Pi, S.; Bedford, J.; Whyte, S.D. Integrating intraoperative physiology data into outcome analysis for the ACS Pediatric National Surgical Quality Improvement Program. Pediatr. Anesth. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Sessler, D.I. Complications and Treatment of Mild Hypothermia. Anesthesiology 2001, 95, 531–543. [Google Scholar] [CrossRef]
- Walker, S.; Amin, R.; Arca, M.J.; Datta, A. Effects of intraoperative temperatures on postoperative infections in infants and neonates. J. Pediatr. Surg. 2020, 55, 80–85. [Google Scholar] [CrossRef]
- Pearce, B.; Christensen, R.; Voepel-Lewis, T. Perioperative Hypothermia in the Pediatric Population: Prevalence, Risk Factors and Outcomes. J. Anesth. Clin. Res. 2010, 1, 1000102. Available online: https://www.omicsonline.org/perioperative-hypothermia-in-the-pediatric-population-prevalence-risk-factors-and-outcomes-2155-6148.1000102.php?aid=157 (accessed on 22 August 2023). [CrossRef]
- Brindle, M.E.; McDiarmid, C.; Short, K.; Miller, K.; MacRobie, A.; Lam, J.Y.K.; Brockel, M.; Raval, M.V.; Howlett, A.; Lee, K.-S.; et al. Consensus Guidelines for Perioperative Care in Neonatal Intestinal Surgery: Enhanced Recovery after Surgery (ERAS®) Society Recommendations. World J. Surg. 2020, 44, 2482–2492. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.; Sessler, D.I.; Lenhardt, R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N. Engl. J. Med. 1996, 334, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Mittnacht, A.J.C.; Lin, H.M.; Liu, X.; Wax, D. New-onset intra-operative hyperthermia in a large surgical patient population: A retrospective observational study. Eur. J. Anaesthesiol. 2021, 38, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Torossian, A.; Becke, K.; Bein, B.; Bräuer, A.; Gantert, D.; Greif, R.; Höcker, J.; Horn, E.P.; Kimberger, O.; Klar, E.; et al. S3 Leitlinie “Vermeidung von perioperativer Hypothermie”. Aktualisierung 2019, 46. Available online: https://www.awmf.org/uploads/tx_szleitlinien/001-018l_S3_Vermeidung_perioperativer_Hypothermie_2019-08.pdf (accessed on 22 August 2023).
- García-Mato, E.; Varela-Aneiros, I.; Abeleira-Pazos, M.; Outumuro-Rial, M.; Diz-Dios, P.; Limeres-Posse, J.; Diniz-Freitas, M. Is It Useful to Determine the Temperature of Children for COVID-19 Screening in the Dental Setting? J. Clin. Med. 2022, 11, 976. [Google Scholar] [CrossRef]
- Herlich, A. Perioperative temperature elevation: Not all hyperthermia is malignant hyperthermia. Pediatr. Anesth. 2013, 23, 842–850. [Google Scholar] [CrossRef]
- O’grady, N.P.; Barie, P.S.; Bartlett, J.G.; Bleck, T.; Carroll, K.; Kalil, A.C.; Linden, P.; Maki, D.G.; Nierman, D.; Pasculle, W.; et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit. Care Med. 2008, 36, 1330–1349. [Google Scholar] [CrossRef]
- Sessler, D.I. Perioperative Temperature Monitoring. Anesthesiology 2021, 134, 111–118. [Google Scholar] [CrossRef]
- Eshraghi, Y.; Nasr, V.; Parra-Sanchez, I.; Van Duren, A.; Botham, M.; Santoscoy, T.; Sessler, D.I. An evaluation of a zero-heat-flux cutaneous thermometer in cardiac surgical patients. Obstet. Anesth. Dig. 2014, 119, 543–549. [Google Scholar] [CrossRef]
- Ikeda, T.; Sessler, D.I.; Marder, D.; Xiong, J. Influence of thermoregulatory vasomotion and ambient temperature variation on the accuracy of core-temperature estimates by cutaneous liquid-crystal thermometers. Anesthesiology 1997, 86, 603–612. [Google Scholar] [CrossRef]
- Fox, R.H.; Solman, A.J.; Isaacs, R.; Fry, A.J.; MacDonald, I.C. A new method for monitoring deep body temperature from the skin surface. Clin. Sci. 1973, 44, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Guschlbauer, M.; Maul, A.C.; Yan, X.; Herff, H.; Annecke, T.; Sterner-Kock, A.; Böttiger, B.W.; Schroeder, D.C. Zero-heat-flux thermometry for non-invasive measurement of core body temperature in pigs. PLoS ONE 2016, 11, e0150759. [Google Scholar] [CrossRef] [PubMed]
- Iden, T.; Horn, E.P.; Bein, B.; Böhm, R.; Beese, J.; Höcker, J. Intraoperative temperature monitoring with zero heat flux technology (3M SpotOn sensor) in comparison with sublingual and nasopharyngeal temperature: An observational study. Eur. J. Anaesthesiol. 2015, 32, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, L.P.J.; Klewer, J.; de Haan, A.; de Koning, J.J.; Daanen, H.A.M. Non-invasive continuous core temperature measurement by zero heat flux. Physiol. Meas. 2011, 32, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Kimberger, O.; Saager, L.; Egan, C.; Sanchez, I.P.; Dizili, S.; Koch, J.; Kurz, A. The accuracy of a disposable noninvasive core thermometer. Can. J. Anesth. Can. Anesth. 2013, 60, 1190–1196. [Google Scholar] [CrossRef]
- Bräuer, A.; Fazliu, A.; Perl, T.; Heise, D.; Meissner, K.; Brandes, I.F. Accuracy of zero-heat-flux thermometry and bladder temperature measurement in critically ill patients. Sci. Rep. 2020, 10, 21746. [Google Scholar] [CrossRef]
- Kimberger, O.; Thell, R.; Schuh, M.; Koch, J.; Sessler, D.; Kurz, A. Accuracy and precision of a novel non-invasive core thermometer. Br. J. Anaesth. 2009, 103, 226–231. [Google Scholar] [CrossRef]
- Evron, S.; Weissman, A.; Toivis, V.; Shahaf, D.B.; You, J.; Sessler, D.I.; Ezri, T. Evaluation of the Temple Touch Pro, a Novel Noninvasive Core-Temperature Monitoring System. Obstet. Anesth. Dig. 2017, 125, 103–109. [Google Scholar] [CrossRef]
- Carvalho, H.; Najafi, N.; Poelaert, J. Intra-operative temperature monitoring with cutaneous zero-heat- flux-thermometry in comparison with oesophageal temperature: A prospective study in the paediatric population. Pediatr. Anesth. 2019, 29, 865–871. [Google Scholar] [CrossRef]
- Nemeth, M.; Klose, K.; Asendorf, T.; Pancaro, C.; Mielke, B.; Fazliu, A.; Saager, L.; Bräuer, A.; Miller, C. Evaluation of the non-invasive Temple Touch Pro temperature monitoring system compared with oesophageal temperature in paediatric anaesthesia (PETER PAN): A prospective observational study. Eur. J. Anaesthesiol. 2023, 40, 198–207. [Google Scholar] [CrossRef]
- Draeger Medical Inc. Tcore System Core Temperature Sensor Instructions for Use. Available online: https://www.draeger.com/de_at/Home (accessed on 15 August 2023).
- Smiths Medical. ER400-9 ER400-12 Level I Esophageal/Rectal Temperature Probe with 400 Series Thermistor Probe. Instructions for Use; Smiths Medical ASD Inc.: Olive Branch, MS, USA, 2006. [Google Scholar]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Zou, G. Confidence interval estimation for the Bland-Altman limits of agreement with multiple observations per individual. Stat. Methods Med. Res. 2013, 22, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Sessler, D.I.; Warner, D.S.; Warner, M.A. Temperature Monitoring and Perioperative Thermoregulation. Anesthesiology 2008, 109, 318–338. [Google Scholar] [CrossRef] [PubMed]
- Morey, T.E.; Gravenstein, N.; Rice, M.J. Let’s Think Clinically Instead of Mathematically about Device Accuracy. Obstet. Anesth. Dig. 2011, 113, 89–91. [Google Scholar] [CrossRef]
- Sessler, D.I.; Pei, L.; Li, K.; Cui, S.; Chan, M.T.V.; Huang, Y.; Wu, J.; He, X.; Bajracharya, G.R.; Rivas, E.; et al. Aggressive intraoperative warming versus routine thermal management during non-cardiac surgery (PROTECT): A multicentre, parallel group, superiority trial. Lancet 2022, 399, 1799–1808. [Google Scholar] [CrossRef]
- Reback, J.; McKinney, W.; Van Den Bossche, J.; Augspurger, T.; Cloud, P.; Hawkins, S.; Roeschke, M.; Klein, A.; Petersen, T.; She, C.; et al. Pandas-dev/Pandas: Pandas 1.3.4. Zenodo. 2021. Available online: https://zenodo.org/record/5574486 (accessed on 21 July 2023).
- Sinha, P.K.; Kaushik, S.; Neema, P.K. Massive epistaxis after nasopharyngeal temperature probe insertion after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2004, 18, 123–124. [Google Scholar] [CrossRef]
- Bräuer, A.; Martin, J.-D.; Schuhmann, M.U.; Braun, U.; Weyland, W. Genauigkeit derBlasentemperaturmessungbei intraabdominellen Eingriffen. AINS Anästhesiol. Intensiv. Notfallmed. Schmerzther. 2000, 35, 435–439. [Google Scholar] [CrossRef]
- Tsadok, I.; Scheinowitz, M.; Shpitzer, S.A.; Ketko, I.; Epstein, Y.; Yanovich, R. Assessing rectal temperature with a novel non-invasive sensor. J. Therm. Biol. 2021, 95, 102788. [Google Scholar] [CrossRef]
- Bräuer, A.; Fazliu, A.; Brandes, I.F.; Vollnhals, F.; Grote, R.; Menzel, M. Evaluation of the Temple Touch ProTM noninvasive core-temperature monitoring system in 100 adults under general anesthesia: A prospective comparison with esophageal temperature. J. Clin. Monit. Comput. 2023, 37, 29–36. [Google Scholar] [CrossRef]
- Lefrant, J.-Y.; Muller, L.; De La Coussaye, J.E.; Benbabaali, M.; LeBris, C.; Zeitoun, N.; Mari, C.; Saïssi, G.; Ripart, J.; Eledjam, J.-J. Temperature measurement in intensive care patients: Comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med. 2003, 29, 414–418. [Google Scholar] [CrossRef]
- Sessler, D.I. Forced-air warming in infants and children. Pediatr. Anesth. 2013, 23, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, M.; Lovric, M.; Asendorf, T.; Bräuer, A.; Miller, C. Intraoperative zero-heat-flux thermometry overestimates esophageal temperature by 0.26 °C: An observational study in 100 infants and young children. J. Clin. Monit. Comput. 2021, 35, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Ajčević, M.; Stella, A.B.; Furlanis, G.; Caruso, P.; Naccarato, M.; Accardo, A.; Manganotti, P. A Novel Non-Invasive Thermometer for Continuous Core Body Temperature: Comparison with Tympanic Temperature in an Acute Stroke Clinical Setting. Sensors 2022, 22, 4760. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Aratake, S.; Naito, H.; Nogawa, M.; Nemoto, T.; Togawa, T.; Tanaka, S. Development of a Core Body Thermometer Applicable for High-Temperature Environment Based on the Zero-Heat-Flux Method. Sensors 2023, 23, 1970. [Google Scholar] [CrossRef] [PubMed]

| N = 51 Patients | |
|---|---|
| Age in Months (Quartiles) | |
| 18.80 (9.37–44.73) | |
| Sex (n) | 5 females |
| 46 males | |
| Weight in kg (SD) | |
| 11.00 (8.50–14.00) | |
| ASA (n) | |
| 1 | 45 |
| 2 | 4 |
| 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeiner, S.; Zadrazil, M.; Willschke, H.; Wiegele, M.; Marhofer, P.; Hammerle, F.P.; Laxar, D.; Gleiss, A.; Kimberger, O. Accuracy of a Dual-Sensor Heat-Flux (DHF) Non-Invasive Core Temperature Sensor in Pediatric Patients Undergoing Surgery. J. Clin. Med. 2023, 12, 7018. https://doi.org/10.3390/jcm12227018
Zeiner S, Zadrazil M, Willschke H, Wiegele M, Marhofer P, Hammerle FP, Laxar D, Gleiss A, Kimberger O. Accuracy of a Dual-Sensor Heat-Flux (DHF) Non-Invasive Core Temperature Sensor in Pediatric Patients Undergoing Surgery. Journal of Clinical Medicine. 2023; 12(22):7018. https://doi.org/10.3390/jcm12227018
Chicago/Turabian StyleZeiner, Sebastian, Markus Zadrazil, Harald Willschke, Marion Wiegele, Peter Marhofer, Fabian Peter Hammerle, Daniel Laxar, Andreas Gleiss, and Oliver Kimberger. 2023. "Accuracy of a Dual-Sensor Heat-Flux (DHF) Non-Invasive Core Temperature Sensor in Pediatric Patients Undergoing Surgery" Journal of Clinical Medicine 12, no. 22: 7018. https://doi.org/10.3390/jcm12227018





