Predicting the Outcome of Patients with Aneurysmal Subarachnoid Hemorrhage: A Machine-Learning-Guided Scorecard
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistics
2.3. Model Analysis
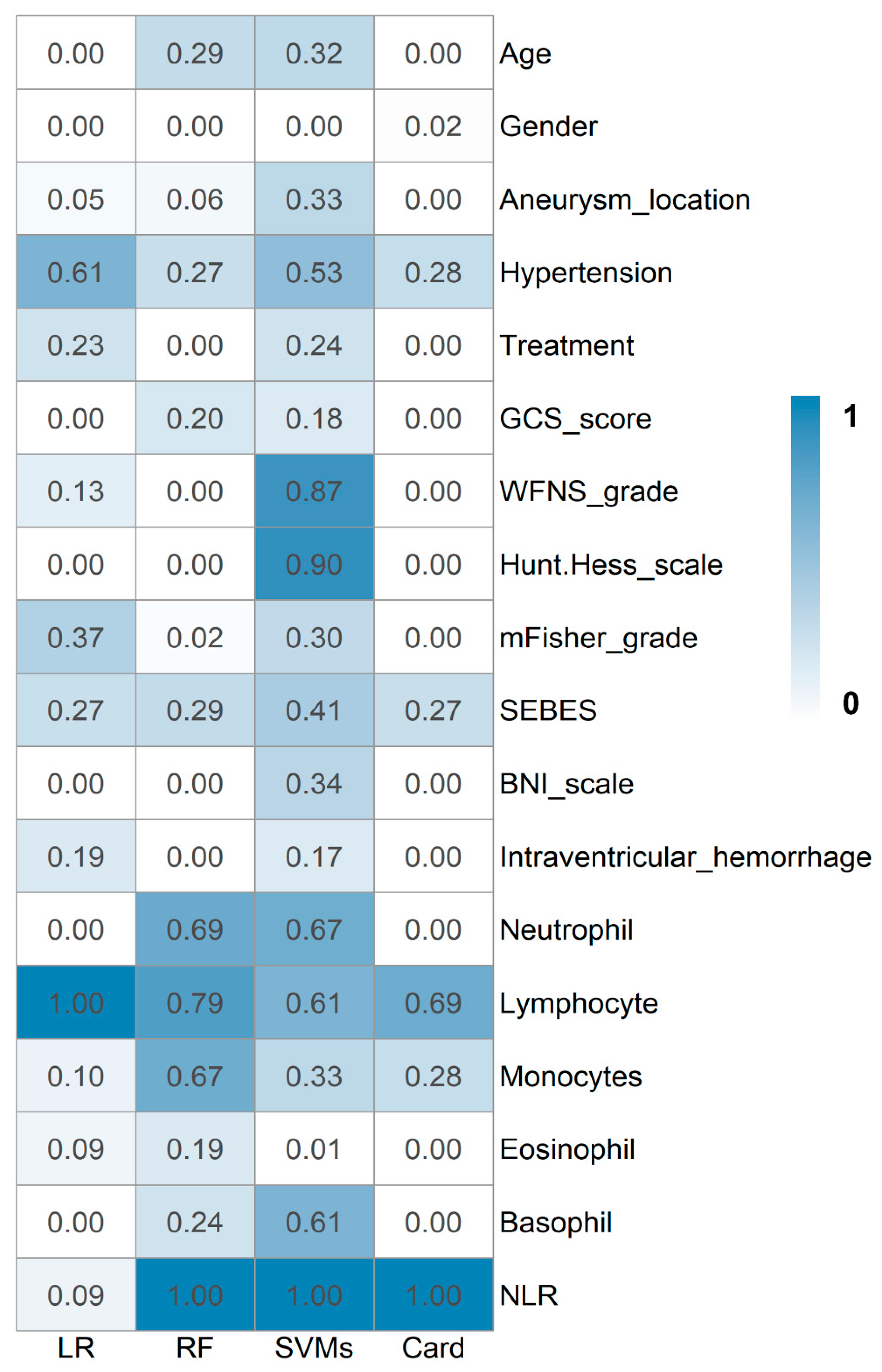
3. Results
3.1. Patient Data Collection and Demographics
3.2. Prediction Models
3.3. Scorecard Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aSAH | aneurysmal subarachnoid hemorrhage |
| BNI | Barrow neurological institute |
| FCNN | fully connected neural networks |
| GCS | Glasgow Coma Scale |
| IV | information value |
| LR | logistic regression model |
| NLR | the neutrophil-to-lymphocyte ratio |
| RF | random forest model |
| SEBES | subarachnoid hemorrhage early brain edema score |
| SVM | support vector machine |
| WFNS | World Federation of Neurological Surgeons |
| VIF | variance inflation factor |
References
- Feigin, V.L.; Lawes, C.M.; Bennett, D.A.; Barker-Collo, S.L.; Parag, V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009, 8, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Springer, M.V.; Schmidt, J.M.; Wartenberg, K.E.; Frontera, J.A.; Badjatia, N.; Mayer, S.A. Predictors of global cognitive impairment 1 year after subarachnoid hemorrhage. Neurosurgery 2009, 65, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Jaja, B.N.; Saposnik, G.; Lingsma, H.F.; Macdonald, E.; Thorpe, K.E.; Mamdani, M.; Steyerberg, E.W.; Molyneux, A.; de Oliveira Manoel, A.L.; Schatlo, B.; et al. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: The SAHIT multinational cohort study. BMJ 2018, 360, j5745. [Google Scholar] [CrossRef]
- Naval, N.S.; Kowalski, R.G.; Chang, T.R.; Caserta, F.; Carhuapoma, J.R.; Tamargo, R.J.; Diseases, C. The SAH score: A comprehensive communication tool. J. Stroke Cerebrovasc. Dis. 2014, 23, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Jaja, B.N.; Cusimano, M.D.; Etminan, N.; Hanggi, D.; Hasan, D.; Ilodigwe, D.; Lantigua, H.; Le Roux, P.; Lo, B.; Louffat-Olivares, A.; et al. Clinical prediction models for aneurysmal subarachnoid hemorrhage: A systematic review. Neurocritical Care 2013, 18, 143–153. [Google Scholar] [CrossRef] [PubMed]
- de Jong, G.; Aquarius, R.; Sanaan, B.; Bartels, R.; Grotenhuis, J.A.; Henssen, D.; Boogaarts, H.D. Prediction Models in Aneurysmal Subarachnoid Hemorrhage: Forecasting Clinical Outcome with Artificial Intelligence. Neurosurgery 2021, 88, E427–E434. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.; Shan, B.; He, M.; Xu, J. XGBoost Machine Learning Algorithm for Prediction of Outcome in Aneurysmal Subarachnoid Hemorrhage. Neuropsychiatr. Dis. Treat. 2022, 18, 659–667. [Google Scholar] [CrossRef]
- Yu, D.; Williams, G.W.; Aguilar, D.; Yamal, J.M.; Maroufy, V.; Wang, X.; Zhang, C.; Huang, Y.; Gu, Y.; Talebi, Y.; et al. Machine learning prediction of the adverse outcome for nontraumatic subarachnoid hemorrhage patients. Ann. Clin. Transl. Neurol. 2020, 7, 2178–2185. [Google Scholar] [CrossRef]
- Gaastra, B.; Barron, P.; Newitt, L.; Chhugani, S.; Turner, C.; Kirkpatrick, P.; MacArthur, B.; Galea, I.; Bulters, D. CRP (C-Reactive Protein) in Outcome Prediction after Subarachnoid Hemorrhage and the Role of Machine Learning. Stroke 2021, 52, 3276–3285. [Google Scholar] [CrossRef]
- Jamali, S.A.; Turnbull, M.T.; Kanekiyo, T.; Vishnu, P.; Zubair, A.C.; Raper, C.C.; Tawk, R.G.; Freeman, W.D.; Diseases, C. Elevated neutrophil-lymphocyte ratio is predictive of poor outcomes following aneurysmal subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 2020, 29, 104631. [Google Scholar] [CrossRef]
- Cai, L.; Zeng, H.; Tan, X.; Wu, X.; Qian, C.; Chen, G. The role of the blood neutrophil-to-lymphocyte ratio in aneurysmal subarachnoid hemorrhage. Front. Neurol. 2021, 12, 671098. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Acharjee, A.; Ament, Z.; Schleicher, R.; Bevers, M.; Stapleton, C.; Patel, A.; Kimberly, W.T. Machine learning-driven metabolomic evaluation of cerebrospinal fluid: Insights into poor outcomes after aneurysmal subarachnoid hemorrhage. Neurosurgery 2021, 88, 1003. [Google Scholar] [CrossRef]
- Ahn, S.-H.; Savarraj, J.P.; Pervez, M.; Jones, W.; Park, J.; Jeon, S.-B.; Kwon, S.U.; Chang, T.R.; Lee, K.; Kim, D.H.; et al. The subarachnoid hemorrhage early brain edema score predicts delayed cerebral ischemia and clinical outcomes. Neurosurgery 2018, 83, 137–145. [Google Scholar] [CrossRef]
- Wilson, D.A.; Nakaji, P.; Abla, A.A.; Uschold, T.D.; Fusco, D.J.; Oppenlander, M.E.; Albuquerque, F.C.; McDougall, C.G.; Zabramski, J.M.; Spetzler, R.F. A simple and quantitative method to predict symptomatic vasospasm after subarachnoid hemorrhage based on computed tomography. Neurosurgery 2012, 71, 869–876. [Google Scholar] [CrossRef]
- McKinney, W. pandas: A foundational Python library for data analysis and statistics. Python High Perform. Sci. Comput. 2011, 14, 1–9. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Kolde, R. pheatmap: Pretty Heatmaps; R Package Version 1.0. 12; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Dong, G.; Lai, K.K.; Yen, J. Credit scorecard based on logistic regression with random coefficients. Procedia Comput. Sci. 2010, 1, 2463–2468. [Google Scholar] [CrossRef]
- Shachar, N.; Mitelpunkt, A.; Kozlovski, T.; Galili, T.; Frostig, T.; Brill, B.; Marcus-Kalish, M.; Benjamini, Y. The importance of nonlinear transformations use in medical data analysis. JMIR Med. Inform. 2018, 6, e7992. [Google Scholar] [CrossRef]
- Biau, G.; Scornet, E. A random forest guided tour. TEST 2016, 25, 197–227. [Google Scholar] [CrossRef]
- Patle, A.; Chouhan, D.S. SVM kernel functions for classification. In Proceedings of the 2013 International Conference on Advances in Technology and Engineering (ICATE), Mumbai, India, 23–25 January 2013; pp. 1–9. [Google Scholar]
- Scabini, L.F.; Bruno, O.M. Structure and performance of fully connected neural networks: Emerging complex network properties. Phys. A Stat. Mech. Its Appl. 2023, 615, 128585. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; Wu, H.; Krafft, P.R.; Wang, Z.; Zhang, J.H. The harmful effects of subarachnoid hemorrhage on extracerebral organs. Biomed Res. Int. 2014, 2014, 858496. [Google Scholar] [CrossRef] [PubMed]
- Cahill, J.; Calvert, J.W.; Zhang, J.H. Mechanisms of early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2006, 26, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Wang, J.; Hu, X.; Ma, J.; Li, H.; You, C. Clinical value of neutrophil to lymphocyte and platelet to lymphocyte ratio after aneurysmal subarachnoid hemorrhage. Neurocritical Care 2017, 26, 393–401. [Google Scholar] [CrossRef]
- Friedrich, V.; Flores, R.; Muller, A.; Bi, W.; Peerschke, E.I.; Sehba, F. Reduction of neutrophil activity decreases early microvascular injury after subarachnoid haemorrhage. J. Neuroinflammation 2011, 8, 103. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, Y.; Lenahan, C.; Chen, S. The role of immune inflammation in aneurysmal subarachnoid hemorrhage. Exp. Neurol. 2021, 336, 113535. [Google Scholar] [CrossRef]
- Chaichana, K.L.; Pradilla, G.; Huang, J.; Tamargo, R.J. Role of inflammation (leukocyte-endothelial cell interactions) in vasospasm after subarachnoid hemorrhage. World Neurosurg. 2010, 73, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Qin, B.; Zhou, S.; Li, J.; Guo, Y.; Cai, L.; Cao, S.; Zhou, H.; Chen, H.; Zhuang, J.; et al. Neutrophil extracellular traps, released from neutrophil, promote microglia inflammation and contribute to poor outcome in subarachnoid hemorrhage. Aging 2021, 13, 13108–13123. [Google Scholar]
- Saraffzadeh, A.; Schlenk, F.; Meisel, A.; Dreier, J.; Vajkoczy, P.; Meisel, C. Immunodepression after aneurysmal subrachnoid hemorrhage. Stroke 2011, 42, 53–58. [Google Scholar] [CrossRef]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy 2021, 122, 474–488. [Google Scholar] [CrossRef]
- Gee, J.M.; Kalil, A.; Shea, C.; Becker, K.J. Lymphocytes: Potential mediators of postischemic injury and neuroprotection. Stroke 2007, 38, 783–788. [Google Scholar] [CrossRef]
- Ayer, R.E.; Ostrowski, R.P.; Sugawara, T.; Ma, Q.; Jafarian, N.; Tang, J.; Zhang, J.H. Statin-induced T-lymphocyte modulation and neuroprotection following experimental subarachnoid hemorrhage. In Vasospasm: Neurovascular Events after Subarachnoid Hemorrhage; Springer Nature: Berlin, Germany, 2013; pp. 259–266. [Google Scholar]
- Giede-Jeppe, A.; Reichl, J.; Sprügel, M.I.; Lücking, H.; Hoelter, P.; Eyüpoglu, I.Y.; Kuramatsu, J.B.; Huttner, H.B.; Gerner, S.T. Neutrophil-to-lymphocyte ratio as an independent predictor for unfavorable functional outcome in aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2019, 132, 400–407. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Feghali, J.; Kim, J.; Gami, A.; Rapaport, S.; Caplan, J.M.; McDougall, C.G.; Huang, J.; Tamargo, R.J.; Jackson, C.M. Monocyte-based inflammatory indices predict outcomes following aneurysmal subarachnoid hemorrhage. Neurosurg. Rev. 2021, 44, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Dubow, J.; Fink, M.E. Impact of hypertension on stroke. Curr. Atheroscler. Rep. 2011, 13, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Rosengart, A.J.; Schultheiss, K.E.; Tolentino, J.; Macdonald, R.L. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke 2007, 38, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Juvela, S.; Siironen, J.; Kuhmonen, J. Hyperglycemia, excess weight, and history of hypertension as risk factors for poor outcome and cerebral infarction after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2005, 102, 998–1003. [Google Scholar] [CrossRef]
- Claassen, J.; Carhuapoma, J.R.; Kreiter, K.T.; Du, E.Y.; Connolly, E.S.; Mayer, S.A. Global cerebral edema after subarachnoid hemorrhage: Frequency, predictors, and impact on outcome. Stroke 2002, 33, 1225–1232. [Google Scholar] [CrossRef]
- Eibach, M.; Won, S.-Y.; Bruder, M.; Keil, F.; Herrmann, E.; Berkefeld, J.; Seifert, V.; Konczalla, J. Age dependency and modification of the subarachnoid hemorrhage early brain edema score. J. Neurosurg. 2020, 1, 1–7. [Google Scholar] [CrossRef]
- Said, M.; Gümüs, M.; Herten, A.; Dinger, T.F.; Chihi, M.; Darkwah Oppong, M.; Deuschl, C.; Wrede, K.H.; Kleinschnitz, C.; Sure, U.; et al. Subarachnoid Hemorrhage Early Brain Edema Score (SEBES) as a radiographic marker of clinically relevant intracranial hypertension and unfavorable outcome after subarachnoid hemorrhage. Eur. J. Neurol. 2021, 28, 4051–4059. [Google Scholar] [CrossRef]

| Predictors | Development Cohort (n = 240) | Validation Cohort (n = 61) |
|---|---|---|
| Age (y) | 56.18 ± 11.69 | 54.77 ± 12.47 |
| Female | 150 (62.5%) | 37 (60.66%) |
| Aneurysm location | ||
| Anterior cerebral artery | 102 (42.5%) | 24 (39.34%) |
| Middle cerebral artery | 39 (16.25%) | 17 (27.87%) |
| Posterior circulation | 84 (35%) | 12 (19.67%) |
| Internal carotid artery | 15 (6.25%) | 8 (13.11%) |
| Hypertension | 112 (46.67%) | 26 (42.62%) |
| Treatment | ||
| Coil | 91 (37.92%) | 21 (34.43%) |
| Clip | 148 (61.66%) | 40 (65.57%) |
| Conservative treatment | 1 (0.42%) | 0 (0%) |
| GCS score | ||
| 3–8 | 33 (13.75%) | 10 (16.39%) |
| 9–12 | 6 (2.5%) | 3 (4.92%) |
| 13–15 | 201 (83.75%) | 48 (78.69%) |
| WFNS grade | ||
| 1 | 174 (72.5%) | 42 (68.85%) |
| 2 | 24 (10%) | 5 (8.20%) |
| 3 | 3 (1.25%) | 1 (1.64%) |
| 4 | 24 (10%) | 4 (6.56%) |
| 5 | 15 (6.25%) | 9 (14.75%) |
| Hunt and Hess scale | ||
| 1 | 81 (33.75%) | 6 (9.84%) |
| 2 | 83 (34.58%) | 34 (55.74%) |
| 3 | 46 (19.17%) | 11 (18.03%) |
| 4 | 26 (10.83%) | 7 (11.48%) |
| 5 | 4 (1.67%) | 3 (4.92%) |
| Neuroimaging assessment | ||
| mFisher grade | 3 (2–4) | 3 (2–4) |
| SEBES | 1 (0–2) | 2 (0–3) |
| BNI scale | 5 (4–5) | 5 (4–5) |
| Intraventricular hemorrhage | 132 (55%) | 41 (67.21%) |
| Laboratory indexes | ||
| Neutrophil (×109/L) | 11.05 ± 3.95 | 12.55 ± 5.14 |
| Lymphocyte (×109/L) | 1.10 ± 0.54 | 1.02 ± 0.43 |
| Monocytes (×109/L) | 0.52 ± 0.33 | 0.59 ± 0.36 |
| Eosinophil (×109/L) | 0.01 ± 0.03 | 0.01 ± 0.02 |
| Basophil (×109/L) | 0.03 ± 0.02 | 0.02 ± 0.01 |
| NLR | 11.77 ± 5.85 | 14.45 ± 8.43 |
| Models | Accuracy | AUC | |
|---|---|---|---|
| Logistic Regression | 0.792 (0.745–0.828) | 0.739 (0.645–0.834) | |
| Random Forest | 0.783 (0.770–0.786) | 0.749 (0.664–0.834) | |
| Support Vector Machine | linear | 0.796 (0.742–0.849) | 0.762 (0.679–0.844) |
| rbf | 0.783 (0.771–0.796) | 0.774 (0.696–0.852) | |
| sigmoid | 0.821 (0.766–0.875) | 0.793 (0.723–0.863) | |
| FCNNs | 128 | 0.950 (0.927–0.972) | 0.946 (0.918–0.974) |
| 256 | 0.957 (0.938–0.976) | 0.952 (0.920–0.983) | |
| 512 | 0.960 (0.940–0.979) | 0.946 (0.911–0.981) | |
| 1024 | 0.962 (0.947–0.978) | 0.947 (0.916–0.978) | |
| Variables | Range | Score |
|---|---|---|
| NLR | (−inf, 8.301] | −14 |
| (8.301, 17.233] | −1 | |
| (17.233, inf) | 15 | |
| Lymphocyte count | (−inf, 0.68] | 7 |
| (0.68, 1.523] | −1 | |
| (1.523, inf) | −8 | |
| Monocyte count | (−inf, 0.179] | 12 |
| (0.179, inf) | −1 | |
| Hypertension | No | −5 |
| Yes | 4 | |
| SEBES | (−inf, 2.0] | −3 |
| (2.0, inf) | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zeng, H.; Zhou, H.; Li, J.; Wang, T.; Guo, Y.; Cai, L.; Hu, J.; Zhang, X.; Chen, G. Predicting the Outcome of Patients with Aneurysmal Subarachnoid Hemorrhage: A Machine-Learning-Guided Scorecard. J. Clin. Med. 2023, 12, 7040. https://doi.org/10.3390/jcm12227040
Zhang Y, Zeng H, Zhou H, Li J, Wang T, Guo Y, Cai L, Hu J, Zhang X, Chen G. Predicting the Outcome of Patients with Aneurysmal Subarachnoid Hemorrhage: A Machine-Learning-Guided Scorecard. Journal of Clinical Medicine. 2023; 12(22):7040. https://doi.org/10.3390/jcm12227040
Chicago/Turabian StyleZhang, Yi, Hanhai Zeng, Hang Zhou, Jingbo Li, Tingting Wang, Yinghan Guo, Lingxin Cai, Junwen Hu, Xiaotong Zhang, and Gao Chen. 2023. "Predicting the Outcome of Patients with Aneurysmal Subarachnoid Hemorrhage: A Machine-Learning-Guided Scorecard" Journal of Clinical Medicine 12, no. 22: 7040. https://doi.org/10.3390/jcm12227040






