Short-Term Evaluation of Bone–ACL–Bone Complex Allograft in ACL Reconstruction in a Rabbit Model
Abstract
:1. Introduction
2. Methods
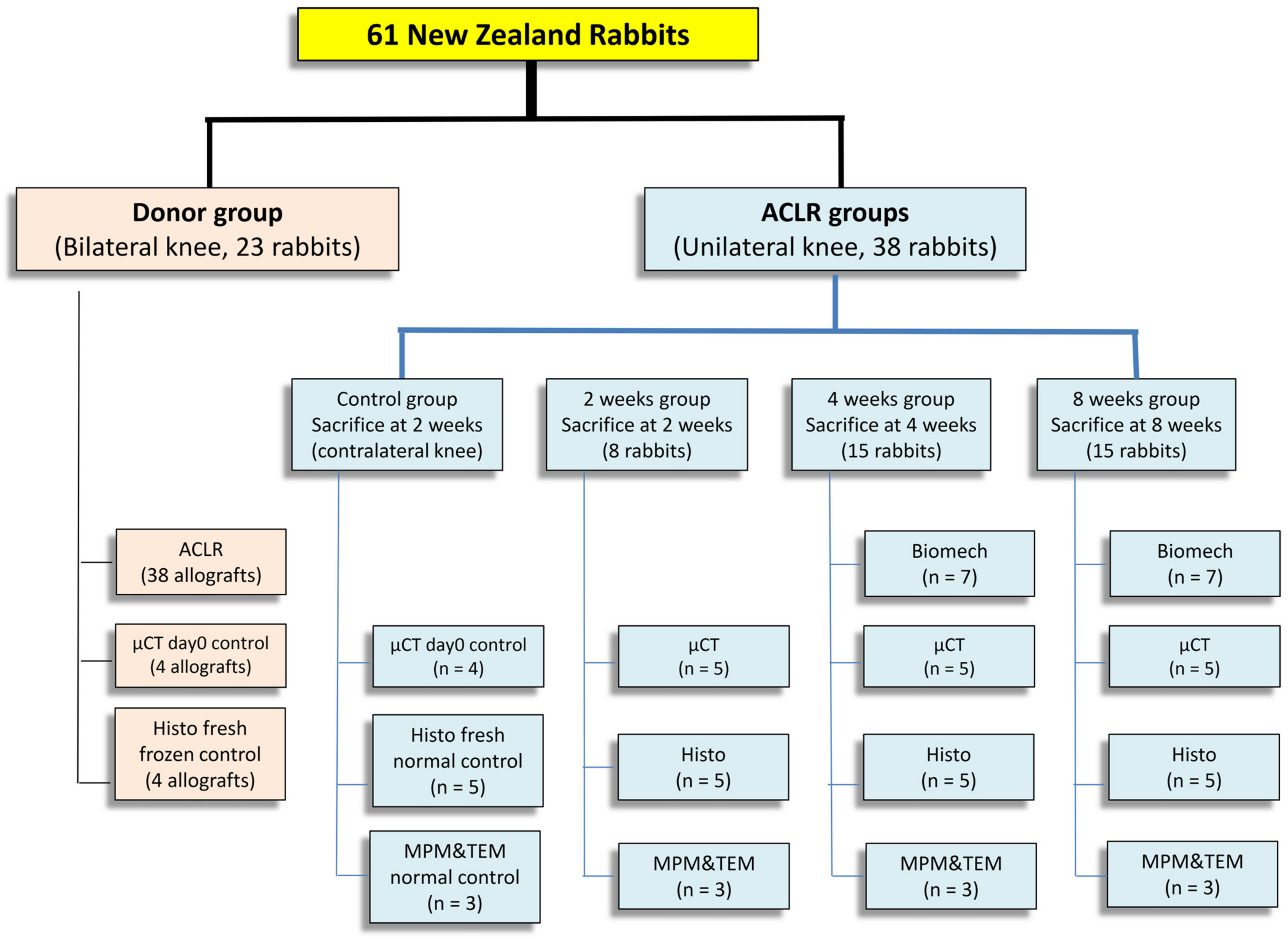
2.1. Study Design
2.2. Surgical Procedure
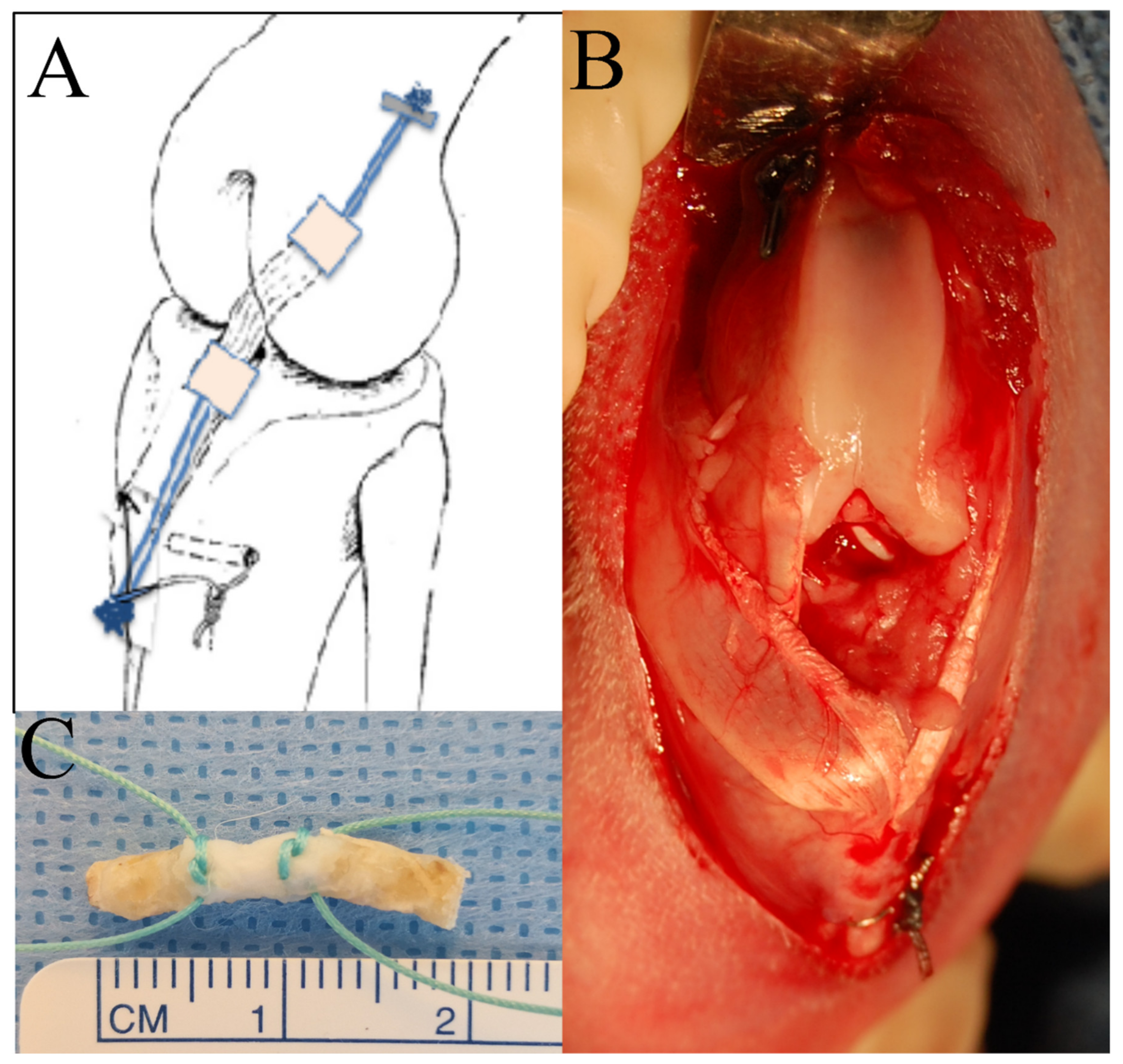
2.2.1. Femur–ACL–Tibia Allograft Harvest and Preparation
2.2.2. ACL Reconstruction Procedure
2.2.3. Radiographic Imaging
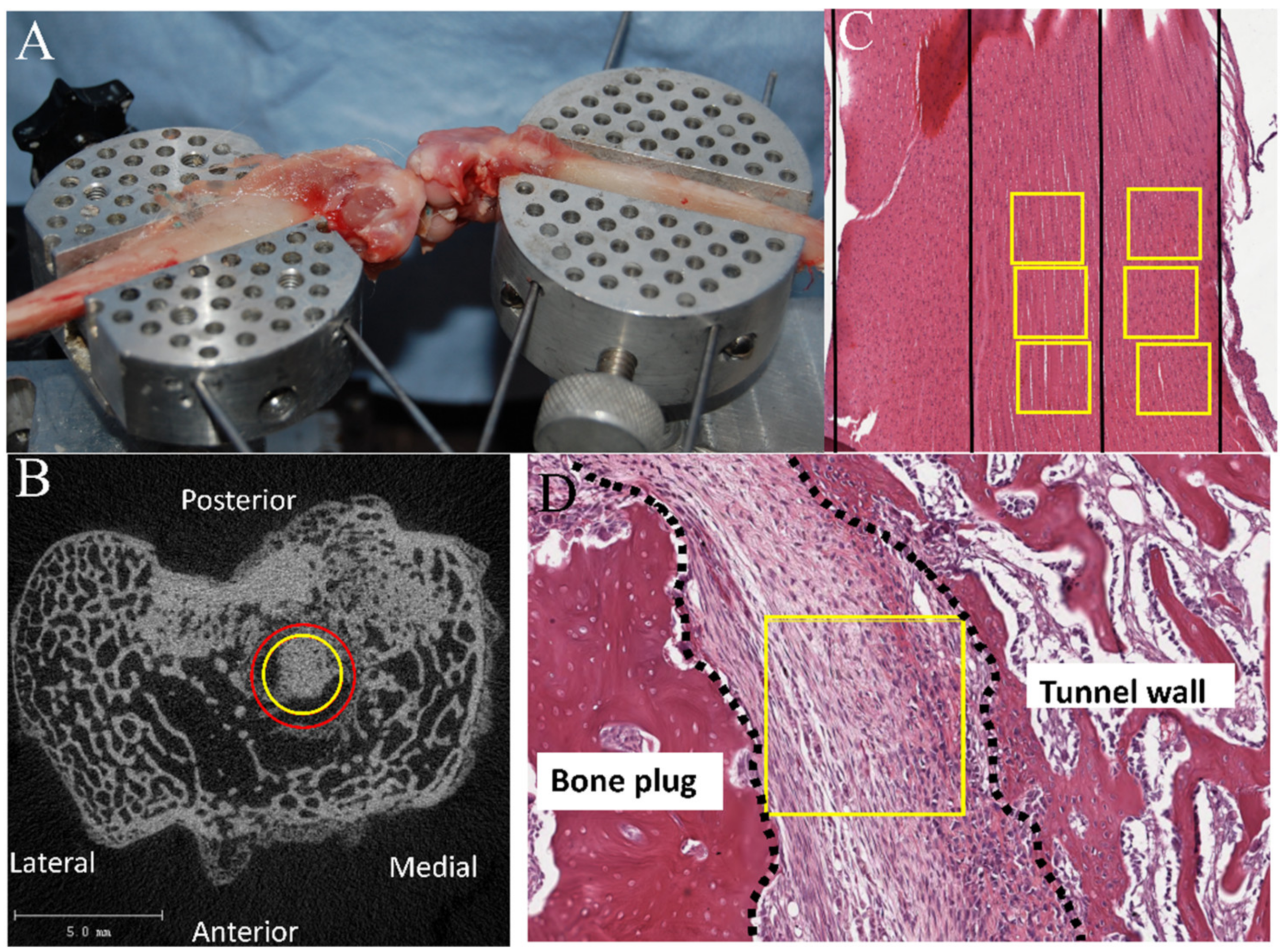
2.2.4. Biomechanical Testing
2.2.5. μCT Analysis
2.2.6. Histology and Histomorphometry
2.3. Multi-Photon Microscopy and Transmission Electron Microscopy
2.4. Statistical Analysis
3. Results
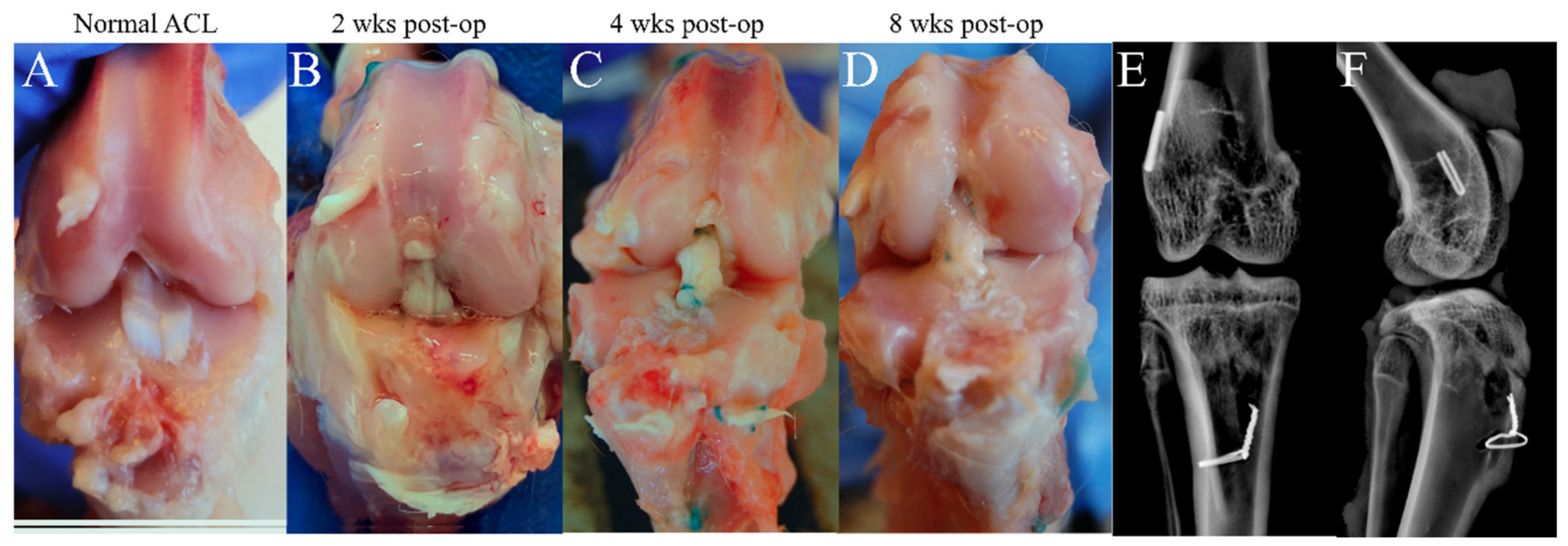
3.1. Macroscopic Observation and Radiographic Imaging
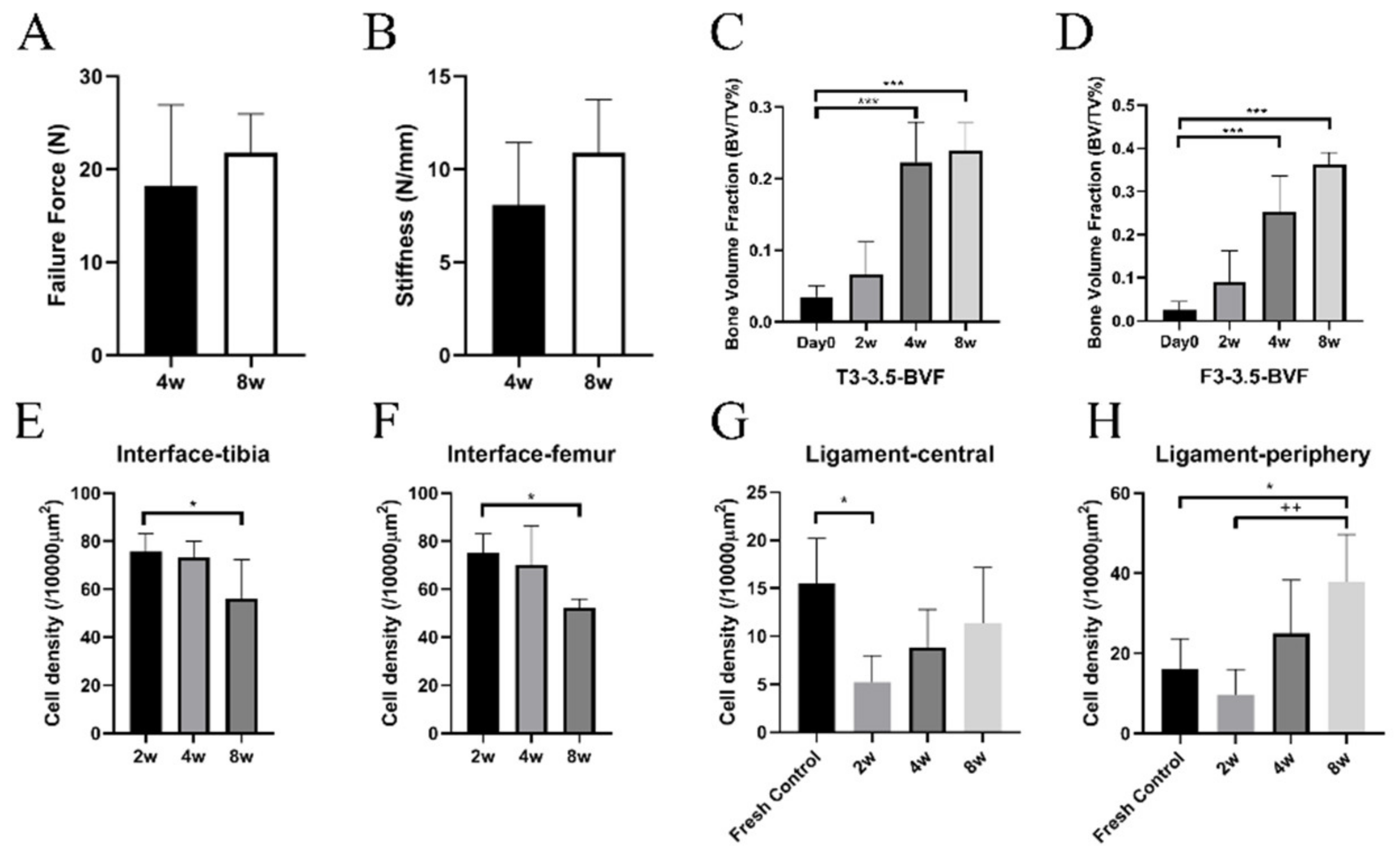
3.2. Biomechanical Analysis
3.3. μCT Analysis
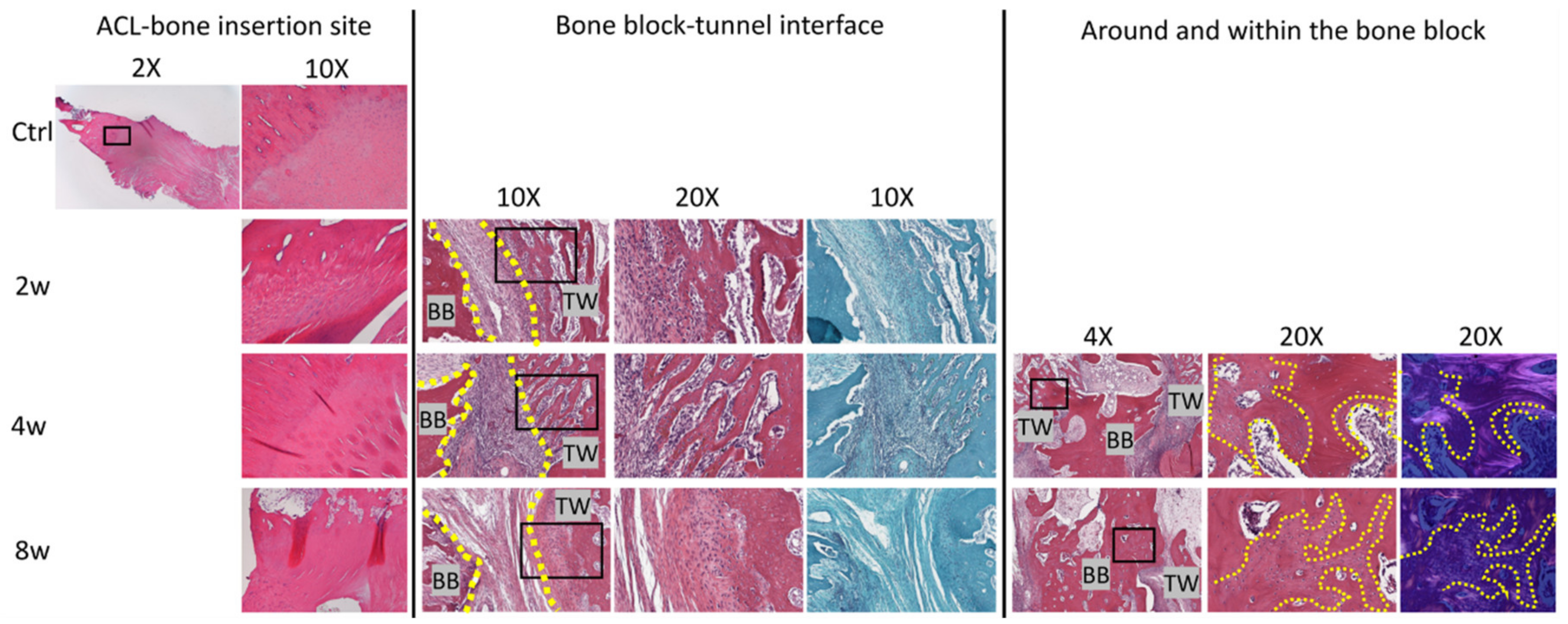
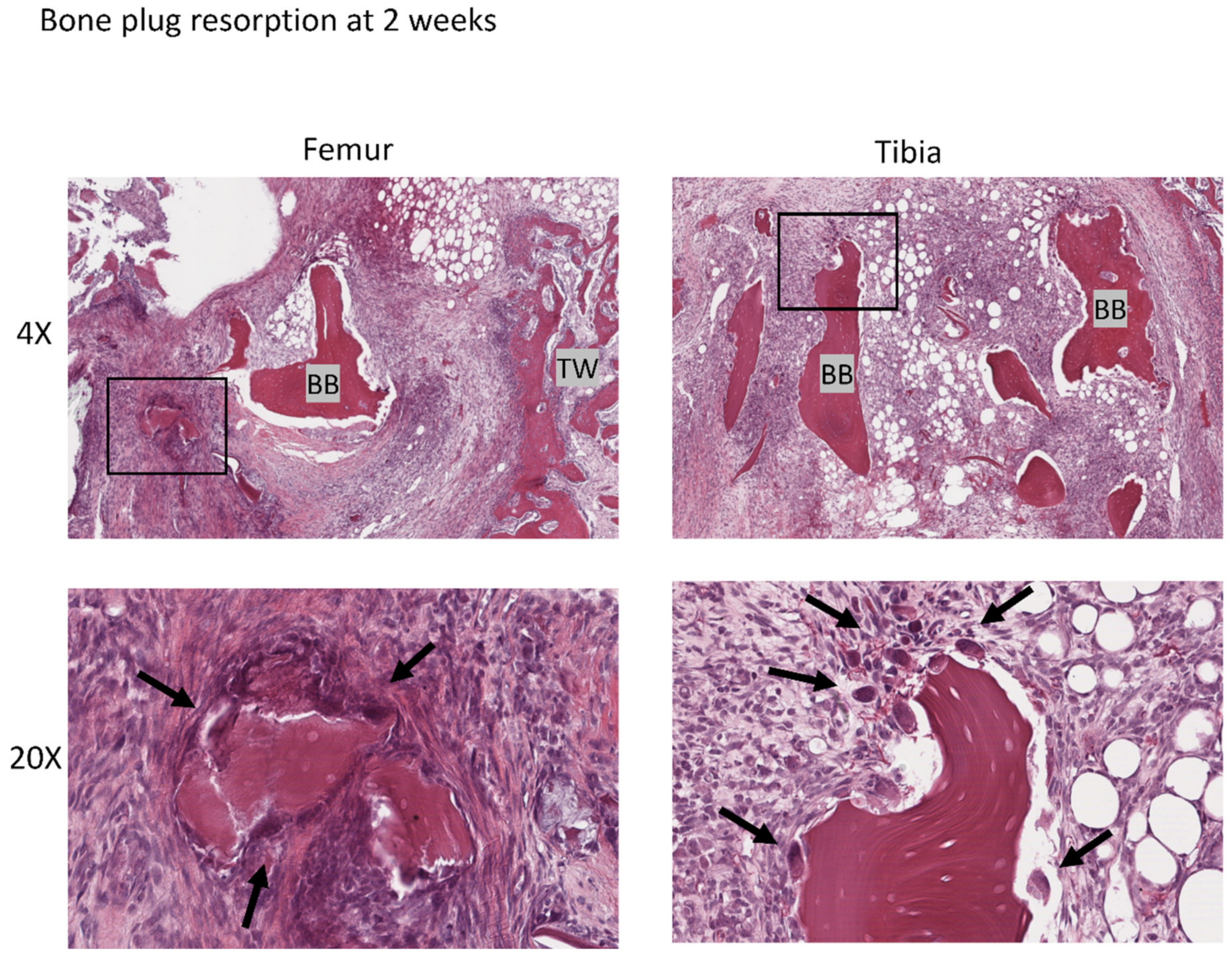
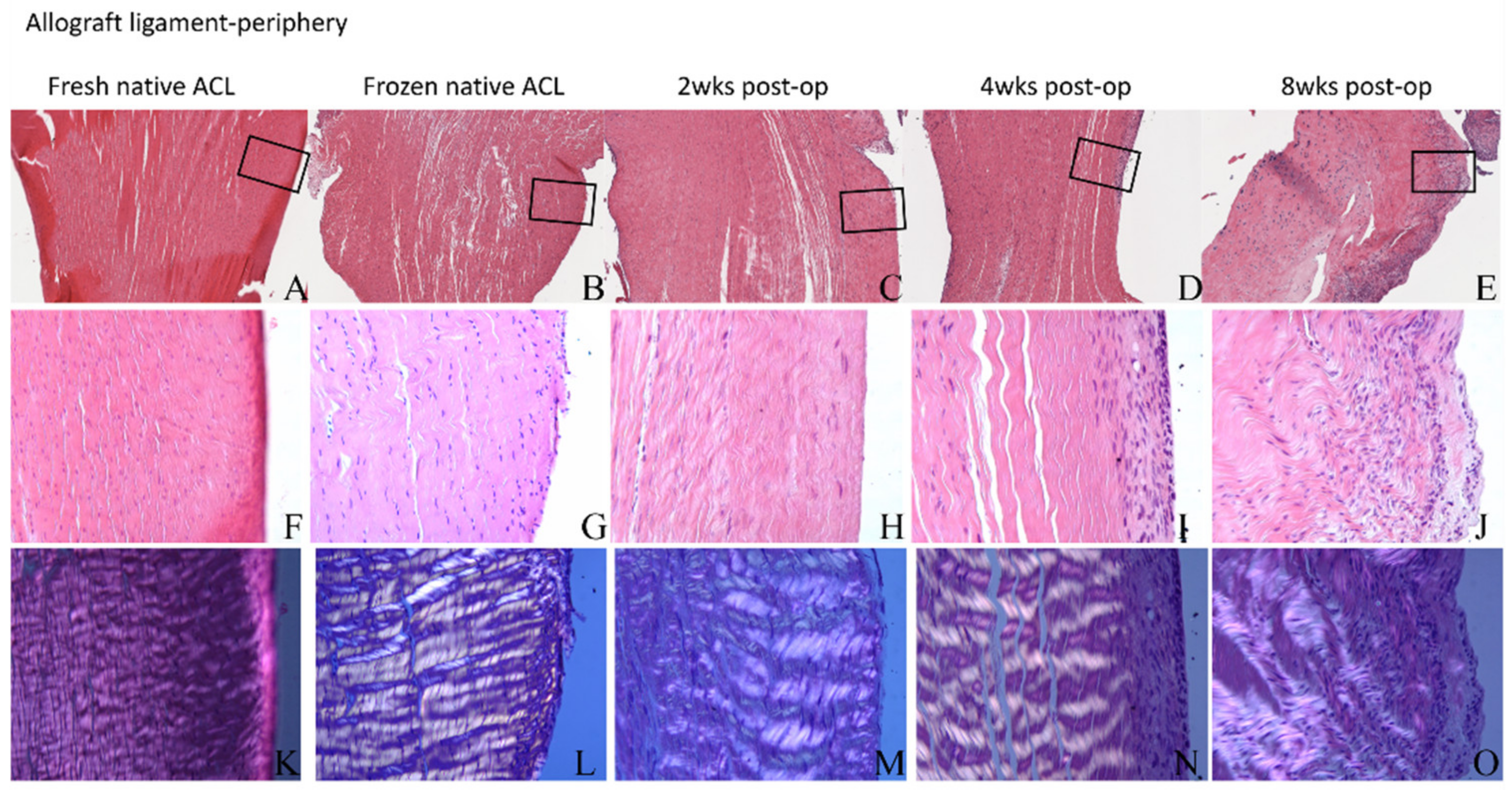
3.4. Histological Analysis
3.4.1. Bone Plug-to-Tunnel Healing
3.4.2. Bone Plug Remodeling
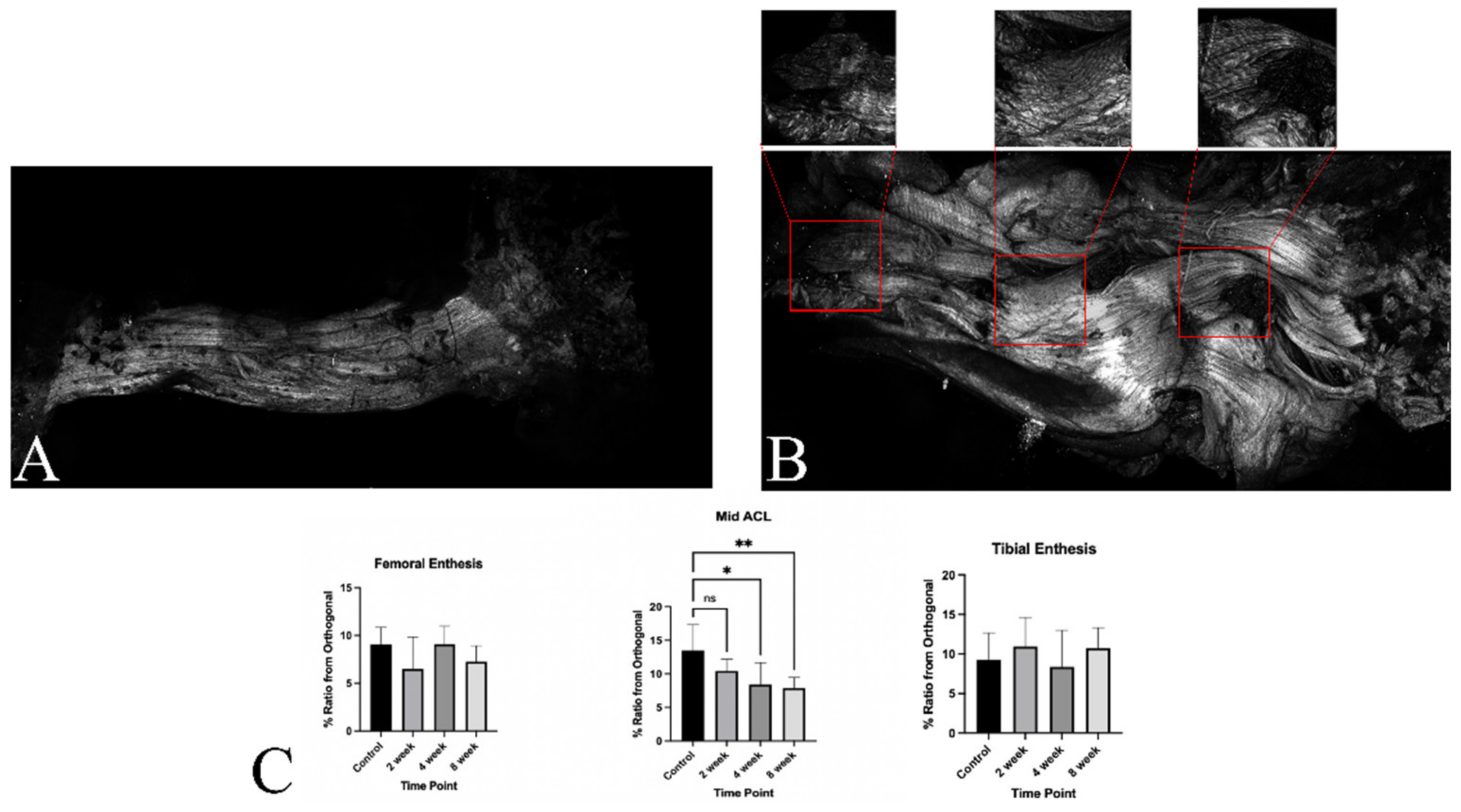
3.4.3. ACL Mid-Substance Remodeling
3.5. Ultrastructural Evaluation with Multi-Photon Microscopy and TEM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, P.J.; Alexander, J.W.; Gold, J.E.; Icenogle, K.D.; Noble, P.C.; Lowe, W.R. Anatomic Double-Bundle Anterior Cruciate Ligament Reconstruction: Kinematics and Knee Flexion Angle–Graft Tension Relation. Arthroscopy 2010, 26, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Rovere, G.D.; Adair, D.M. Anterior cruciate-deficient knees: A review of the literature. Am. J. Sports Med. 1983, 11, 412–419. [Google Scholar] [CrossRef]
- Gulotta, L.V.; Rodeo, S.A. Biology of Autograft and Allograft Healing in Anterior Cruciate Ligament Reconstruction. Clin. Sports Med. 2007, 26, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Rodeo, S.A.; Arnoczky, S.P.; Torzilli, P.A.; Hidaka, C.; Warren, R.F. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J. Bone Jt. Surg. 1993, 75, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Rodeo, S.A.; Kawamura, S.; Ma, C.B.; Deng, X.; Sussman, P.S.; Patrick, S.; Hays, P.; Ying, L. The effect of osteoclastic activity on tendon-to-bone healing: An experimental study in rabbits. J. Bone Jt. Surg. Am. 2007, 89, 2250–2259. [Google Scholar]
- Grana, W.A.; Egle, D.M.; Mahnken, R.; Goodhart, C.W. An analysis of autograft fixation after anterior cruciate ligament recon-struction in a rabbit model. Am. J. Sports Med. 1994, 22, 344–351. [Google Scholar] [CrossRef]
- Goradia, V.K.; Rochat, M.C.; A Grana, W.; Rohrer, M.D.; Prasad, H.S. Tendon-to-bone healing of a semitendinosus tendon autograft used for ACL reconstruction in a sheep model. Am. J. Knee Surg. 2000, 13, 143–151. [Google Scholar]
- Goertzen, M.; Dellmann, A.; Gruber, J.; Clahsen, H.; Bürrig, K.F. Anterior cruciate ligament allograft transplantation for intraar-ticular ligamentous reconstruction. Arch. Orthop. Trauma Surg. 1992, 111, 273–279. [Google Scholar] [CrossRef]
- Goertzen, M.J.; Clahsen, H.; Schulitz, K.-P.; Bürrig, K.F. Anterior cruciate ligament reconstruction using cryopreserved irradiated bone-ACL-bone-allograft transplants. Knee Surg. Sports Traumatol. Arthrosc. 1994, 2, 150–157. [Google Scholar] [CrossRef]
- Jackson, D.W.; Grood, E.S.; Arnoczky, S.P.; Butler, D.L.; Simon, T.M. Freeze dried anterior cruciate ligament allografts. Preliminary studies in a goat model. Am. J. Sports Med. 1987, 15, 295–303. [Google Scholar] [CrossRef]
- Jackson, D.W.; Windler, G.E.; Simon, T.M. Intraarticular reaction associated with the use of freeze-dried, ethylene oxide-sterilized bone-patella tendon-bone allografts in the reconstruction of the anterior cruciate ligament. Am. J. Sports Med. 1990, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.W.; Grood, E.S.; Wilcox, P.; Butler, D.L.; Simon, T.M.; Holden, J.P. The effects of processing techniques on the mechanical properties of bone-anterior cruciate ligament-bone allografts. An experimental study in goats. Am. J. Sports Med. 1988, 16, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Cai, S.; Niu, J.; Liu, C.; Dong, C.; Wang, F. Biomechanical properties of reinforced bone-anterior cruciate ligament-bone allografts for anterior cruciate ligament reconstruction in rabbits. Int. J. Clin. Exp. Med. 2018, 11, 9189–9199. [Google Scholar]
- Nikolaou, P.K.; Seaber, A.V.; Glisson, R.R.; Ribbeck, B.M.; Bassett, F.H., 3rd. Anterior cruciate ligament allograft transplantation. Long-term function, histology, revascularization, and operative technique. Am. J. Sports Med. 1986, 14, 348–360. [Google Scholar] [CrossRef]
- Vasseur, P.B.; Rodrigo, J.J.; Stevenson, S.; Clark, G.; Sharkey, N. Replacement of the anterior cruciate ligament with a bone-ligament-bone anterior cruciate ligament allograft in dogs. Clin. Orthop. Relat. Res. 1987, 219, 268–277. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Taylor, S.E.; Cao, T.; Talauliker, P.M.; Lifshitz, J. Objective Morphological Quantification of Microscopic Images Using a Fast Fourier Transform (FFT) Analysis. Curr. Protoc. Essent. Lab. Tech. 2013, 95 (Suppl. 7), 9.5.1–9.5.12. [Google Scholar] [CrossRef]
- Shimizu, T.; Cheng, Z.; Samaan, M.A.; Tanaka, M.S.; Souza, R.B.; Li, X.; Ma, C.B. Increases in Joint Laxity After Anterior Cruciate Ligament Reconstruction Are As-sociated With Sagittal Biomechanical Asymmetry. Arthroscopy 2019, 35, 2072–2079. [Google Scholar] [CrossRef]
- Wang, J.H.; Kato, Y.; Ingham, S.J.; Maeyama, A.; Linde-Rosen, M.; Smolinski, P.; Fu, F.H. Measurement of the End-to-End Distances Between the Femoral and Tibial Insertion Sites of the Anterior Cruciate Ligament During Knee Flexion and with Rotational Torque. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 1524–1532. [Google Scholar] [CrossRef]
- Jackson, D.W.; Grood, E.S.; Goldstein, J.D.; Rosen, M.A.; Kurzweil, P.R.; Cummings, J.F.; Simon, T.M. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am. J. Sports Med. 1993, 21, 176–185. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Fan, H.-B.; Xu, H.; Li, Q.-H.; Guo, L. Histological comparison of fate of ligamentous insertion after reconstruction of anterior cruciate ligament: Autograft vs allograft. Chin. J. Traumatol. 2006, 9, 72–76. [Google Scholar] [PubMed]
- Harris, N.L.; Indelicato, P.A.; Bloomberg, M.S.; Meister, K.; Wheeler, D.L. Radiographic and Histologic Analysis of the Tibial Tunnel after Allograft Anterior Cruciate Ligament Reconstruction in Goats. Am. J. Sports Med. 2002, 30, 368–373. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Havasy, J.; Green, S.; Deng, X.-H.; Chen, D.; Piacentini, A.; Rodeo, S.A. Short-Term Evaluation of Bone–ACL–Bone Complex Allograft in ACL Reconstruction in a Rabbit Model. J. Clin. Med. 2023, 12, 7057. https://doi.org/10.3390/jcm12227057
Liu Y, Havasy J, Green S, Deng X-H, Chen D, Piacentini A, Rodeo SA. Short-Term Evaluation of Bone–ACL–Bone Complex Allograft in ACL Reconstruction in a Rabbit Model. Journal of Clinical Medicine. 2023; 12(22):7057. https://doi.org/10.3390/jcm12227057
Chicago/Turabian StyleLiu, Yulei, Janice Havasy, Samuel Green, Xiang-Hua Deng, Daoyun Chen, Alexander Piacentini, and Scott A. Rodeo. 2023. "Short-Term Evaluation of Bone–ACL–Bone Complex Allograft in ACL Reconstruction in a Rabbit Model" Journal of Clinical Medicine 12, no. 22: 7057. https://doi.org/10.3390/jcm12227057




