Implementation of an Enhanced Recovery after Surgery Pathway for Transgender and Gender-Diverse Individuals Undergoing Chest Reconstruction Surgery: An Observational Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Framework
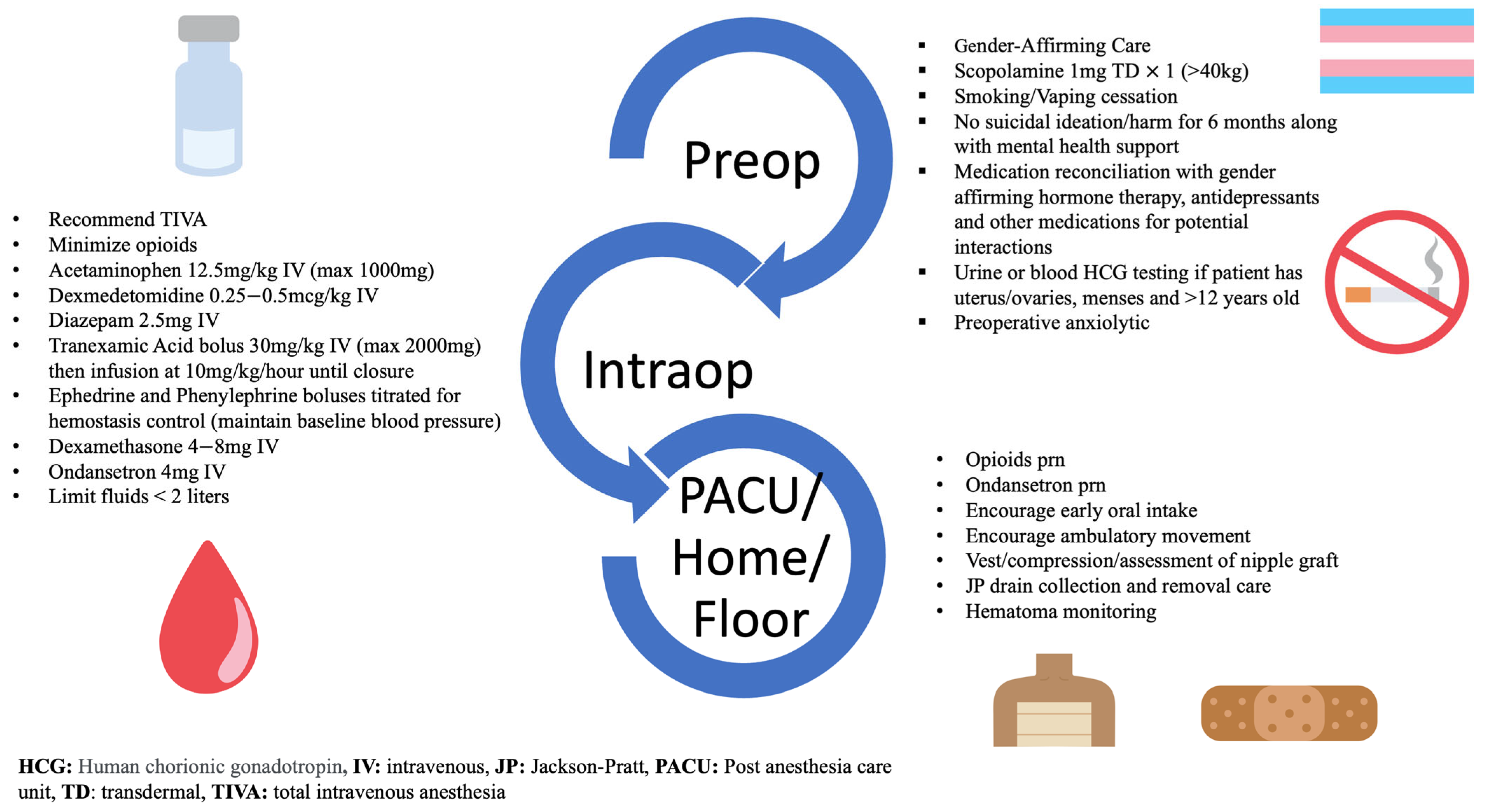
2.2. Interventions
2.3. Traditional Group
2.4. Partial ERAS Implementation Group
2.5. ERAS Group
2.6. Primary and Secondary Outcome Measures
2.7. Statistical Analysis
3. Results
3.1. Demographics and Patient Characteristics
3.2. Group Implementation
3.3. Intraoperative Predictors of Length of Stay
3.4. Tranexamic Acid Comparisons
3.5. Intraoperative Predictors of Hematoma or Return to OR
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herman, J.L.; Flores, A.R.; O’Neill, K.K. How Many Adults and Youth Identify as Transgender in the United States? The Williams Institute, UCLA School of Law: Los Angeles, CA, USA, 2022; p. 26. [Google Scholar]
- Almazan, A.N.; Keuroghlian, A.S. Association Between Gender-Affirming Surgeries and Mental Health Outcomes. JAMA Surg. 2021, 156, 611. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.; Ives, G.C.; Sluiter, E.C.; Waljee, J.F.; Yao, T.-H.; Hu, H.M.; Kuzon, W.M. Trends in Gender-Affirming Surgery in Insured Patients in the United States. Plast. Reconstr. Surg.-Glob. Open 2018, 6, e1738. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Perdikis, G.; Al Kassis, S.; Drolet, B.C. Gender-Affirming Chest Reconstruction Among Transgender and Gender-Diverse Adolescents in the US From 2016 to 2019. JAMA Pediatr. 2023, 177, 89. [Google Scholar] [CrossRef] [PubMed]
- Astanehe, A.; Temple-Oberle, C.; Nielsen, M.; de Haas, W.; Lindsay, R.; Matthews, J.; McKenzie, D.C.; Yeung, J.; Schrag, C. An Enhanced Recovery after Surgery Pathway for Microvascular Breast Reconstruction Is Safe and Effective. Plast. Reconstr. Surg.-Glob. Open 2018, 6, e1634. [Google Scholar] [CrossRef]
- Chiu, C.; Aleshi, P.; Esserman, L.J.; Inglis-Arkell, C.; Yap, E.; Whitlock, E.L.; Harbell, M.W. Improved Analgesia and Reduced Post-Operative Nausea and Vomiting after Implementation of an Enhanced Recovery after Surgery (ERAS) Pathway for Total Mastectomy. BMC Anesthesiol. 2018, 18, 41. [Google Scholar] [CrossRef]
- Steinthorsdottir, K.J.; Awada, H.N.; Abildstrøm, H.; Kroman, N.; Kehlet, H.; Kvanner Aasvang, E. Dexamethasone Dose and Early Postoperative Recovery after Mastectomy. Anesthesiology 2020, 132, 678–691. [Google Scholar] [CrossRef]
- Temple-Oberle, C.; Shea-Budgell, M.A.; Tan, M.; Semple, J.L.; Schrag, C.; Barreto, M.; Blondeel, P.; Hamming, J.; Dayan, J.; Ljungqvist, O. Consensus Review of Optimal Perioperative Care in Breast Reconstruction: Enhanced Recovery after Surgery (ERAS) Society Recommendations. Plast. Reconstr. Surg. 2017, 139, 1056e–1071e. [Google Scholar] [CrossRef]
- Sanchez, K.J.; Sanchez, R.A.; Ben Khallouq, B.; Ellis, D.B. Perioperative Care of Transgender and Gender-Diverse Patients: A Biopsychosocial Approach. Anesth. Analg. 2023, 137, 234–246. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yoo, J.Y.; Kim, J.Y.; Kim, D.H.; Lee, J.D.; Rho, G.U.; Park, H.; Park, S.Y. Optimal Effect-Site Concentration of Remifentanil for Preventing Cough during Removal of the Double-Lumen Endotracheal Tube from Sevoflurane-Remifentanil Anesthesia: A Prospective Clinical Trial. Medicine (Baltimore) 2016, 95, e3878. [Google Scholar] [CrossRef]
- Son, H.W.; Lee, J.M.; Park, S.H.; Lee, Y.J.; Oh, J.M.; Hwang, S.K. Fentanyl versus Remifentanil for Cough Suppression and Recovery after Video-Assisted Thoracic Surgery. J. Chest Surg. 2021, 54, 200–205. [Google Scholar] [CrossRef]
- Goobie, S.M.; Staffa, S.J.; Meara, J.G.; Proctor, M.R.; Tumolo, M.; Cangemi, G.; Disma, N. High-Dose versus Low-Dose Tranexamic Acid for Paediatric Craniosynostosis Surgery: A Double-Blind Randomised Controlled Non-Inferiority Trial. Br. J. Anaesth. 2020, 125, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Halpern, L.M.; Bronson, W.E.; Kogan, C.J. A New Low Dose of Tranexamic Acid for Decreasing the Rate of Blood Loss in Posterior Spinal Fusion for Adolescent Idiopathic Scoliosis. J. Pediatr. Orthop. 2021, 41, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, J.; Chen, X.; Du, L.; Li, K.; Zhou, Y. Enhanced Recovery after Surgery on Multiple Clinical Outcomes: Umbrella Review of Systematic Reviews and Meta-Analyses. Medicine (Baltimore) 2020, 99, e20983. [Google Scholar] [CrossRef] [PubMed]
- El Tahan, M.R.; Pahade, A.; Gómez-Ríos, M.Á. Enhanced Recovery after Surgery: Comes out to the Sun. BMC Anesthesiol. 2023, 23, 274. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Sola, C.; Saour, A.C.; Chapron, K.; Coruble, L.; Bringuier, S.; Dadure, C. Enhanced Recovery after Surgery (ERAS) for Adolescent Idiopathic Scoliosis: Standardisation of Care Improves Patient Outcomes. Anaesth. Crit. Care Pain Med. 2022, 41, 101116. [Google Scholar] [CrossRef]
- Smith, A.E.; Heiss, K.; Childress, K.J. Enhanced Recovery After Surgery in Pediatric and Adolescent Gynecology: A Pilot Study. J. Pediatr. Adolesc. Gynecol. 2020, 33, 403–409. [Google Scholar] [CrossRef]
- Cuccolo, N.G.; Kang, C.O.; Boskey, E.R.; Ibrahim, A.M.S.; Blankensteijn, L.L.; Taghinia, A.; Lee, B.T.; Lin, S.J.; Ganor, O. Masculinizing Chest Reconstruction in Transgender and Nonbinary Individuals: An Analysis of Epidemiology, Surgical Technique, and Postoperative Outcomes. Aesthetic Plast. Surg. 2019, 43, 1575–1585. [Google Scholar] [CrossRef]
- McEvenue, G.; Xu, F.Z.; Cai, R.; McLean, H. Female-to-Male Gender Affirming Top Surgery: A Single Surgeon’s 15-Year Retrospective Review and Treatment Algorithm. Aesthet. Surg. J. 2018, 38, 49–57. [Google Scholar] [CrossRef]
- Elena Scarafoni, E. A Systematic Review of Tranexamic Acid in Plastic Surgery: What’s New? Plast. Reconstr. Surg.-Glob. Open 2021, 9, e3172. [Google Scholar] [CrossRef]
- Winder, A.A.; McQuillan, P.; Dijkstra, B. Tranexamic Acid Use in Breast Surgery: A Systematic Review and Meta-Analysis. Ann. Breast Surg. 2021, 5, 12. [Google Scholar] [CrossRef]
- Verdecchia, N.; Grunwaldt, L.; Visoiu, M. Pain Outcomes Following Mastectomy or Bilateral Breast Reduction for Transgender and Nontransgender Patients Who Received Pectoralis Nerve Blocks. Pediatr. Anesth. 2020, 30, 1049–1050. [Google Scholar] [CrossRef] [PubMed]
- Sindali, K.; Harries, V.; Borges, A.; Simione, S.; Patel, S.; Vorster, T.; Lawrence, C.; Jones, M. Improved Patient Outcomes Using the Enhanced Recovery Pathway in Breast Microsurgical Reconstruction: A UK Experience. JPRAS Open 2019, 19, 24–34. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall (n = 362) | Traditional Group (n = 144) | Partial ERAS Group (n = 92) | ERAS Group (n = 126) |
|---|---|---|---|---|
| Age (years) | 18 (17, 21) | 18 (17, 20) | 19 (18, 22) | 20 (17, 23) |
| Weight (kg) | 71 (60, 86) | 71 (60, 84) | 69 (60, 87) | 74 (62, 87) |
| BMI (kg/m2) | 27 (23, 32) | 26 (23, 31) | 25 (22, 32) | 27 (23, 32) |
| Gender Identity | ||||
| Non-binary | 34 (9.4%) | 8 (5.6%) | 9 (9.8%) | 17 (13.5%) |
| Transmasculine | 328 (90.6%) | 136 (94.4%) | 83 (90.2%) | 109 (86.5%) |
| Hospital Location | ||||
| Main Hospital | 104 (28.7%) | 30 (20.8%) | 35 (38%) | 39 (31%) |
| Ambulatory Center | 258 (71.3%) | 114 (79.2%) | 57 (62%) | 87 (69%) |
| ASA-PS | ||||
| I | 77 (21.3%) | 35 (24.3%) | 18 (19.6%) | 24 (19.1%) |
| II | 268 (74%) | 102 (70.8%) | 70 (76.1%) | 96 (76.2%) |
| III | 17 (4.7%) | 7 (4.9%) | 4 (4.4%) | 6 (4.8%) |
| Peripheral Nerve Block (Paravertebral block/catheters) | 4 (1.1%) | 3 (2.1%) | 1 (1.1%) | 0 (0%) |
| Discharge Disposition | ||||
| Discharged Home | 172 (47.5%) | 15 (10.4%) | 70 (76.1%) | 87 (69.1%) |
| Inpatient Floor | 190 (52.5%) | 129 (89.6%) | 22 (23.9%) | 39 (31%) |
| Outcomes | Traditional Group (n = 144) | Partial ERAS Group (n = 92) | ERAS Group (n = 126) |
|---|---|---|---|
| Primary Outcome | |||
| Hospital Length of Stay (days) | 1.1 (1, 1.2) | 0.3 (0.3, 0.6) | 0.3 (0.2, 1) |
| Secondary Outcomes | |||
| PACU Pain a | n = 141 | ||
| Low (0–3 NPRS score) | 93 (66%) | 65 (70.7%) | 69 (54.8%) |
| Medium (4–6 NPRS score) | 43 (30.5%) | 22 (23.9%) | 49 (38.9%) |
| High (7–10 NPRS score) | 5 (3.6%) | 5 (5.4%) | 8 (6.4%) |
| Inpatient 24 h Pain Score (Numeric Pain Scale) | 3 (2, 4) | 2 (1, 4) | 3 (2, 5) |
| Inpatient PONV | 19 (13.2%) | 1 (1.1%) | 1 (0.8%) |
| Adverse Event | 17 (11.8%) | 11 (12%) | 7 (5.6%) |
| Hematoma | 8 (5.6%) | 5 (5.4%) | 4 (3.2%) |
| Seroma | 4 (2.8%) | 6 (6.5%) | 2 (1.6%) |
| Cellulitis | 8 (5.6%) | 1 (1.1%) | 1 (0.8%) |
| Return to OR | 5 (3.5%) | 2 (2.2%) | 0 (0%) |
| Covariate | Adjusted Coefficient | 95% CI | p Value |
|---|---|---|---|
| Age (years) | −0.01 | (−0.02, 0.01) | 0.519 |
| Weight (kg) | 0.002 | (−0.002, 0.005) | 0.341 |
| Hospital Location Main Hospital (reference = Ambulatory Center) | 0.17 | (0.04, 0.3) | 0.01 * |
| ASA-PS | |||
| I | Reference | ||
| II | 0.01 | (−0.12, 0.15) | 0.83 |
| III | 0.35 | (0.05, 0.65) | 0.023 * |
| Peripheral Nerve Block (Paravertebral block/catheters) | 1.32 | (0.79, 1.84) | <0.001 * |
| Intraoperative Medications | |||
| Acetaminophen Given | −0.1 | (−0.55, 0.35) | 0.657 |
| Dexmedetomidine Given | 0.05 | (−0.08, 0.18) | 0.475 |
| Diazepam Given | 0.01 | (−0.11, 0.14) | 0.825 |
| Fentanyl Given | 0.04 | (−0.08, 0.16) | 0.514 |
| Hydromorphone Given | 0.19 | (−0.24, 0.63) | 0.377 |
| Ketamine Given | 0.54 | (0.29, 0.79) | <0.001 * |
| Morphine Given | 0.32 | (−0.28, 0.91) | 0.295 |
| Remifentanil Given | 0.03 | (−0.96, 1.03) | 0.947 |
| Nausea Drugs | |||
| Dexamethasone Given | 0.09 | (−0.18, 0.36) | 0.532 |
| Haloperidol Given | 0.46 | (0.01, 0.91) | 0.048 * |
| Ondansetron Given | −0.03 | (−0.35, 0.29) | 0.845 |
| Scopolamine Given | −0.01 | (−0.12, 0.11) | 0.942 |
| Intraoperative Infusions and Halogenated Agents | |||
| Remifentanil Infusion Given | −0.12 | (−1.12, 0.88) | 0.813 |
| Propofol Infusion Given | Cannot calculate-all patients received | ||
| Sevoflurane given | −0.04 | (−0.2, 0.12) | 0.633 |
| Isoflurane given | −0.25 | (−0.71, 0.21) | 0.292 |
| Desflurane given | 0.04 | (−0.08, 0.16) | 0.487 |
| Tranexamic Acid Bolus given | −0.01 | (−0.37, 0.34) | 0.945 |
| Tranexamic Acid Infusion given | −0.68 | (−0.84, −0.53) | <0.001 * |
| Variable | Intraoperative TXA Infusion Given (n = 239) | No Intraoperative TXA Infusion Given (n = 123) | Difference (95% CI) | p Value |
|---|---|---|---|---|
| Age (years) | 19 (17, 22) | 18 (17, 20) | 1 (0.2, 1.8) | 0.014 * |
| Weight (kg) | 71 (60.1, 86.8) | 70.6 (59.8, 82.6) | 0.4 (−5.1, 5.9) | 0.886 |
| ASA-PS | ||||
| I | 43 (18%) | 34 (27.6%) | N/A | 0.065 |
| II | 186 (77.8%) | 82 (66.7%) | ||
| III | 10 (4.2%) | 7 (5.7%) | ||
| Discharge Disposition | ||||
| Discharged Home | 162 (67.8%) | 10 (8.1%) | N/A | <0.001 * |
| Inpatient Floor | 77 (32.2%) | 113 (91.9%) | ||
| Yearly Trends of TXA Administration | ||||
| 2017 | 0 (0%) | 4 (3.3%) | N/A | <0.001 * |
| 2018 | 0 (0%) | 37 (30.1%) | ||
| 2019 | 1 (0.4%) | 77 (62.6%) | ||
| 2020 | 97 (40.6%) | 3 (2.4%) | ||
| 2021 | 97 (40.6%) | 2 (2.4%) | ||
| 2022 | 44 (18.4%) | 0 (0%) | ||
| PACU and Inpatient Drain Output within 24 h | ||||
| PACU JP Drainage Output prior to discharge home (mL/kg) a | 0.23 (0, 0.53) n = 154 | 0.68 (0.18, 1.25) n = 5 | −0.45 (−0.85, −0.05) | 0.048 * |
| Inpatient 24-h JP Drainage Output (inpatient overnight) (mL/kg) | 0.82 (0.61, 1.17) n = 85 | 1.5 (1.06, 2.25) n = 118 | −0.68 (−0.92, −0.48) | <0.001 * |
| Adverse Events | ||||
| Hematoma | 11 (4.6%) | 6 (4.9%) | −0.3% (−4.9%, 4.3%) | 0.999 |
| Seroma | 8 (3.4%) | 4 (3.3%) | 0.1% (3.8%, 4%) | 0.999 |
| Cellulitis | 4 (1.7%) | 6 (4.9%) | −3.2% (−7.4%, 0.1%) | 0.095 |
| Return to operating room in 24 h | 2 (0.8%) | 5 (5.1%) | −4.3% (−8.3%, −0.1%) | 0.047 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquino, N.J.; Goobie, S.M.; Staffa, S.J.; Eastburn, E.; Ganor, O.; Jones, C.T. Implementation of an Enhanced Recovery after Surgery Pathway for Transgender and Gender-Diverse Individuals Undergoing Chest Reconstruction Surgery: An Observational Cohort Study. J. Clin. Med. 2023, 12, 7083. https://doi.org/10.3390/jcm12227083
Aquino NJ, Goobie SM, Staffa SJ, Eastburn E, Ganor O, Jones CT. Implementation of an Enhanced Recovery after Surgery Pathway for Transgender and Gender-Diverse Individuals Undergoing Chest Reconstruction Surgery: An Observational Cohort Study. Journal of Clinical Medicine. 2023; 12(22):7083. https://doi.org/10.3390/jcm12227083
Chicago/Turabian StyleAquino, Nelson J., Susan M. Goobie, Steven J. Staffa, Elizabeth Eastburn, Oren Ganor, and Cathie T. Jones. 2023. "Implementation of an Enhanced Recovery after Surgery Pathway for Transgender and Gender-Diverse Individuals Undergoing Chest Reconstruction Surgery: An Observational Cohort Study" Journal of Clinical Medicine 12, no. 22: 7083. https://doi.org/10.3390/jcm12227083
APA StyleAquino, N. J., Goobie, S. M., Staffa, S. J., Eastburn, E., Ganor, O., & Jones, C. T. (2023). Implementation of an Enhanced Recovery after Surgery Pathway for Transgender and Gender-Diverse Individuals Undergoing Chest Reconstruction Surgery: An Observational Cohort Study. Journal of Clinical Medicine, 12(22), 7083. https://doi.org/10.3390/jcm12227083





