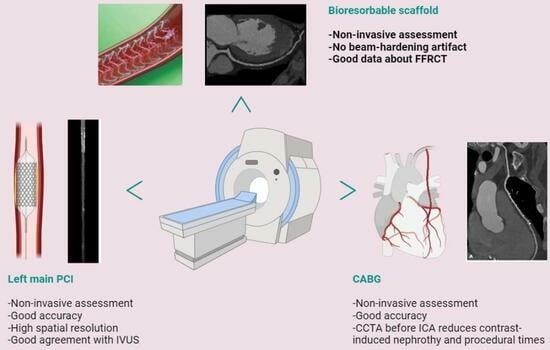
Cardiac Computed Tomography in Monitoring Revascularization
Abstract
1. Introduction
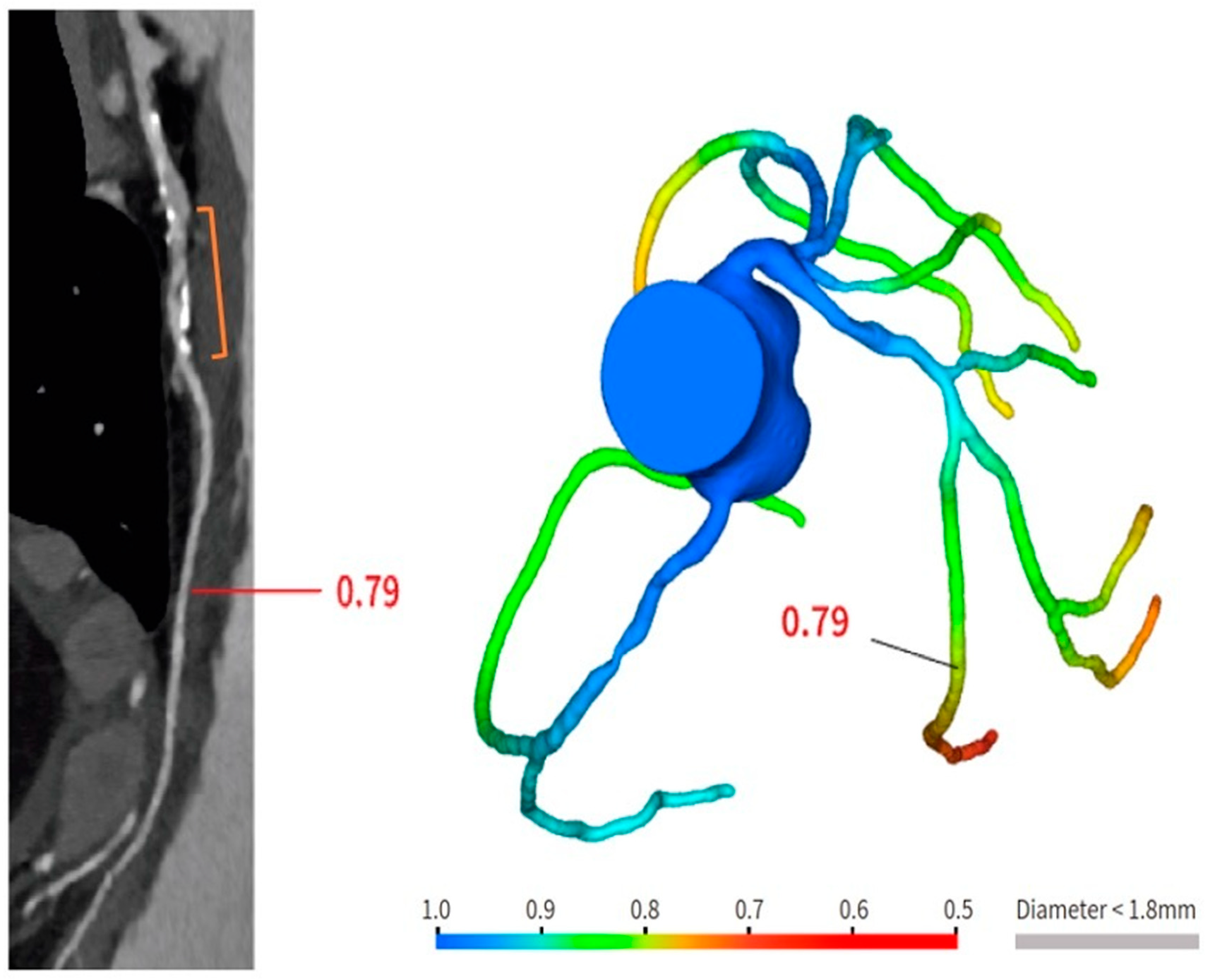
2. New Technologies in CCTA
3. Brief Methodological Considerations
4. CCTA after Complex PCI
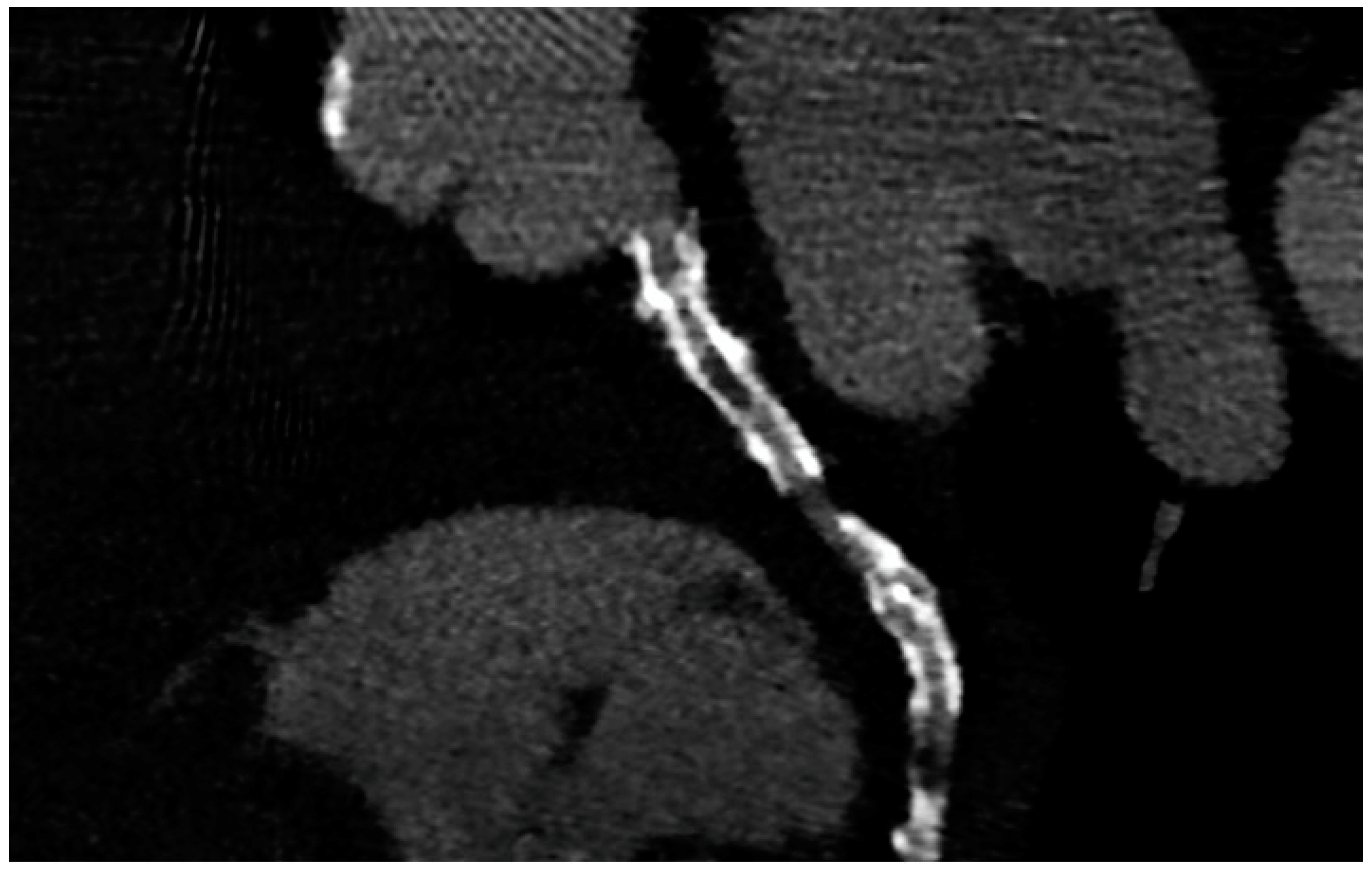
5. CCTA after Scaffold Implantation
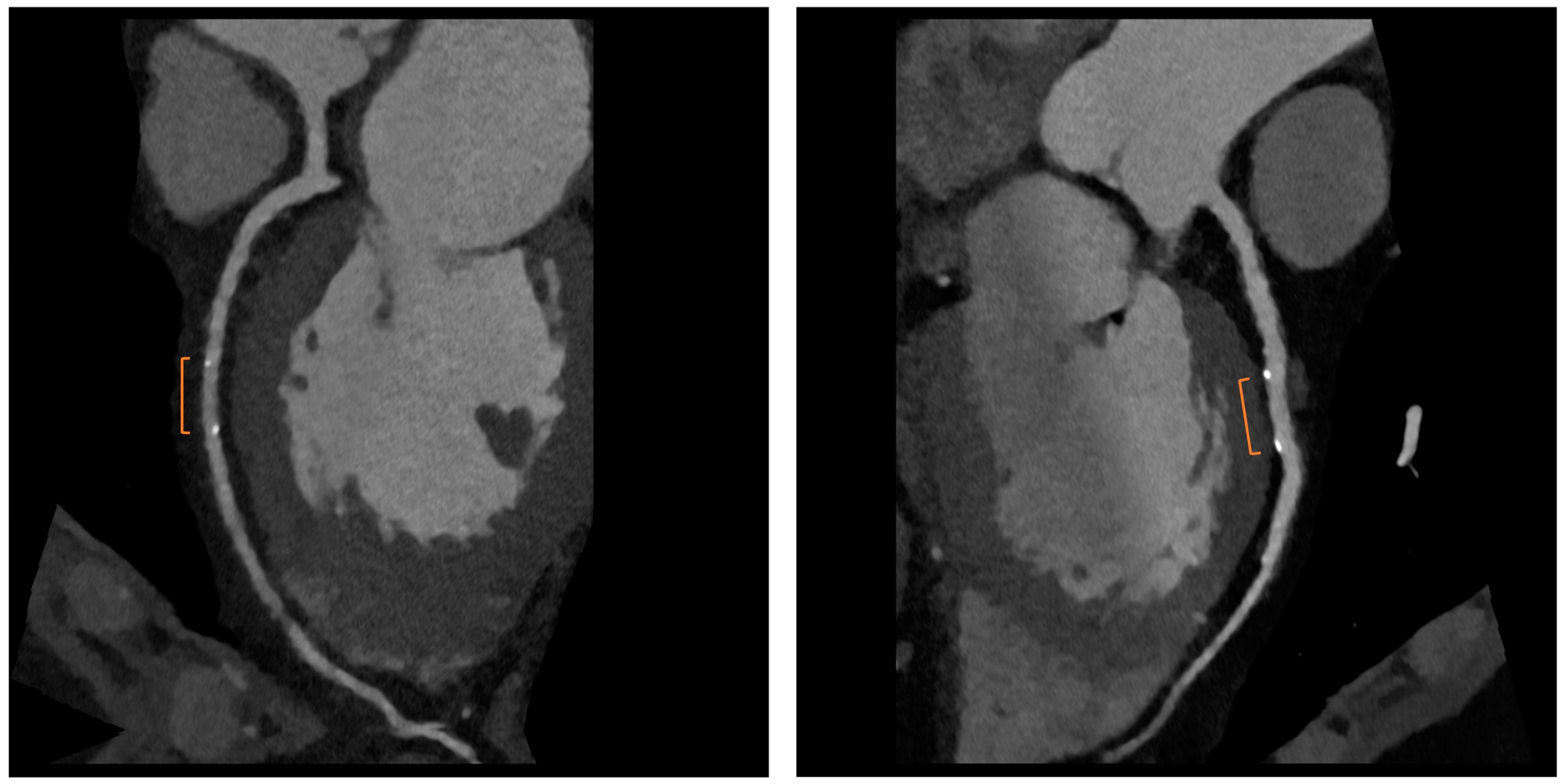
6. CCTA Post-CABG
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Baessato, F.; Guglielmo, M.; Muscogiuri, G.; Baggiano, A.; Fusini, L.; Scafuri, S.; Babbaro, M.; Mollace, R.; Collevecchio, A.; Guaricci, A.I.; et al. The Incremental Role of Coronary Computed Tomography in Chronic Coronary Syndromes. J. Clin. Med. 2020, 9, 3925. [Google Scholar] [CrossRef] [PubMed]
- Roura, G.; Gomez-Lara, J.; Ferreiro, J.L.; Gomez-Hospital, J.A.; Romaguera, R.; Teruel, L.M.; Carreno, E.; Esplugas, E.; Alfonso, F.; Cequier, A. Multislice CT for assessing in-stent dimensions after left main coronary artery stenting: A comparison with three dimensional intravascular ultrasound. Heart 2013, 99, 1106–1112. [Google Scholar] [CrossRef][Green Version]
- Van Mieghem, C.A.G.; Cademartiri, F.; Mollet, N.R.; Malagutti, P.; Valgimigli, M.; Mejboom, W.B.; Pugliese, F.; McFadden, E.P.; Ligthart, J.; Runza, G.; et al. Multislice spiral computed tomography for the evaluation of stent patency after left main coronary artery stenting: A comparison with conventional coronary angiography and intravascular ultrasound. Circulation 2006, 114, 645–653. [Google Scholar] [CrossRef]
- Collet, C.; Chevalier, B.; Cequier, A.; Fajadet, J.; Dominici, M.; Helqvist, S.; Van Boven, A.J.; Dudek, D.; McClean, D.; Almeida, M.; et al. Diagnostic accuracy of coronary CT angiography for the evaluation of bioresorbable vascular scaffolds. J. Am. Coll. Cardiol. Imaging 2018, 11, 722–732. [Google Scholar] [CrossRef]
- Salinas, P.; Pozo-Osinalde, E.; Cerrato, E.; Garcia-Blas, S.; Vaudano, G.P.; Parrilla, C.; Sanchis, J.; Varbella, F.; Escaned, J. Cardiac Computed Tomography Angiography Follow-Up of Resorbable Magnesium Scaffolds. Cardiovasc. Revasc Med. 2021, 29, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Tonet, E.; Cossu, A.; Pompei, G.; Ruggiero, R.; Caglioni, S.; Mele, D.; Boccadoro, A.; Micillo, M.; Cocco, M.; De Raffele, M.; et al. Coronary Computed Tomography Angiography for the Assessment of Sirolimus-Eluting Resorbable Magnesium Scaffold. Life 2022, 12, 1612. [Google Scholar] [CrossRef] [PubMed]
- Malagutti, P.; Nieman, K.; Meijboom, W.B.; van Mieghem, C.A.; Pugliese, F.; Cademartiri, F.; Mollet, N.R.; Boersma, E.; de Jaegere, P.P.; de Feyter, P.J. Use of 64-slice CT in symptomatic patients after coronary bypass surgery: Evaluation of grafts and coronary arteries. Eur. Heart J. 2007, 28, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Barbero, U.; Iannaccone, M.; d’Ascenzo, F.; Barbero, C.; Mohamed, A.; Annone, U.; Benedetto, S.; Celentani, D.; Gagliardi, M.; Moretti, C.; et al. 64 slice-coronary computed tomography sensitivity and specificity in the evaluation of coronary artery bypass graft stenosis: A meta-analysis. Int. J. Cardiol. 2016, 216, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Weustink, A.C.; Nieman, K.; Pugliese, F.; Mollet, N.R.; Meijboom, W.B.; van Mieghem, C.; ten Kate, G.J.; Cademartiri, F.; Krestin, G.P.; de Feyter, P.J. Diagnostic accuracy of computed tomography angiography in patients after bypass grafting: Comparison with invasive coronary angiography. JACC Cardiovasc. Imaging 2009, 2, 816–824, Erratum in JACC Cardiovasc. Imaging 2010, 3, 553. [Google Scholar] [CrossRef]
- Mushtaq, S.; Conte, E.; Pontone, G.; Pompilio, G.; Guglielmo, M.; Annoni, A.; Baggiano, A.; Formenti, A.; Mancini, M.E.; Muscogiuri, G.; et al. Interpretability of coronary CT angiography performed with a novel whole-heart coverage high-definition CT scanner in 300 consecutive patients with coronary artery bypass grafts. J. Cardiovasc. Comput. Tomogr. 2020, 14, 137–143. [Google Scholar] [CrossRef]
- Jones, D.A.; Beirne, A.M.; Kelham, M.; Rathood, K.S.; Andiapen, M.; Wynne, L.; Godec, T.; Forooghi, N.; Ramaseshan, R.; Moon, J.C.; et al. Computed Tomography Cardiac Angiography Before Invasive Coronary Angiography in Patients with Previous Bypass Surgery: The BYPASS-CTCA Trial. Circulation 2023, 148, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.; Head, S.J.; Juni, P.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology/ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2014, 35, 2541–2619. [Google Scholar] [PubMed]
- Valgimigli, M.; Chieffo, A.; Lefevre, T.; Colombo, A.; Morice, M.C.; Serruys, P.W. Revisiting the incidence and temporal distribution of cardiac and sudden death in patients undergoing elective intervention for unprotected left main coronary artery stenosis in the drug eluting stent era. Eurointervention 2007, 2, 435–443. [Google Scholar]
- Bennett, J.; De Hemptinne, Q.; McCutcheon, K. Magmaris Resorbable Magnesium Scaffold for the Treatment of Coronary Heart Disease: Overview of Its Safety and Efficacy. Expert. Rev. Med. Devices 2019, 16, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, C.; Brassart, N.; Delmotte, P.; Brunner, P.; Dghoughi, S.; Carlier, S. Bioresorbable Magnesium-Based Stent: Real-World Clinical Experience and Feasibility of Follow-Up by Coronary Computed Tomography: A New Window to Look at New Scaffolds. Biomedicines 2023, 11, 1150. [Google Scholar] [CrossRef] [PubMed]
- Cigarroa, J.E. Coronary CT Angiography and Bioresorbable Vascular Scaffolds: Is There Clarity? JACC Cardiovasc. Imaging 2018, 11, 733–735. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, X.; Sun, S.; Wu, D.; Bai, J.; Yin, Y.; Liu, X.; Zhang, H.; de Albuquerque, V.H.C. Learning physical properties in complex visual scenes: An intelligent machine for perceiving blood flow dynamics from static CT angiography imaging. Neural Netw. 2020, 123, 82–93. [Google Scholar] [CrossRef]
- Tan, E.S.; van der Meer, J.; Jan de Kam, P.; Dunselman, P.H.; Mulder, B.J.; Ascoop, C.A.; Pfisterer, M.; Lie, K.I. Worse clinical outcome but similar graft patency in women versus men one year after coronary artery bypass graft surgery owing to an excess of exposed risk factors in women. CABADAS. Research Group of the Interuniversity Cardiology Institute of The Netherlands. Coronary Artery Bypass graft occlusion by Aspirin, Dipyridamole and Acenocoumarol/phenoprocoumon Study. J. Am. Coll. Cardiol. 1999, 34, 1760–1768. [Google Scholar]
- Fitzgibbon, G.M.; Kafka, H.P.; Leach, A.J.; Keon, W.J.; Hooper, G.D.; Burton, J.R. Coronary bypass graft fate and patient outcome: Angiographic follow-up of 5, 065 grafts related to survival and reoperation in 1388 patients during 25 years. J. Am. Coll. Cardiol. 1996, 28, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Alderman, E.L.; Kip, K.E.; Whitlow, P.L.; Bashore, T.; Fortin, D.; Bourassa, M.G.; Lesperance, J.; Schwartz, L.; Stadius, M. Native coronary disease progression exceeds failed revascularization as cause of angina after five years in the Bypass Angioplasty Revascularization Investigation (BARI). J. Am. Coll. Cardiol. 2004, 44, 766–774. [Google Scholar] [CrossRef]
- Masroor, M.; Ahmad, A.; Wang, Y.; Dong, N. Assessment of the Graft Quality and Patency during and after Coronary Artery Bypass Grafting. Diagnostics 2023, 13, 1891. [Google Scholar] [CrossRef]
- Taylor, A.J.; Cerqueira, M.; Hodgson, J.M.; Mark, D.; Min, J.; O’Gara, P.; Rubin, G.D.; American College of Cardiology Foundation Appropriate Use Criteria Task Force; Society of Cardiovascular Computed Tomography; American College of Radiology; et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of cardiology foundation appropriate use criteria task force, the society of cardiovascular computed tomography, the American College of Radiology, the American heart association, the American society of echocardiography, the American society of nuclear cardiology, the North American society for cardiovascular imaging, the society for cardiovascular angiography and interventions, and the society for cardiovascular magnetic resonance. J. Cardiovasc. Comput. Tomogr. 2010, 4, 1864–1894. [Google Scholar]
- De Graaf, F.R.; van Velzen, J.E.; Witkowska, A.J.; Schuijf, J.D.; van der Bijl, N.; Kroft, L.J.; de Roos, A.; Reiber, J.H.C.; Bax, J.J.; de Grooth, G.J.; et al. Diagnostic performance of 320-slice multidetector computed tomography coronary angiography in patients after coronary artery bypass grafting. Eur. Radiol. 2011, 21, 2285–2296. [Google Scholar] [CrossRef] [PubMed]
- Tsigkas, G.; Apostolos, A.; Synetos, A.; Latsios, G.; Toutouzas, K.; Xenogiannis, I.; Hamilos, M.; Sianos, G.; Ziakas, A.; Tsiafoutis, I.; et al. Computer tomoGRaphy guidEd invasivE Coronary angiography in patiEnts with a previous coronary artery bypass graft surgery trial (GREECE trial): Rationale and design of a multicenter, randomized control trial. Hell. J. Cardiol. 2021, 62, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Andreini, D.; Pontone, G.; Bertella, E.; Bartorelli, A.L.; Conte, E.; Baggiano, A.; Annoni, A.; Formenti, A.; Trabattoni, D.; et al. Prognostic value of coronary CTA in coronary bypass patients: A long-term followup study. JACC Cardiovasc. Imag. 2014, 7, 580–589. [Google Scholar] [CrossRef]
- Choi, A.D.; Brar, V.; Kancherla, K.; Fatemi, O.; Pinto, G.; Boyce, S.; Bafi, A.; Corso, P.J.; Tefera, E.; Taylor, A.J.; et al. Prospective evaluation of cardiac CT in reoperative cardiac surgery. JACC Cardiovasc. Imaging 2016, 9, 1356–1357. [Google Scholar] [CrossRef]
- Narula, J.; Chandrashekhar, Y.; Ahmadi, A.; Abbara, S.; Berman, D.S.; Blankstein, R.; Leipsic, J.; Newby, D.; Nicol, E.D.; Nieman, K.; et al. SCCT 2021 Expert consensus document on coronary computed tomographic angiography: A report of the society of cardiovascular computed tomography. J. Cardiovasc. Comput. Tomogr. 2021, 15, 192–217. [Google Scholar] [CrossRef]
- Usmanij, E.A.; Senden, P.J.; Meiss, L.; de Klerk, J.M.H. Myocardial ischaemia due to subclavian stenosis after coronary artery bypass graft: A case report. Eur. Heart J. Case Rep. 2018, 2, yty069. [Google Scholar] [CrossRef]
- Opolski, M.P.; Achenbach, S.; Schuhback, A.; Rolf, A.; Mollmann, H.; Nef, H.; Rixe, J.; Renker, M.; Witkowskii, A.; Kepka, C.; et al. Coronary computed tomographic prediction rule for time-efficient guidewire crossing through chronic total occlusion: Insights from the CTRECTOR multicenter registry (Computed Tomography Registry of Chronic Total Occlusion Revascularization). JACC Cardiovasc. Interv. 2015, 8, 257–267. [Google Scholar] [CrossRef]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, e368–e454. [Google Scholar]

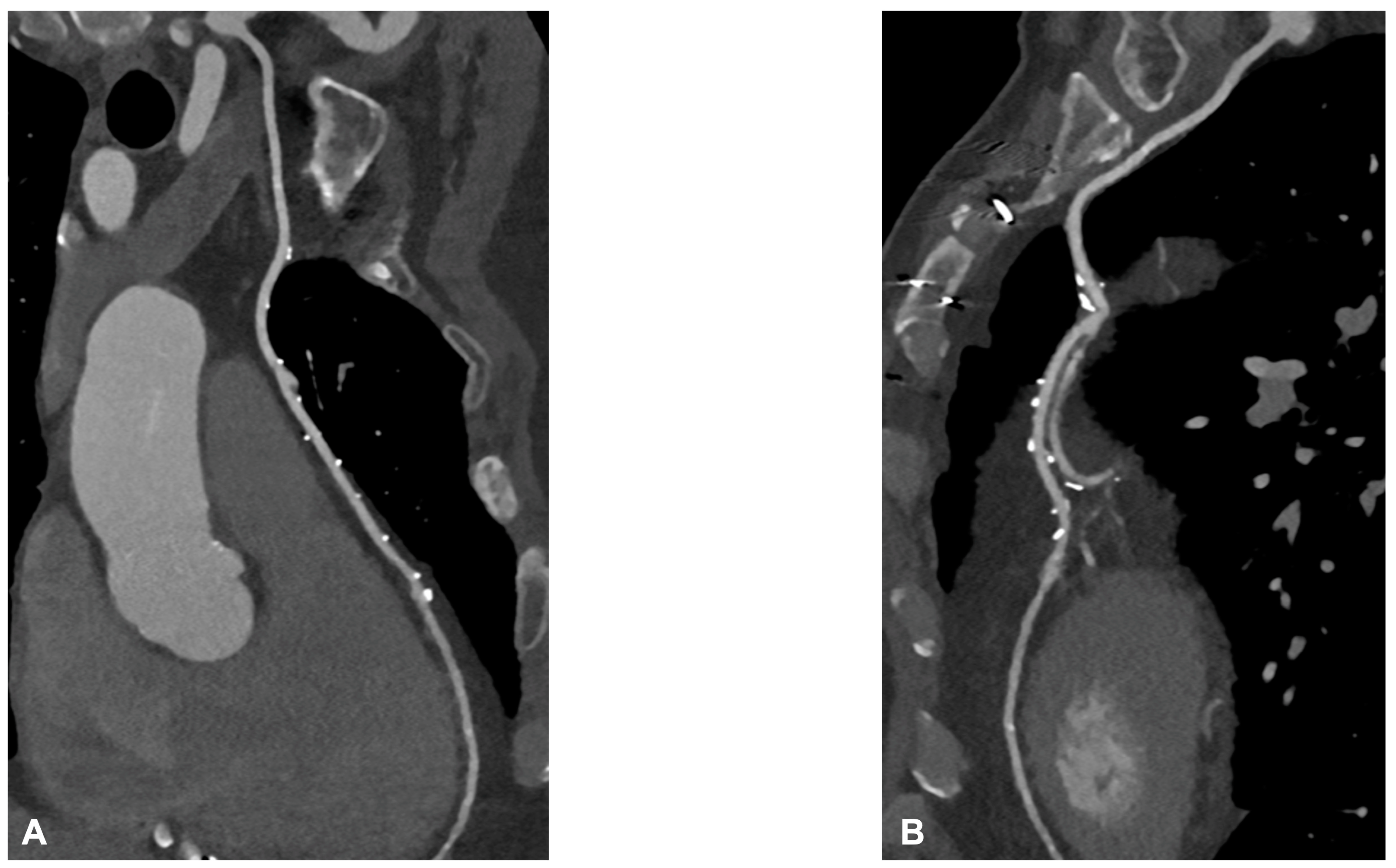
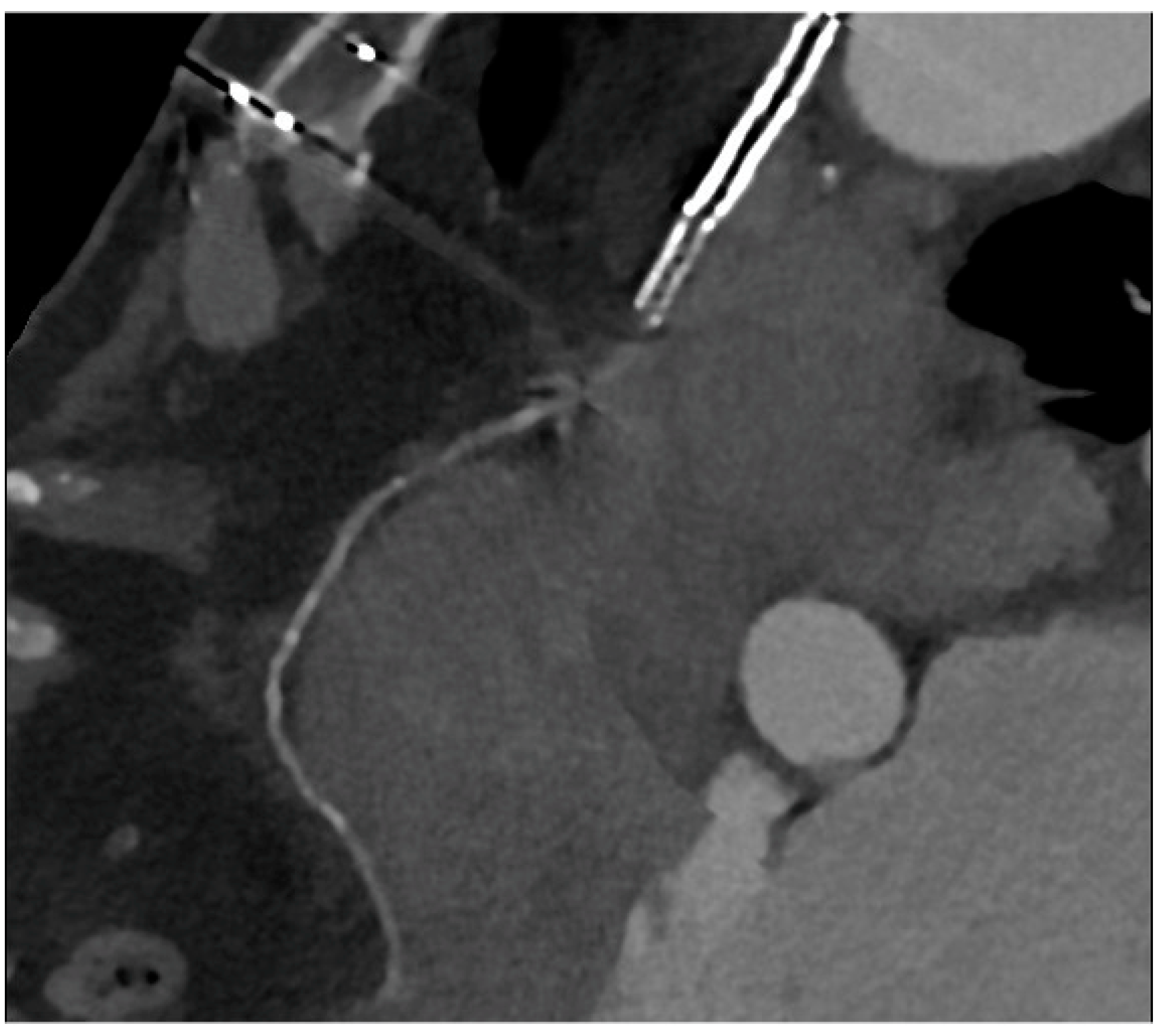
| CCTA after Complex PCI | |
| Roura G et al., 2013 [4] | Good agreement between CCTA and IVUS in the assessment of in-stent lumen diameters and lumen area of LM stents. |
| Van Mieghem CAG et al., 2006 [5] | CCTA showed a good performance in pointing out LM ISR with an accuracy of 98% (ICA as reference). Good correlation with IVUS in the evaluation of stent diameter and area. |
| CCTA after scaffold implantation | |
| Collet C et al., 2018 [6] | At 3-year follow-up after BRS implantation, CCTA diagnostic accuracy for detecting in-scaffold obstruction and luminal dimensions was similar compared with invasive coronary angiography (ICA) and IVUS. Analyzing scaffold segments, the sensitivity, specificity, and negative predictive values were 71%, 82%, and 97%, respectively, using IVUS as a reference. |
| Salinas P et al., 2020 [7] | CCTA is feasible in scaffolded coronary segments. |
| Tonet E et al., 2022 [8] | CCTA and FFR-CT in 26 patients treated with Magnesium bioresorbable scaffold. FFR-CT demonstrated to be feasible in scaffolded segments. |
| CCTA post-CABG | |
| Malagutti P et al., 2007 [9] | CCTA exhibited a sensitivity of 100% and a specificity of 98.3% in detecting graft patency when compared to ICA. Additionally, their findings indicated that overestimation of obstruction was more likely in native coronary arteries, particularly in the presence of calcification. |
| Barbero U et al., 2016 [10] | The sensitivity and specificity of CCTA for identifying any coronary artery bypass graft with stenosis greater than 50% were found to be 0.98 (95% CI: 0.97–0.99) and 0.98 (95% CI: 0.96–0.98), respectively. |
| Weustink AC et al., 2010 [11] | CCTA showed a diagnostic accuracy of 100% in identifying or ruling out significant stenosis in grafts. The specificity, sensitivity, PPV, and NPV all yielded 100% accuracy in the detection of significant stenosis. |
| Mushtaq S et al., 2020 [12] | CCTA successfully interpreted 100% of the bypass grafts. When compared to ICA, CCTA exhibited the ability to identify occlusion and significant stenosis in all CABG segments, with a sensitivity, specificity, PPV, and NPV of 100% each for the grafts. |
| Jones DA et al., 2023 [13] | In a randomized cohort, CCTA before ICA leads to reductions in procedure time and contrast-induced nephropathy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonet, E.; Amantea, V.; Lapolla, D.; Assabbi, P.; Boccadoro, A.; Berloni, M.L.; Micillo, M.; Marchini, F.; Chiarello, S.; Cossu, A.; et al. Cardiac Computed Tomography in Monitoring Revascularization. J. Clin. Med. 2023, 12, 7104. https://doi.org/10.3390/jcm12227104
Tonet E, Amantea V, Lapolla D, Assabbi P, Boccadoro A, Berloni ML, Micillo M, Marchini F, Chiarello S, Cossu A, et al. Cardiac Computed Tomography in Monitoring Revascularization. Journal of Clinical Medicine. 2023; 12(22):7104. https://doi.org/10.3390/jcm12227104
Chicago/Turabian StyleTonet, Elisabetta, Veronica Amantea, Davide Lapolla, Paolo Assabbi, Alberto Boccadoro, Maria Letizia Berloni, Marco Micillo, Federico Marchini, Serena Chiarello, Alberto Cossu, and et al. 2023. "Cardiac Computed Tomography in Monitoring Revascularization" Journal of Clinical Medicine 12, no. 22: 7104. https://doi.org/10.3390/jcm12227104
APA StyleTonet, E., Amantea, V., Lapolla, D., Assabbi, P., Boccadoro, A., Berloni, M. L., Micillo, M., Marchini, F., Chiarello, S., Cossu, A., & Campo, G. (2023). Cardiac Computed Tomography in Monitoring Revascularization. Journal of Clinical Medicine, 12(22), 7104. https://doi.org/10.3390/jcm12227104