Saphenous Vein Graft Failure: Current Challenges and a Review of the Contemporary Percutaneous Options for Management
Abstract
:1. Introduction
2. Choice of Surgical Conduit
Arterial versus Venous Conduit in Coronary Artery Bypass Grafting
3. Saphenous Vein Graft Failure
3.1. The Natural History of Saphenous Venous Grafts Following Anastomosis
3.2. The Natural History of Native Artery Coronary Atherosclerosis Following Grafting
4. Diagnosis of Graft Failure
5. Management of Graft Failure
5.1. Medical Therapies to Improve Saphenous Vein Graft Patency
5.2. Redo CABG in the Context of Failed Saphenous Vein Grafts
5.3. Percutaneous Coronary Intervention in the Context of Failed Saphenous Vein Grafts
5.4. Percutaneous Coronary Intervention to a Failing Saphenous Vein Graft
5.5. Percutaneous Coronary Intervention to the Previously Bypassed Native Coronary Artery
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- British Heart Foundation. Heart & Circulatory Disease Statistics, 2023 Compendium: British Heart Foundation. 2023. Available online: https://www.bhf.org.uk/what-we-do/our-research/heart-statistics/heart-statistics-publications/cardiovascular-disease-statistics-2023 (accessed on 25 September 2023).
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018, 40, 87–165. [Google Scholar] [CrossRef]
- Taggart, D.P.; D’Amico, R.; Altman, D.G. Effect of arterial revascularisation on survival: A systematic review of studies comparing bilateral and single internal mammary arteries. Lancet 2001, 358, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Taggart, D.P.; Benedetto, U.; Gerry, S.; Altman, D.G.; Gray, A.M.; Lees, B.; Gaudino, M.; Zamvar, V.; Bochenek, A.; Buxton, B.; et al. Bilateral versus Single Internal-Thoracic-Artery Grafts at 10 Years. N. Engl. J. Med. 2019, 380, 437–446. [Google Scholar] [CrossRef]
- Barner, H.B.; Barnett, M.G. Fifteen- to twenty-one-year angiographic assessment of internal thoracic artery as a bypass conduit. Ann. Thorac. Surg. 1994, 57, 1526–1528. [Google Scholar] [CrossRef] [PubMed]
- Tatoulis, J.; Buxton, B.F.; Fuller, J.A. Patencies of 2127 arterial to coronary conduits over 15 years. Ann. Thorac. Surg. 2004, 77, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, T.; Saso, S.; Rao, C.; Vecht, J.; Grapsa, J.; Dunning, J.; Lemma, M.; Casula, R. Radial artery versus saphenous vein conduits for coronary artery bypass surgery: Forty years of competition—Which conduit offers better patency? A systematic review and meta-analysis. Eur. J. Cardio-Thorac. Surg. 2011, 40, 208–220. [Google Scholar] [CrossRef]
- Tranbaugh, R.F.; Schwann, T.A.; Swistel, D.G.; Dimitrova, K.R.; Al-Shaar, L.; Hoffman, D.M.; Geller, C.M.; Engoren, M.; Balaram, S.K.; Puskas, J.D.; et al. Coronary Artery Bypass Graft Surgery Using the Radial Artery, Right Internal Thoracic Artery, or Saphenous Vein as the Second Conduit. Ann. Thorac. Surg. 2017, 104, 553–559. [Google Scholar] [CrossRef]
- Taggart, D.P. Current status of arterial grafts for coronary artery bypass grafting. Ann. Cardiothorac. Surg. 2013, 2, 427–430. [Google Scholar] [CrossRef]
- Goldman, S.; Zadina, K.; Moritz, T.; Ovitt, T.; Sethi, G.; Copeland, J.G.; Thottapurathu, L.; Krasnicka, B.; Ellis, N.; Anderson, R.J.; et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: Results from a Department of Veterans Affairs Cooperative Study. J. Am. Coll. Cardiol. 2004, 44, 2149–2156. [Google Scholar] [CrossRef]
- Fitzgibbon, G.M.; Kafka, H.P.; Leach, A.J.; Keon, W.J.; Hooper, G.D.; Burton, J.R. Coronary bypass graft fate and patient outcome: An-giographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J. Am. Coll. Cardiol. 1996, 28, 616–626. [Google Scholar] [CrossRef]
- Tabata, M.; Grab, J.D.; Khalpey, Z.; Edwards, F.H.; O’Brien, S.M.; Cohn, L.H.; Bolman, R.M., III. Prevalence and variability of internal mammary artery graft use in contemporary multivessel coronary artery bypass graft surgery: Analysis of the Society of Thoracic Surgeons National Cardiac Database. Circulation 2009, 120, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Khot, U.N.; Friedman, D.T.; Pettersson, G.; Smedira, N.G.; Li, J.; Ellis, S.G. Radial artery bypass grafts have an increased oc-currence of angiographically severe stenosis and occlusion compared with left internal mammary arteries and saphenous vein grafts. Circulation 2004, 109, 2086–2091. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, A.; Habib, R.H.; Schwann, T.A.; Riordan, C.J.; Durham, S.J.; Shah, A. Improved Survival With Radial Artery Versus Vein Conduits in Coronary Bypass Surgery with Left Internal Thoracic Artery to Left Anterior Descending Artery Grafting. Circulation 2004, 109, 1489–1496. [Google Scholar] [CrossRef]
- Deb, S.; Cohen, E.A.; Singh, S.K.; Une, D.; Laupacis, A.; Fremes, S.E. Radial Artery and Saphenous Vein Patency More Than 5 Years after Coronary Artery Bypass Surgery: Results from RAPS (Radial Artery Patency Study). J. Am. Coll. Cardiol. 2012, 60, 28–35. [Google Scholar] [CrossRef]
- Possati, G.; Gaudino, M.; Prati, F.; Alessandrini, F.; Trani, C.; Glieca, F.; Mazzari, M.A.; Luciani, N.; Schiavoni, G. Long-Term Results of the Radial Artery Used for Myocardial Revascularization. Circulation 2003, 108, 1350–1354. [Google Scholar] [CrossRef]
- Achouh, P.; Isselmou, K.O.; Boutekadjirt, R.; D’Alessandro, C.; Pagny, J.-Y.; Fouquet, R.; Fabiani, J.-N.; Acar, C. Reappraisal of a 20-year experience with the radial artery as a conduit for coronary bypass grafting. Eur. J. Cardio-Thorac. Surg. 2012, 41, 87–92. [Google Scholar] [CrossRef]
- Loop, F.D.; Lytle, B.W.; Cosgrove, D.M.; Stewart, R.W.; Goormastic, M.; Williams, G.W.; Golding, L.A.; Gill, C.C.; Taylor, P.C.; Sheldon, W.C.; et al. Influence of the inter-nal-mammary-artery graft on 10-year survival and other cardiac events. N. Engl. J. Med. 1986, 314, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Taggart, D.; Suma, H.; Puskas, J.D.; Crea, F.; Massetti, M. The Choice of Conduits in Coronary Artery Bypass Surgery. J. Am. Coll. Cardiol. 2015, 66, 1729–1737. [Google Scholar] [CrossRef]
- Gaudino, M.; Benedetto, U.; Fremes, S.; Biondi-Zoccai, G.; Sedrakyan, A.; Puskas, J.D.; Angelini, G.D.; Buxton, B.; Frati, G.; Hare, D.L.; et al. Radial-Artery or Saphenous-Vein Grafts in Coronary-Artery Bypass Surgery. N. Engl. J. Med. 2018, 378, 2069–2077. [Google Scholar] [CrossRef]
- Bridgewater, B.; Keogh, B.; Kinsman, R.; Walton, P. Sixth National Adult Cardiac Surgical Database Report 2008; The Society of Cardiothoracic Surgeons of Great Britain and Ireland: London, UK, 2009. [Google Scholar]
- Campeau, L.; Enjalbert, M.; Lesperance, J.; Vaislic, C.; Grondin, C.M.; Bourassa, M.G. Atherosclerosis and late closure of aor-tocoronary saphenous vein grafts: Sequential angiographic studies at 2 weeks, 1 year, 5 to 7 years, and 10 to 12 years after surgery. Circulation 1983, 68 Pt 2, II1-7. [Google Scholar]
- Levisman, J.M.; Budoff, M.J.; Karlsberg, R.P. Long-term coronary artery graft patency as evaluated by 64-slice coronary computed tomographic angiography. Coron. Artery Dis. 2011, 22, 521–525. [Google Scholar] [CrossRef]
- Shah, P.J.; Gordon, I.; Fuller, J.; Seevanayagam, S.; Rosalion, A.; Tatoulis, J.; Raman, J.S.; Buxton, B.F. Factors affecting saphenous vein graft patency: Clinical and angiographic study in 1402 symptomatic patients operated on between 1977 and 1999. J. Thorac. Cardiovasc. Surg. 2003, 126, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.K.; Otsuka, F.; Nakano, M.; Ladich, E.; Virmani, R. Pathology of Saphenous Vein Grafts. Interv. Cardiol. Clin. 2013, 2, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Beijk, M.A.; Harskamp, R.E. Treatment of Coronary Artery Bypass Graft Failure. In Artery Bypass; Wilbert, S.A., Ed.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Campeau, L.; Enjalbert, M.; Lespérance, J.; Bourassa, M.G.; Kwiterovich, P.; Wacholder, S.; Sniderman, A. The Relation of Risk Factors to the Development of Atherosclerosis in Saphenous-Vein Bypass Grafts and the Progression of Disease in the Native Circulation. A Study 10 Years after Aortocoronary Bypass Surgery. N. Engl. J. Med. 1984, 311, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Cashin, W.L.; Sanmarco, M.E.; Nessim, S.A.; Blankenhorn, D.H. Accelerated Progression of Atherosclerosis in Coronary Vessels with Minimal Lesions That Are Bypassed. N. Engl. J. Med. 1984, 311, 824–828. [Google Scholar] [CrossRef]
- Kroncke, G.M.; Kosolcharoen, P.; Clayman, J.A.; Peduzzi, P.N.; Detre, K.; Takaro, T. Five-year changes in coronary arteries of medical and surgical patients of the Veterans Administration Randomized Study of Bypass Surgery. Circulation 1988, 78 Pt 2, I144–I150. [Google Scholar] [PubMed]
- Hamada, Y.; Kawachi, K.; Yamamoto, T.; Nakata, T.; Kashu, Y.; Watanabe, Y.; Sato, M. Effect of coronary artery bypass grafting on native coronary artery stenosis. Comparison of internal thoracic artery and saphenous vein grafts. J. Cardiovasc. Surg. 2001, 42, 159–164. [Google Scholar]
- Michael, T.T.; Karmpaliotis, D.; Brilakis, E.S.; Abdullah, S.M.; Kirkland, B.L.; Mishoe, K.L.; Lembo, N.; Kalynych, A.; Carlson, H.; Banerjee, S.; et al. Impact of prior coronary artery bypass graft surgery on chronic total occlusion revascularisation: Insights from a multicentre US registry. Heart 2013, 99, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Alexandrou, M.; Kostantinis, S.; Rempakos, A.; Simsek, B.; Karacsonyi, J.; Choi, J.W.; Poommipanit, P.; Alaswad, K.; Basir, M.B.; Megaly, M.; et al. Outcomes of Chronic Total Occlusion Percutaneous Coronary Interventions in Patients with Previous Coronary Artery Bypass Graft Surgery. Am. J. Cardiol. 2023, 205, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Budassi, S.; Zivelonghi, C.; Dens, J.; Bagnall, A.J.; Knaapen, P.; Avran, A.; Spratt, J.C.; Walsh, S.; Faurie, B.; Agostoni, P.; et al. Impact of prior coronary artery bypass grafting in patients undergoing chronic total occlusion-percutaneous coronary intervention: Procedural and clinical outcomes from the REgistry of Crossboss and Hybrid procedures in FrAnce, the NetheRlands, BelGium, and UnitEd Kingdom (RECHARGE). Catheter. Cardiovasc. Interv. 2021, 97, E51–E60. [Google Scholar]
- Weintraub, W.S.; Clements, S.D., Jr.; Crisco, L.V.T.; Guyton, R.A.; Craver, J.M.; Jones, E.L.; Hatcher, C.R., Jr. Twenty-year survival after coronary artery surgery: An institutional perspective from Emory University. Circulation 2003, 107, 1271–1277. [Google Scholar] [CrossRef]
- Adelborg, K.; Horváth-Puhó, E.; Schmidt, M.; Munch, T.; Pedersen, L.; Nielsen, P.H.; Bøtker, H.E.; Toft Sørensen, H. Thirty-Year Mortality after Coronary Artery Bypass Graft Surgery: A Danish Nationwide Population-Based Cohort Study. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e002708. [Google Scholar] [CrossRef]
- Fosbøl, E.L.; Zhao, Y.; Shahian, D.M.; Grover, F.L.; Edwards, F.H.; Peterson, E.D. Repeat coronary revascularization after coronary artery bypass surgery in older adults: The Society of Thoracic Surgeons’ national experience, 1991–2007. Circulation 2013, 127, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Xenogiannis, I.; Tajti, P.; Hall, A.B.; Alaswad, K.; Rinfret, S.; Nicholson, W.; Karmpaliotis, D.; Mashayekhi, K.; Furkalo, S.; Cavalcante, J.L.; et al. Update on Cardiac Catheterization in Patients with Prior Coronary Artery Bypass Graft Surgery. JACC Cardiovasc. Interv. 2019, 12, 1635–1649. [Google Scholar] [CrossRef] [PubMed]
- Gobel, F.L.; Stewart, W.J.; Campeau, L.; Hickey, A.; Herd, J.A.; Forman, S.; White, C.W.; Rosenberg, Y. Safety of coronary arteriography in clinically stable patients following coronary bypass surgery. Post CABG Clinical Trial Investigators. Catheter. Cardiovasc. Diagn. 1998, 45, 376–381. [Google Scholar] [CrossRef]
- Delewi, R.; Hoebers, L.P.; Råmunddal, T.; Henriques, J.P.; Angerås, O.; Stewart, J.; Robertsson, L.; Wahlin, M.; Petursson, P.; Piek, J.J.; et al. Clinical and procedural characteristics asso-ciated with higher radiation exposure during percutaneous coronary interventions and coronary angiography. Circ. Cardiovasc. Interv. 2013, 6, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, T.; Lagerqvist, B.; Tornvall, P. Coronary angiography of patients with a previous coronary artery by-pass operation is associated with a three times increased risk for neurological complications. A report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Scand. Cardiovasc. J. 2009, 43, 374–379. [Google Scholar] [CrossRef]
- Barbero, U.; Iannaccone, M.; D’Ascenzo, F.; Barbero, C.; Mohamed, A.; Annone, U.; Benedetto, S.; Celentani, D.; Gagliardi, M.; Moretti, C.; et al. 64 slice-coronary computed tomography sensitivity and specificity in the evaluation of coronary artery bypass graft stenosis: A meta-analysis. Int. J. Cardiol. 2016, 216, 52–57. [Google Scholar] [CrossRef]
- Mushtaq, S.; Conte, E.; Pontone, G.; Pompilio, G.; Guglielmo, M.; Annoni, A.; Baggiano, A.; Formenti, A.; Mancini, M.E.; Muscogiuri, G.; et al. Interpretability of coronary CT angiography per-formed with a novel whole-heart coverage high-definition CT scanner in 300 consecutive patients with coronary artery bypass grafts. J. Cardiovasc. Comput. Tomogr. 2020, 14, 137–143. [Google Scholar] [CrossRef]
- Jones, D.A.; Beirne, A.-M.; Kelham, M.; Rathod, K.S.; Andiapen, M.; Wynne, L.; Godec, T.; Forooghi, N.; Ramaseshan, R.; Moon, J.C.; et al. Computed Tomography Cardiac Angiography Before Invasive Coronary Angiography in Patients with Previous Bypass Surgery: The BYPASS-CTCA Trial. Circulation 2023, 148, 1371–1380. [Google Scholar] [CrossRef]
- Zimmermann, N.; Gams, E.; Hohlfeld, T. Aspirin in coronary artery bypass surgery: New aspects of and alternatives for an old antithrombotic agent. Eur. J. Cardio-Thorac. Surg. 2008, 34, 93–108. [Google Scholar] [CrossRef]
- Kulik, A.; Le May, M.R.; Voisine, P.; Tardif, J.-C.; DeLarochelliere, R.; Naidoo, S.; Wells, G.A.; Mesana, T.G.; Ruel, M. Aspirin plus clopidogrel versus aspirin alone after coronary artery bypass grafting: The clopidogrel after surgery for coronary artery disease (CASCADE) Trial. Circulation 2010, 122, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, Y.; Xu, Z.; Cheng, Z.; Mei, J.; Chen, X.; Wang, X. Effect of Ticagrelor Plus Aspirin, Ticagrelor Alone, or Aspirin Alone on Saphenous Vein Graft Patency 1 Year after Coronary Artery Bypass Grafting: A Randomized Clinical Trial. JAMA 2018, 319, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, L.M.; Janssen, P.W.; Peper, J.; Soliman-Hamad, M.A.; van Straten, A.H.; Klein, P.; Hackeng, C.M.; Sonker, U.; Bekker, M.W.; von Birgelen, C.; et al. Effect of Adding Ticagrelor to Standard Aspirin on Saphenous Vein Graft Patency in Patients Undergoing Coronary Artery Bypass Grafting (POPular CABG): A Randomized, Double-Blind, Placebo-Controlled Trial. Circulation 2020, 142, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, J.; Hillege, H.L.; van Gilst, W.H.; Lie, K.I.; Kootstra, G.J.; Ascoop, C.A.P.L.; Pfisterer, M. Prevention of one-year vein-graft occlusion after aortocoronary-bypass surgery: A comparison of low-dose aspirin, low-dose aspirin plus dipyridamole, and oral antico-agulants. The CABADAS Research Group of the Interuniversity Cardiology Institute of The Netherlands. Lancet 1993, 342, 257–264. [Google Scholar]
- Pfisterer, M.; Jockers, G.; Regenass, S.; Schmitt, H.; Skarvan, K.; Hasse, J.; Burkart, F.; Meyer, B.; Burckhardt, D.; Müller-Brand, J.; et al. Trial of low-dose aspirin plus dipyridamole versus anticoagulants for prevention of aortocoronary vein graft occlusion. Lancet 1989, 334, 1–7. [Google Scholar] [CrossRef]
- Lamy, A.; Eikelboom, J.; Sheth, T.; Connolly, S.; Bosch, J.; Fox, K.A.; Zhu, J.; Lonn, E.; Dagenais, G.; Widimsky, P.; et al. Rivaroxaban, Aspirin, or Both to Prevent Early Coronary Bypass Graft Occlusion: The COMPASS-CABG Study. J. Am. Coll. Cardiol. 2019, 73, 121–130. [Google Scholar] [CrossRef]
- Campeau, L.; Knatterud, G.L.; Domanski, M.; Hunninghake, D.B.; White, C.W.; Geller, N.L.; Rosenberg, Y. The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N. Engl. J. Med. 1997, 336, 153–162. [Google Scholar]
- Liakopoulos, O.J.; Choi, Y.H.; Haldenwang, P.L.; Strauch, J.; Wittwer, T.; Dörge, H.; Stamm, C.; Wassmer, G.; Wahlers, T. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: A meta-analysis of over 30,000 patients. Eur. Heart J. 2008, 29, 1548–1559. [Google Scholar] [CrossRef]
- Eisen, A.; Cannon, C.P.; Blazing, M.A.; Bohula, E.A.; Park, J.-G.; Murphy, S.A.; White, J.A.; Giugliano, R.P.; Braunwald, E. The benefit of adding ezetimibe to statin therapy in patients with prior coronary artery bypass graft surgery and acute coronary syndrome in the IMPROVE-IT trial. Eur. Heart J. 2016, 37, 3576–3584. [Google Scholar] [CrossRef]
- Kulik, A.; Voisine, P.; Mathieu, P.; Masters, R.G.; Mesana, T.G.; Le May, M.R.; Ruel, M. Statin Therapy and Saphenous Vein Graft Disease after Coronary Bypass Surgery: Analysis from the CASCADE Randomized Trial. Ann. Thorac. Surg. 2011, 92, 1284–1291, discussion 90-1. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, H.-B.; Xiao, J.-Y.; Ren, M.; Reilly, K.H.; Li, Y.-M.; Liu, Y. Association between proprotein convertase subtilisin/kexin type 9 and late saphenous vein graft disease after coronary artery bypass grafting: A cross-sectional study. BMJ Open 2018, 8, e021951. [Google Scholar] [CrossRef] [PubMed]
- Ghanta, R.K.; Kaneko, T.; Gammie, J.S.; Sheng, S.; Aranki, S.F. Evolving trends of reoperative coronary artery bypass grafting: An analysis of the Society of Thoracic Surgeons Adult Cardiac Surgery Database. J. Thorac. Cardiovasc. Surg. 2013, 145, 364–372. [Google Scholar] [CrossRef]
- Sabik, J.F., 3rd; Blackstone, E.H.; Gillinov, A.M.; Smedira, N.G.; Lytle, B.W. Occurrence and risk factors for reintervention after coronary artery bypass grafting. Circulation 2006, 114 (Suppl. S1), I454–I460. [Google Scholar] [CrossRef]
- Sajja, L.R. A narrative review of redo coronary artery bypass grafting. AME Med. J. 2020, 6, 20. [Google Scholar] [CrossRef]
- Elbadawi, A.; Hamed, M.; Elgendy, I.Y.; Omer, M.A.; Ogunbayo, G.O.; Megaly, M.; Denktas, A.; Ghanta, R.; Jimenez, E.; Brilakis, E.; et al. Outcomes of Reoperative Coronary Artery Bypass Graft Surgery in the United States. J. Am. Heart Assoc. 2020, 9, e016282. [Google Scholar] [CrossRef] [PubMed]
- Harskamp, R.E.; Beijk, M.A.; Damman, P.; Kuijt, W.J.; Woudstra, P.; Grundeken, M.J.; Kloek, J.J.; Tijssen, J.G.; de Mol, B.A.; de Winter, R.J. Clinical outcome after surgical or percuta-neous revascularization in coronary bypass graft failure. J. Cardiovasc. Med. 2013, 14, 438–445. [Google Scholar] [CrossRef]
- Abdelrahman, A.; Dębski, M.; More, R.; Abdelaziz, H.K.; Choudhury, T.; Eichhofer, J.; Patel, B. One-year outcomes of percutaneous coronary intervention in native coronary arteries versus saphenous vein grafts in patients with prior coronary artery bypass graft surgery. Cardiol. J. 2022, 29, 396–404. [Google Scholar] [CrossRef]
- Cohen, N.S.; Dinh, D.; Ajani, A.; Clark, D.; Brennan, A.; Tie, E.N.; Dagan, M.; Hamilton, G.; Sebastian, M.; Shaw, J.; et al. Outcomes after percutaneous coronary intervention (PCI) in patients with prior coronary artery bypass grafting (cabg). Eur. Heart J. 2021, 42 (Suppl. S1), ehab724-2121. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter. Cardiovasc. Interv. 2013, 82, E266–E355. [Google Scholar] [CrossRef]
- Kolh, P.; Wijns, W.; Danchin, N.; Di Mario, C.; Falk, V.; Folliguet, T.; Garg, S.; Huber, K.; James, S.; Knuuti, J.; et al. Guidelines on myocardial revascularization. Eur. J. Cardio-Thorac. Surg. 2010, 38, S1–S52. [Google Scholar] [CrossRef] [PubMed]
- Baim, D.S.; Wahr, D.; George, B.; Leon, M.B.; Greenberg, J.; Cutlip, D.E.; Kaya, U.; Popma, J.J.; Ho, K.K.; Kuntz, R.E. Randomized Trial of a Distal Embolic Protection Device During Percutaneous Intervention of Saphenous Vein Aorto-Coronary Bypass Grafts. Circulation 2002, 105, 1285–1290. [Google Scholar] [CrossRef]
- Dixon, S.R.; Mann, J.T.; Lauer, M.A.; Casale, P.N.; Dippel, E.J.; Strumpf, R.K.; Feldman, R.L.; Shear, W.; Resar, J.R.; Zimmer, S.D.; et al. A randomized, controlled trial of saphenous vein graft intervention with a filter-based distal embolic protection device: TRAP trial. J. Interv. Cardiol. 2005, 18, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Golwala, H.; Hawkins, B.M.; Stavrakis, S.; Abu-Fadel, M.S. Embolic protection device use and outcomes in patients receiving sa-phenous vein graft interventions—A single-center experience. J. Invasive Cardiol. 2012, 24, 1–3. [Google Scholar] [PubMed]
- Iqbal, M.B.; Nadra, I.J.; Ding, L.; Fung, A.; Aymong, E.; Chan, A.W.; Hodge, S.; Della Siega, A.; Robinson, S.D.; British Columbia Cardiac Registry Investigators. Embolic protection device use and its association with pro-cedural safety and long-term outcomes following saphenous vein graft intervention: An analysis from the British Columbia Cardiac registry. Catheter. Cardiovasc. Interv. 2016, 88, 73–83. [Google Scholar] [CrossRef]
- Brennan, J.M.; Al-Hejily, W.; Dai, D.; Shaw, R.E.; Trilesskaya, M.; Rao, S.V.; Brilakis, E.S.; Anstrom, K.J.; Messenger, J.C.; Peterson, E.D.; et al. Three-year outcomes associated with embolic protec-tion in saphenous vein graft intervention: Results in 49 325 senior patients in the Medicare-linked National Cardiovascular Data Registry CathPCI Registry. Circ. Cardiovasc. Interv. 2015, 8, e001403. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; Ni, J.; Shou, W.; Fang, Y.; Fu, S. Outcomes Following Percutaneous Coronary Intervention in Saphenous Vein Grafts with and without Embolic Protection Devices: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 8, 726579. [Google Scholar] [CrossRef]
- Brilakis, E.S.; Edson, R.; Bhatt, D.L.; Goldman, S.; Holmes, D.R.; Rao, S.V.; Shunk, K.; Rangan, B.V.; Mavromatis, K.; Ramanathan, K.; et al. Drug-eluting stents versus bare-metal stents in sa-phenous vein grafts: A double-blind, randomised trial. Lancet 2018, 391, 1997–2007. [Google Scholar] [CrossRef]
- Patel, N.J.; Bavishi, C.; Atti, V.; Tripathi, A.; Nalluri, N.; Cohen, M.G.; Kini, A.S.; Sharma, S.K.; Dangas, G.; Bhatt, D.L. Drug-Eluting Stents versus Bare-Metal Stents in Saphenous Vein Graft Intervention. Circ. Cardiovasc. Interv. 2018, 11, e007045. [Google Scholar] [CrossRef]
- Al-Lamee, R.; Ielasi, A.; Latib, A.; Godino, C.; Ferraro, M.; Arioli, F.; Mussardo, M.; Piraino, D.; Figini, F.; Carlino, M.; et al. Clinical and Angiographic Outcomes after Percutaneous Recanalization of Chronic Total Saphenous Vein Graft Occlusion Using Modern Techniques. Am. J. Cardiol. 2010, 106, 1721–1727. [Google Scholar] [CrossRef]
- Escaned, J. Secondary revascularization after CABG surgery. Nat. Rev. Cardiol. 2012, 9, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Björkman, P.; Kokkonen, T.; Albäck, A.; Venermo, M. Drug-Coated versus Plain Balloon Angioplasty in Bypass Vein Grafts (the DRECOREST I-Study). Ann. Vasc. Surg. 2019, 55, 36–44. [Google Scholar] [CrossRef]
- Lin, L.; Lu, W.; Wang, X.; Pan, L.; Wang, X.; Zheng, X.; Li, R.; Shan, Y.; Peng, M.; Qiu, C. Short-term outcomes of drug-coated balloon versus drug-eluting stent for de novo saphenous vein graft lesions in coronary heart disease. Front. Cardiovasc. Med. 2023, 10, 982880. [Google Scholar] [CrossRef] [PubMed]
- Jeroudi, O.M.; Alomar, M.E.; Michael, T.T.; El Sabbagh, A.; Patel, V.G.; Mogabgab, O.; Fuh, E.; Sherbet, D.; Lo, N.; Roesle, M.; et al. Prevalence and management of coronary chronic total occlusions in a tertiary veterans affairs hospital. Catheter. Cardiovasc. Interv. 2014, 84, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, M.; Ohara, Y.; Navas, J.P.; Nishida, K.; Murphy, T.J.; Alexander, R.W.; Nerem, R.M.; Harrison, D.G.; Casey, D.P.; Ueda, K.; et al. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am. J. Physiol. 1995, 269 Pt 1, C1371–C1378. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.-J.; Chien, S.; Venturini, G.; Malagrino, P.A.; Padilha, K.; Tanaka, L.Y.; Laurindo, F.R.; Dariolli, R.; Carvalho, V.M.; Cardozo, K.H.M.; et al. Effects of Disturbed Flow on Vascular Endothelium: Pathophysiological Basis and Clinical Perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef]
- Asakura, T.; Karino, T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ. Res. 1990, 66, 1045–1066. [Google Scholar] [CrossRef]
- Samady, H.; Eshtehardi, P.; McDaniel, M.C.; Suo, J.; Dhawan, S.S.; Maynard, C.; Timmins, L.H.; Quyyumi, A.A.; Giddens, D.P. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation 2011, 124, 779–788. [Google Scholar] [CrossRef]
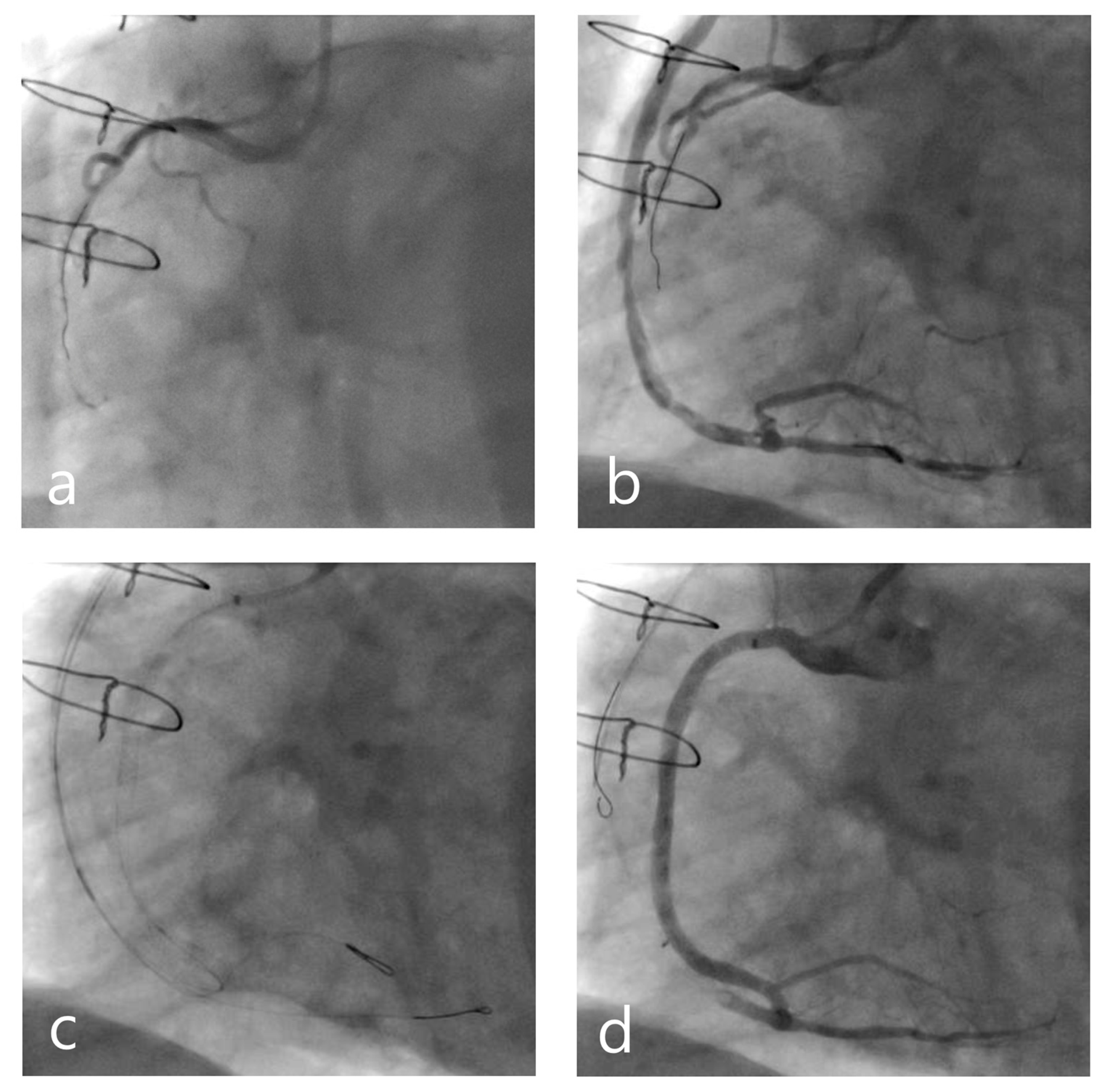
- Wilson, S.J.; Hanratty, C.G.; Spence, M.S.; Owens, C.G.; Rigger, J.; Spratt, J.C.; Walsh, S.J. Saphenous Vein Graft Sacrifice Following Native Vessel PCI is Safe and Associated with Favourable Longer-Term Outcomes. Cardiovasc. Revascularization Med. 2019, 20, 1048–1052. [Google Scholar] [CrossRef]
- Brilakis, E.S.; Rao, S.V.; Banerjee, S.; Goldman, S.; Shunk, K.A.; Holmes, D.R.; Honeycutt, E.; Roe, M.T. Percutaneous Coronary Intervention in Native Arteries versus Bypass Grafts in Prior Coronary Artery Bypass Grafting Patients: A Report from the National Cardiovascular Data Registry. JACC Cardiovasc. Interv. 2011, 4, 844–850. [Google Scholar] [CrossRef]
- Brilakis, E.S.; O’Donnell, C.I.; Penny, W.; Armstrong, E.J.; Tsai, T.; Maddox, T.M.; Plomondon, M.E.; Banerjee, S.; Rao, S.V.; Garcia, S.; et al. Percutaneous Coronary Intervention in Native Coronary Arteries Versus Bypass Grafts in Patients with Prior Coronary Artery Bypass Graft Surgery: Insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. JACC Cardiovasc. Interv. 2016, 9, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomos, A.M.; Tushar, K.; Omar, C.; Jonathan, H.; Roby, D.R. Superior long term outcome associated with native vessel versus graft vessel PCI following secondary PCI in patients with prior CABG. Int. J. Cardiol. 2017, 228, 563–569. [Google Scholar]
- De Winter, R.W.; Walsh, S.J.; Hanratty, C.G.; Spratt, J.C.; Sprengers, R.W.; Twisk, J.W.; Vegting, I.; Schumacher, S.P.; Bom, M.J.; Hoek, R.; et al. Percutaneous coronary intervention of native coronary artery versus saphenous vein graft in patients with prior coronary artery bypass graft surgery: Rationale and design of the multicenter, randomized PROCTOR trial. Am. Heart J. 2023, 257, 20–29. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Back, L.; Ladwiniec, A. Saphenous Vein Graft Failure: Current Challenges and a Review of the Contemporary Percutaneous Options for Management. J. Clin. Med. 2023, 12, 7118. https://doi.org/10.3390/jcm12227118
Back L, Ladwiniec A. Saphenous Vein Graft Failure: Current Challenges and a Review of the Contemporary Percutaneous Options for Management. Journal of Clinical Medicine. 2023; 12(22):7118. https://doi.org/10.3390/jcm12227118
Chicago/Turabian StyleBack, Liam, and Andrew Ladwiniec. 2023. "Saphenous Vein Graft Failure: Current Challenges and a Review of the Contemporary Percutaneous Options for Management" Journal of Clinical Medicine 12, no. 22: 7118. https://doi.org/10.3390/jcm12227118





