A Quick and Practical Approach to Secure a Chronic Fatigue Syndrome Diagnosis: The Novel Functional Limitation Index
Abstract
:1. Introduction
2. Material and Methods
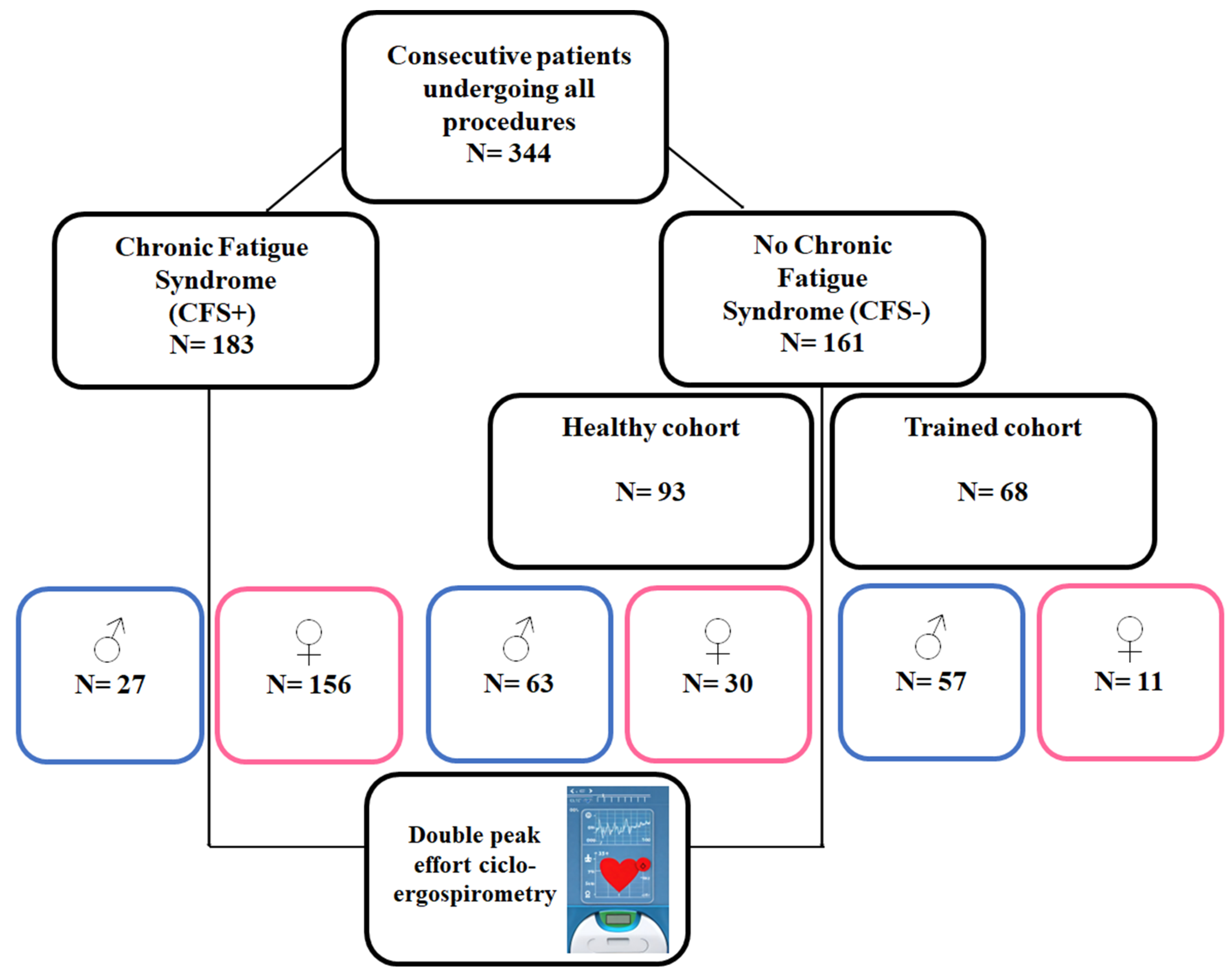
2.1. Study Group
- CFS group: Patients already diagnosed with a definitive CFS by other clinical specialists, following the Fukuda diagnostic criteria [5] (Appendix A). These patients had limited symptoms, including those mentioned above and were referred to our clinic for that reason. Many of them required a study to assess disabilities. Patients with doubtful or incomplete CFS diagnoses were not included.
- Healthy cohort: Healthy patients who wanted to collaborate, without any limiting disease or condition and who did not perform regular or scheduled physical activity. They were studied in the context of routine health checkups.
- Sportspeople/trained cohort: Healthy athlete patients at an amateur level, understood as such, who trained weekly for more than 8–10 h of medium–high intensity (Mitchell Classification) [4].
2.2. Study Protocol and Procedures
- Three minutes no-load pedaling warm-up.
- Maximum test with progressive load of 15 w/m until the end of the test (maximum O2 consumption, VO2 max).
- Rest of three minutes with rest on the bicycle or minimal pedaling, at the patient’s decision.
- Supramaximal test started at the power equivalent to 50% of the maximum power reached in the test with a progression of 15 w every 10 s, until the exhaustion of the patient (supramaximal O2 consumption, VO2 smax).
- Rest period, without pedaling, until the patient recovered completely.
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. General Considerations
4.2. Cardiopulmonary Exercise Testing in CFS
4.3. CFS Pathophysiology
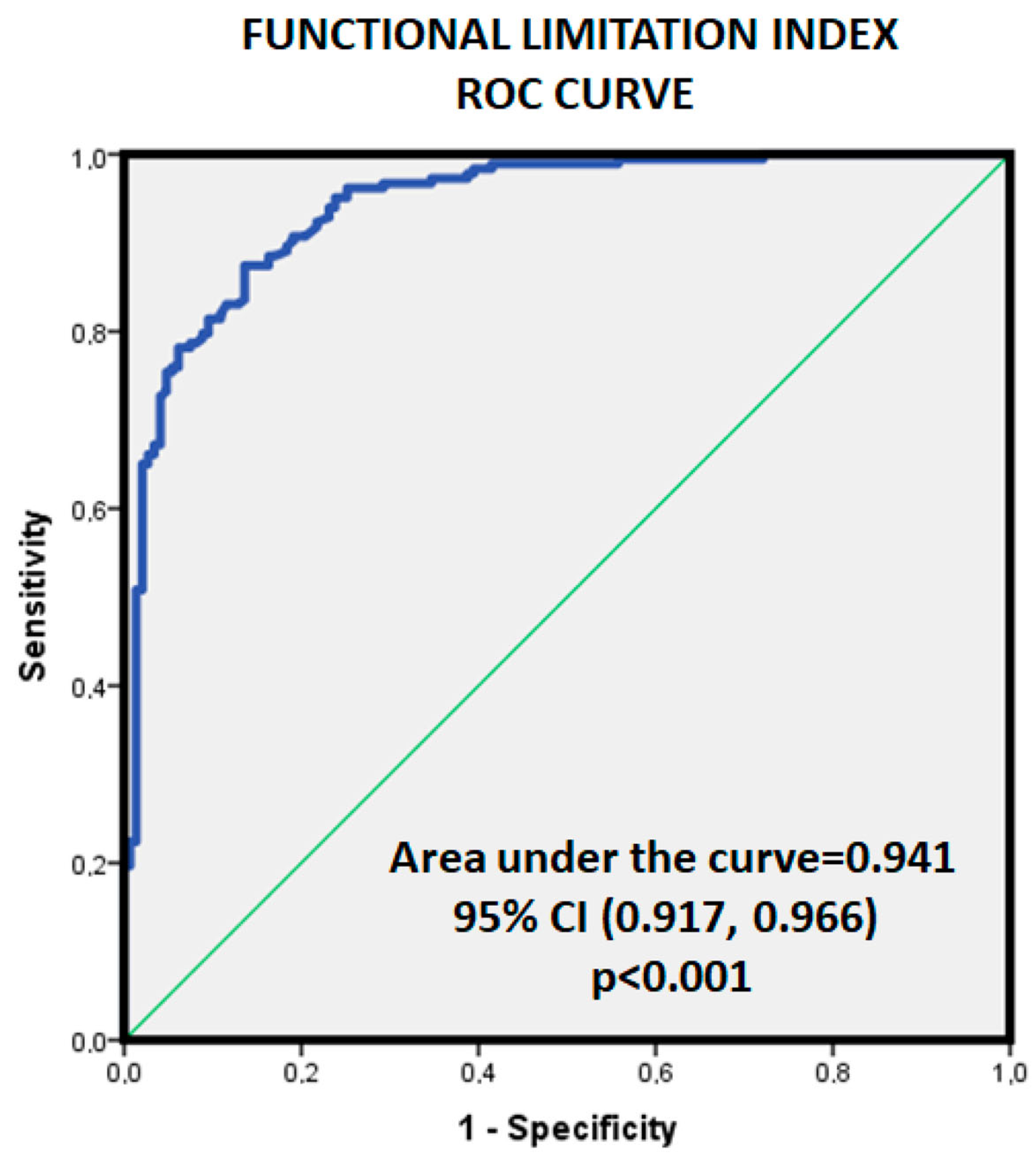
4.4. CFS and FLI Rationale
5. Limitations
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BR < 35% | Ventilatory reserve (maximum ventilation vs. voluntary ventilation VMV) |
| EQO2 | O2 equivalent |
| FEV1 | Forced Expiratory volume in one second (spirometry) |
| FLI | Functional limitation index |
| FVC | Forced Vital Capacity |
| HR AT | Heart rate at the anaerobic threshold (VT1) |
| HR M/T | Maximum heart rate achieved with respect to the theoretical maximum |
| Pulse O2 | O2 pulse vs. heart rate (inotropic function) |
| MVV | Maximum voluntary ventilation (FEV1*40, convention) |
| VE | Ventilation |
| V.ESTIM | Estimated ventilation. |
| VO2m | Peak (maximum) O2 consumption |
| VO2m/t | Peak O2 consumption reached compared with the theoretical |
| VO2m/sm | Relationship between maximum O2 consumption and supramaximal (second peak) |
| VO2sm/t | Supramaximal consumption (2 peak) vs. theoretical |
| VO2sm/m | Supramaximal O2 consumption (second peak) vs. máximum (first peak) |
| VE/VCO2 (EQ. CO2) | CO2 equivalent |
| VO2/W | Relationship between O2 consumption and power |
| Wmax | Peak (Maximum) power |
| W m/t | Peak (Maximum) power compared with the theoretical |
| W/K | Power to weight ratio |
Appendix A
Appendix A.1. Data (Prospectively) Recorded by Protocol in the Present Study
- −
- Age, gender.
- −
- Anthropometric Data: Weight, height, BMI, % fat, muscle and water, Fat/Muscle Ratio, BMI-Corrected.
- −
- Spirometric Data: FVC, FEV1, FEV1/FVC, FEV at 25.50 and 75%, classified later in the Muller graph.
- −
- Ergospirometric data: They were analyzed according to the 9 Wassermman graphs and all the parameters derived from them.
- VO2 peak, VO2 in AT (first threshold QR = 1), VO2 entry into the lactic anaerobic phase (QR = 1.3), VO2 entry into the alactic anaerobic phase (QR = 1.10), measured in Supramaximal VO2 (SM VO2) at the end of recovery.
- VO2 peak/VO2 SM
- FLI. Functional Limitation Index (VO2 peak/VO2 SM)/(VO2 SM/VO2 predicted)
- O2 pulse (VO2/HR) at AT and at peak effort.
- VO2/W ratio
- W peak and at each of the above thresholds
- Power/weight ratio
- CO2 production
- O2 and CO2 equivalents
- PTE of O2 and CO2
- VE peak
- MVV (FEV1*40)
- Ventilatory Reserve (BR, Breath Reserve) (VMV − VE peak)/VMV, in %
- Energy expenditure at rest, AT and peak effort
Appendix A.2. Fukuda et al., CFS Diagnostic Criteria (1994) [5]
- Persistent fatigue (at least six months) or intermittent, unexplained, recurring or with a defined onset, not resulting from recent efforts; does not improve with rest; leads to a noticeable reduction in the patient’s previous usual activity.
- Exclusion of other diseases that can cause chronic fatigue.
- Recent concentration or memory impairments.
- Odynophagia.
- Painful cervical or axillary lymph nodes.
- Myalgias.
- Polyarthralgias without signs of inflammation.
- Recently onset headaches or headaches with different characteristics from the usual ones.
- Non-refreshing sleep.
- Post-exertional malaise lasting more than 24 h.
References
- Fulcher, K.Y.; White, P.D. Strength and physiological response to exercise in patients with chronic fatigue syndrome. J. Neurol. Neurosurg. Psychiatry 2000, 69, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Falk Hvidberg, M.; Brinth, L.S.; Olesen, A.V.; Petersen, K.D.; Ehlers, L. The health-related quality of life for patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). PLoS ONE 2015, 10, e0132421. [Google Scholar] [CrossRef] [PubMed]
- Bateman, L.; Bested, A.C.; Bonilla, H.F.; Chheda, B.V.; Chu, L.; Curtin, J.M.; Dempsey, T.T.; Dimmock, M.E.; Dowell, T.G.; Felsenstein, D.; et al. Myalgic encephalomyelitis/chronic fatigue syndrome: Essentials of diagnosis and management. Mayo Clin. Proc. 2021, 96, 2861–2878. [Google Scholar] [CrossRef] [PubMed]
- Alijotas, J.; Alegre, J.; Fernández-Solà, J.; Cots, J.M.; Panisello, J.; Peri, J.M.; Pujol, R. Consensus report on the diagnosis and treatment of chronique fatigue syndrome in Catalonia. Med. Clin. 2002, 118, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Strauss, S.E.; Hickie, I.; Sharp, C.M.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Faro, M.; Aliste, L.; Sáez-Francàs, N.; Calvo, N.; Martínez-Martínez, A.; de Sevilla, T.F.; Alegre, J. Comorbidity in chronic fatigue syndrome/myalgic encephalomyelitis: A nationwide population-based cohort study. Psychosomatics 2017, 58, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Young, P.; Finn, B.C.; Bruetman, J.; Pellegrini, D.; Kremer, A. The chronic asthenia syndrome: A clinical approach. Medicina 2010, 70, 284–292. [Google Scholar]
- Bradley, A.S.; Ford, B.; Bansal, A.S. Altered functional B cell subset populations in patients with chronic fatigue syndrome compared to healthy controls. Clin. Exp. Immunol. 2013, 172, 73–80. [Google Scholar] [CrossRef]
- Rutherford, G.; Manning, P.; Newton, J.L. Understanding Muscle Dysfunction in Chronic Fatigue Syndrome. J. Aging Res. 2016, 2016, 2497348. [Google Scholar] [CrossRef]
- Vanness, J.M.; Snell, C.R.; Strayer, D.R.; Dempsey, L., 4th; Stevens, S.R. Subclassifying chronic fatigue syndrome through exercise testing. Med. Sci. Sports Exerc. 2003, 35, 908–913. [Google Scholar] [CrossRef]
- Fernández, A.A.; Martín, A.P.; Martínez, M.I. Chronic fatigue syndrome. Consensus document. Aten. Primaria 2009, 41, 529–531. [Google Scholar] [CrossRef] [PubMed]
- van Campen, C.L.M.; Rowe, P.C.; Visser, F.C. Two-Day Cardiopulmonary Exercise Testing in Females with a Severe Grade of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Comparison with Patients with Mild and Moderate Disease. Healthcare 2020, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Poca-Dias, V.; Ojanguren Sabán, I.; Pereira dos Santos, C.; Sánchez-Vizcaíno, E.; Ariza Fernández, A.; García-Fructuoso, F. Implicación de la mitocondria en la fatiga crónica. Dolor 2008, 23, 18–24. [Google Scholar]
- Babor, J.A.; Obarz Aznárez, J. Química General Moderna; Ley de Acción de Masas; Editorial Marin, SA: Barcelona, Spain, 1970; pp. 291–293. [Google Scholar]
- López Chicharro, J.; Fernández Vaquero, A. Fisiología del Ejercicio, 3rd ed.; Panamericana: Madrid, Spain, 2010; pp. 197–202. [Google Scholar]
- West, A.P. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology 2017, 391, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Moreno Fernández-Ayala, D.J.; Navas, P.; López-Lluch, G. Age-related mitochondrial dysfunction as a key factor in COVID-19 disease. Exp. Gerontol. 2020, 142, 111147. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.; Snell, C.; Stevens, J.; Keller, B.; VanNess, J.M. Cardiopulmonary Exercise Test Methodology for Assessing Exertion Intolerance in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Pediatr. 2018, 4, 242. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.A.; Jason, L.A.; Evans, M.A.; Flores, S. Contrasting Case Definitions: The ME International Consensus Criteria vs. the Fukuda et al. CFS Criteria. N. Am. J. Psychol. 2013, 15, 103–120. [Google Scholar]
- Twisk, F. Myalgic Encephalomyelitis or What? The International Consensus Criteria. Diagnostics 2018, 9, 1. [Google Scholar] [CrossRef]
- Grach, S.L.; Seltzer, J.; Chon, T.Y.; Ganesh, R. Diagnosis and Management of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Mayo Clin. Proc. 2023, 98, 1544–1551. [Google Scholar] [CrossRef]
- Joseph, P.; Arevalo, C.; Oliveira, R.K.; Faria-Urbina, M.; Felsenstein, D.; Oaklander, A.L.; Systrom, D.M. Insights from Invasive Cardiopulmonary Exercise Testing of Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Chest 2021, 160, 642–651. [Google Scholar] [CrossRef]
- Montoya, J.G.; Dowell, T.G.; Mooney, A.E.; Dimmock, M.E.; Chu, L. Caring for the patient with severe or very severe myalgic encephalomyelitis/chronic fatigue syndrome. Healthcare 2021, 9, 1331. [Google Scholar] [CrossRef]
| Variable | CFS+ | CFS− | p-Value |
|---|---|---|---|
| Gender (Female) | 85.2% | 25.5% | <0.001 |
| Age | 46.2 (9.3) | 41.2 (13.8) | <0.001 |
| Weight | 66.3 (12.9) | 77.3 (14.3) | <0.001 |
| VO2m | 20.4 (6.3) | 38.0 (9.6) | <0.001 |
| VO2m/t | 76.8 (16.7) | 114.8 (24.1) | 0.093 |
| Wmax | 118.2 (33.4) | 220.86 (58.9) | <0.001 |
| W m/t | 87.4 (19.0) | 122.9 (25.5) | <0.001 |
| W/K | 87.4 (19.0) | 3.04 (0.7) | <0.001 |
| FC M/T | 84.7 (9.8) | 93.0 (10.7) | <0.001 |
| FC AT | 120.5 (20.7) | 137.5 (18.1) | <0.001 |
| VO2/W | 11.38 (1.9) | 13.0 (1.7) | <0.001 |
| VO2m/sm | 140.4 (31.2) | 114.3 (13.2) | <0.001 |
| VO2sm/t | 57.1 (15.0) | 100.8 (25.2) | <0.001 |
| FLI | 2.7 (1.26) | 1.2 (0.4) | <0.001 |
| VO2sm/m | 0.7 (0.1) | 0.9 (0.0) | <0.001 |
| VE | 46.1 (14.3) | 90.1 (25.1) | <0.001 |
| EQO2 | 33.5 (5.5) | 31.5 (4.1) | <0.001 |
| VE/VCO2 | 27.2 (5.3) | 24.8 (3.6) | <0.001 |
| Pulse O2 | 9.0 (2.5) | 17.3 (4.9) | <0.001 |
| VEF1 | 2.65 (0.7) | 3.7 (0.7) | <0.001 |
| MVV | 70.5 (54.7) | 147.4 (30.1) | <0.001 |
| V.ESTIM | 51.5 (12.1) | 85.4 (24.5) | <0.001 |
| BR < 35% | 55.2 (12.3) | 37.9 (13.3) | <0.001 |
| Variable | CFS+ Female | CFS+ Male | Healthy Female | Healthy Male | Trained Female | Trained Male | p-Value * |
|---|---|---|---|---|---|---|---|
| Age | 46.8 (8.9) | 42.8 (11.3) | 43.6 (9.4) | 46.1 (13.7) | 32.7 (10.3) | 36.1 (14.2) | <0.001 |
| Weight | 64.6 (11.9) | 77.2 (14.4) | 61.3 (12.9) | 81.2 (12.0) | 59.4 (5.9) | 77.2 (11.4) | <0.001 |
| VO2m | 19.3 (5.1) | 26.7 (8.5) | 28.6 (6.3) | 33.9 (5.7) | 42.3 (5.9) | 46.6 (7.4) | <0.001 |
| VO2m/t | 77.0 (16.7) | 75.4 (16.5) | 104.6 (19.2) | 105.1 (17.1) | 148.0 (28.5) | 124.54 (23.0) | 0.350 |
| Wmax | 109.5 (24.3) | 168.1 (35.5) | 147.9 (26.6) | 213.3 (38.5) | 200.5 (25.9) | 271.5 (38.6) | <0.001 |
| W m/t | 87.8 (19.2) | 85.1 (18.6) | 118.9 (19.6) | 110.3 (22.7) | 155.5 (20.2) | 132.6 (24.8) | <0.001 |
| W/K | 1.73 (0.4) | 2.27 (0.7) | 2.45 (0.5) | 2.67 (0.4) | 3.39 (0.4) | 3.6 (0.6) | <0.001 |
| FC M/T | 83.9 (9,7) | 89.1 (9.4) | 94.5 (14.2) | 92.7 (7.3) | 92.4 (4.5) | 92.8 (12.6) | <0.001 |
| FC AT | 121.5 (21.6) | 114.8 (14.8) | 129.0 (16.8) | 135.4 (17.9) | 142.6 (33.3) | 142.3 (13.9) | <0.001 |
| VO2sm | 13.9 (4.3) | 22.1 (7.3) | 23.6 (6.3) | 29.9 (5.9) | 38.5 (5.3) | 42.8 (7.6) | <0.001 |
| VO2m/sm | 143.5 (31.9) | 123.2 (19.1) | 123.1 (12.7) | 115.0 (14.9) | 109.0 (6.2) | 109.3 (9.3) | <0.001 |
| VO2sm/t | 56.1 (14.9) | 62.6 (14.1) | 85.9 (19.0) | 91.4 (19.1) | 135.2 (23.1) | 112.9 (22.7) | <0.001 |
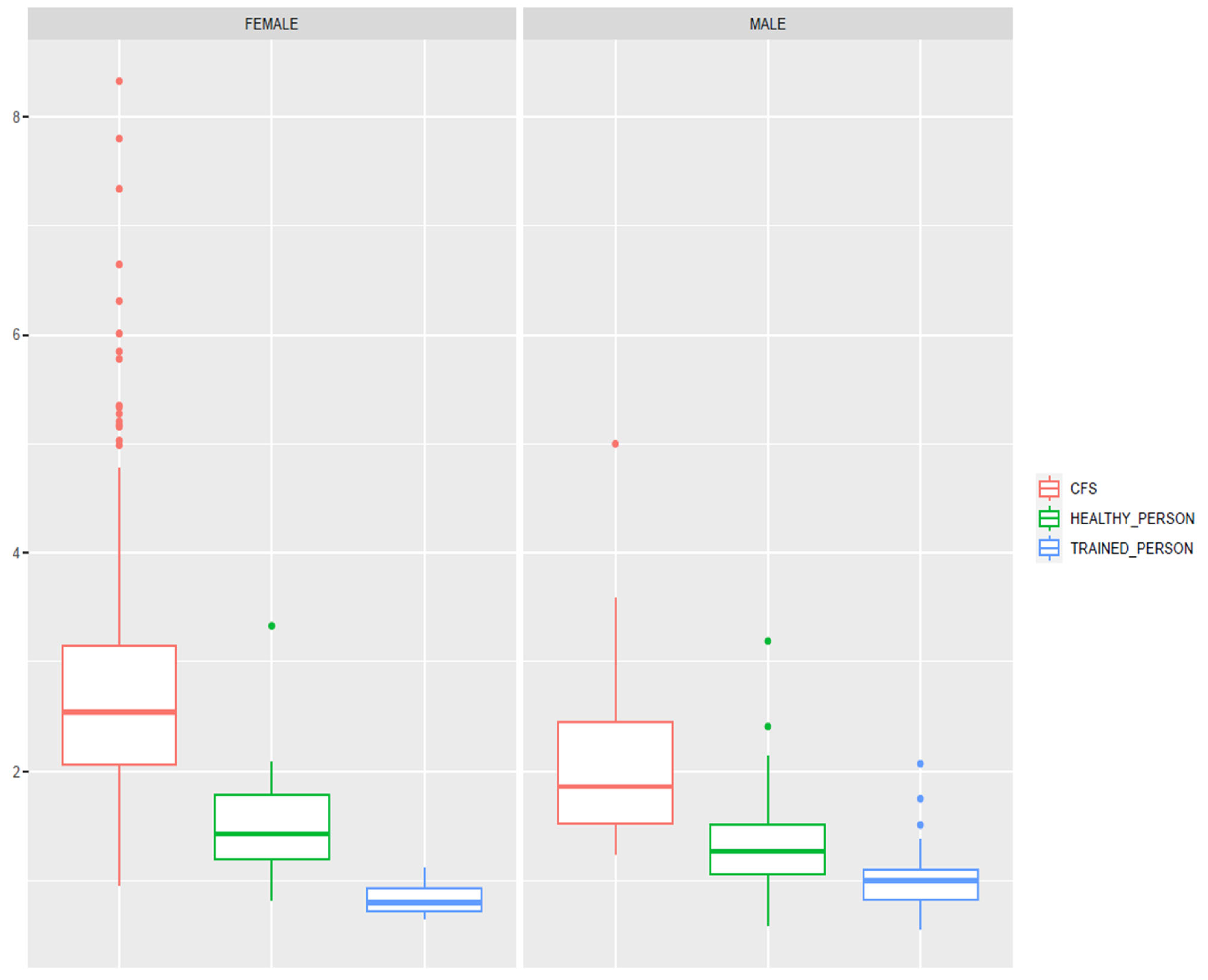
| FLI | 2.84 (1.3) | 2.12 (0.8) | 1.52 (0.4) | 1.33 (0.4) | 0.83 (0.1) | 1.01 (0.27) | <0.001 |
| VO2sm/m | 0.72 (0.1) | 0.82 (0.1) | 0.82 (0.1) | 0.88 (0.1) | 0.91 (0.0) | 0.92 (0.1) | <0.001 |
| VE | 42.8 (11.9) | 65.3 (12.3) | 58.9 (12.5) | 87.9 (16.0) | 84.1 (13.9) | 110.2 (21.1) | <0.001 |
| EQO2 | 33.8 (5.7) | 31.7 (3.8) | 33.6 (4.2) | 31.4 (4.0) | 32.9 (2.6) | 30.4 (3.9) | <0.001 |
| VE/VCO2 | 27.9 (5.2) | 23.7 (4.1) | 26.0 (3.8) | 24.7 (2.9) | 24.3 (2.9) | 24.3 (4.1) | <0.001 |
| Pulse O2 | 8.4 (1.8) | 12.8 (2.6) | 10.3 (1.9) | 17.4 (3.3) | 14.5 (2.1) | 21.5 (2.8) | <0.001 |
| VEF1 | 2.51 (0.5) | 3.89 (0.6) | 2.77 (0.4) | 3.82 (0.6) | 3.01 (0.3) | 4.13 (0.6) | <0.001 |
| MVV | 63.6 (51.3) | 147.7 (23.6) | 110.8 (16.1) | 152.8 (23.0) | 120.4 (13.7) | 165.3 (24.0) | <0.001 |
| V.ESTIM | 48.4 (8.7) | 69.5 (12.8) | 62.2 (9.6) | 85.8 (13.8) | 39.3 (12.3) | 106.7 (13.9) | <0.001 |
| BR < 35% | 55.2 (12.6) | 55.2 (10.5) | 46.3 (12.2) | 41.2 (11.0) | 29.7 (11.4) | 32.6 (13.2) | <0.001 |
| Study Group | FLI Mean Z-Score (SD) | p-Value |
|---|---|---|
| Gender (all) | <0.001 | |
| 0.38 (1.07) −0.56 (0.52) | |
| Health status | <0.001 | |
| 0.55 (1.02) −0.68 (0.35) | |
| CFS + Gender | 0.006 | |
| 0.63 (1.05) 0.05 (0.67) | |
| CFS – Gender | 0.072 | |
| 0.43 (0.43) 0.32 (0.32) | |
| Training level | <0.001 * | |
| 0.55 (1.02) −0.54 (0.37) −0.88 (0.21) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbalán, J.A.; Feltes, G.; Silva, D.; Gómez-Utrero, E.; Núñez-Gil, I.J. A Quick and Practical Approach to Secure a Chronic Fatigue Syndrome Diagnosis: The Novel Functional Limitation Index. J. Clin. Med. 2023, 12, 7157. https://doi.org/10.3390/jcm12227157
Corbalán JA, Feltes G, Silva D, Gómez-Utrero E, Núñez-Gil IJ. A Quick and Practical Approach to Secure a Chronic Fatigue Syndrome Diagnosis: The Novel Functional Limitation Index. Journal of Clinical Medicine. 2023; 12(22):7157. https://doi.org/10.3390/jcm12227157
Chicago/Turabian StyleCorbalán, Juan Antonio, Gisela Feltes, Daniela Silva, Eduardo Gómez-Utrero, and Iván J. Núñez-Gil. 2023. "A Quick and Practical Approach to Secure a Chronic Fatigue Syndrome Diagnosis: The Novel Functional Limitation Index" Journal of Clinical Medicine 12, no. 22: 7157. https://doi.org/10.3390/jcm12227157
APA StyleCorbalán, J. A., Feltes, G., Silva, D., Gómez-Utrero, E., & Núñez-Gil, I. J. (2023). A Quick and Practical Approach to Secure a Chronic Fatigue Syndrome Diagnosis: The Novel Functional Limitation Index. Journal of Clinical Medicine, 12(22), 7157. https://doi.org/10.3390/jcm12227157






