The Current Knowledge of Cerebral Magnetic Resonance Imaging in Monochorionic Twins: A Systematic Review of the Last 20 Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Quality Assessment
3. Results
3.1. Study Selection, Quality Assessment, and Study Characteristics
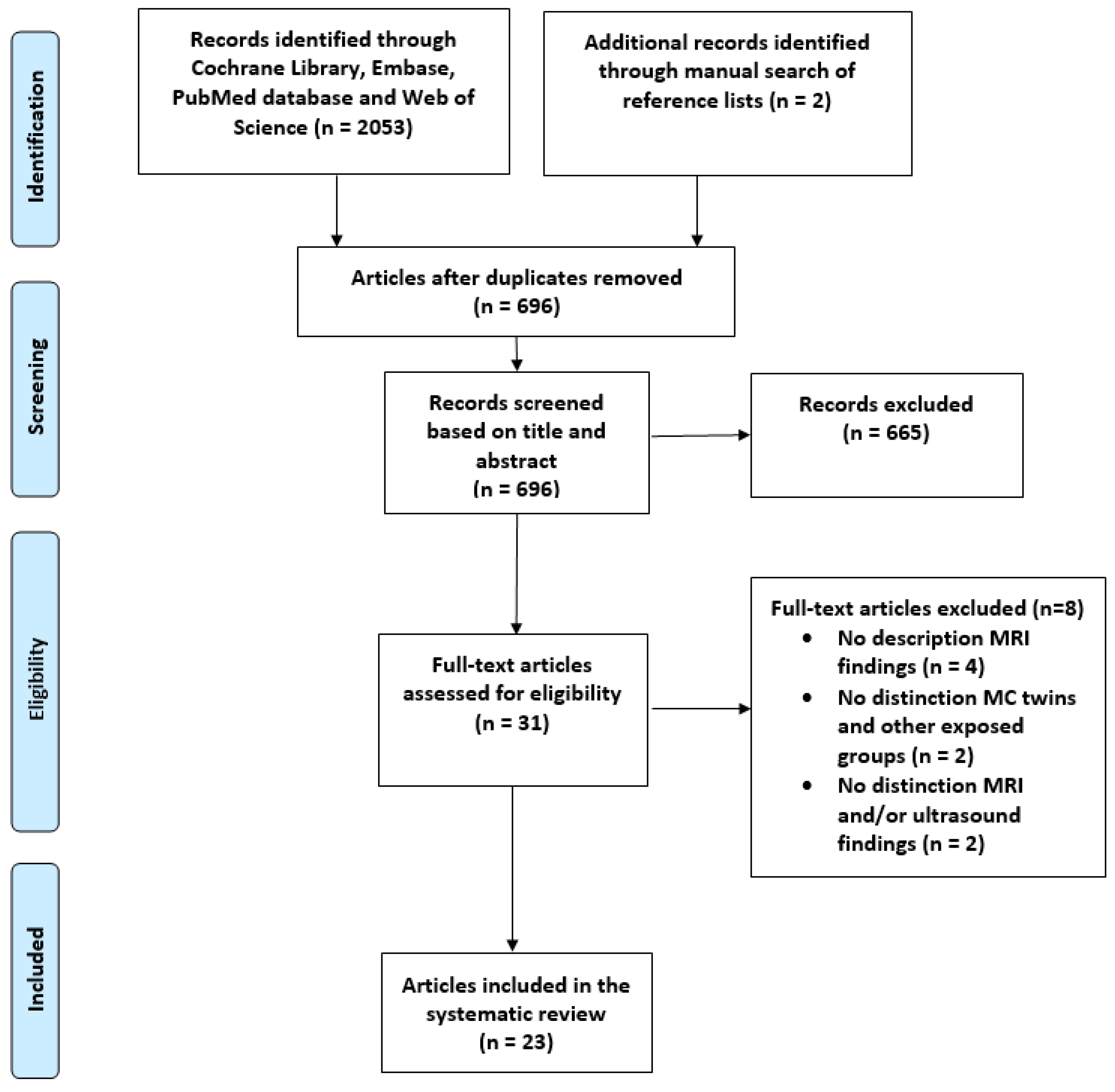
3.1.1. Study Selection
3.1.2. Quality Assessment
3.1.3. Study Characteristics
| First Author (Year) | Country | Study Design | Study Period | Number of Fetuses/Neonates with MRI (N) | Population | Validity |
|---|---|---|---|---|---|---|
| Shinar (2022) [14] | International | R | 2008–2020 | 47 a | sIUFD * | Good |
| Rosen (2022) [15] | Israel | R | 2013–2021 | 46 a | TAPS | Good |
| Gebb (2022) [16] | USA | R | 2009–2021 | 40 a | sIUFD | Good |
| Segev (2022) [8] | Israel | R | 2017–2020 | 29 a | sIUFD | Good |
| Moradi (2022) [17] | Iran | P | NR | 43 a | sIUFD | Good |
| Anh (2022) [18] | Vietnam | P | 2019–2021 | 21 b | TTTS | Good |
| Halevy (2021) [19] | Israel | P | 2010–2015 | 10 a | Uncomplicated | Good |
| Hochberg (2021) [20] | Israel | R | 2011–2019 | 153 a | TTTS | Good |
| Aertsen (2021) [21] | Belgium | R | 2010–2017 | 125 a | TTTS | Good |
| Kocaoglu (2020) [7] | USA | R | 2014–2018 | 366 a | TTTS | Good |
| Stirnemann (2018) [22] | France | R | 2003–2015 | 720 a | TTTS | Good |
| Conte (2018) [23] | Italy | R | 2002–2015 | 430 a | sIUFD | Good |
| Robinson (2017) [24] | Australia | R | 2007–2016 | 48 a | Mixed (**) | Fair |
| van Klink (2015) [10] | The Netherlands | R | 2002–2013 | 50 a,b | sIUFD | Good |
| Jatzko (2015) [25] | Austria | R | 2005–2012 | 11 a | sIUFD | Good |
| Griffiths (2015) [26] | UK | R | 2004–2013 | 68 a | sIUFD | Fair |
| Weisz (2014) [27] | Israel | P | 2009–2012 | 52 a | TTTS | Good |
| Hoffmann (2013) [28] | Israel | P | 2007–2010 | 34 a | sIUFD | Good |
| O’Donoghue (2009) [13] | UK | R | 2000–2007 | 76 a,b | sIUFD | Good |
| Fichera (2009) [29] | Italy | R | 2001–2006 | 13 a | sIUFD * | Good |
| Jelin (2008) [30] | USA | R | 1997–2007 | 21 a | sIUFD | Good |
| Kline-Fath (2007) [31] | USA | R | 2003–2005 | 48 a | TTTS | Good |
| Hu (2006) [12] | USA | R | NR | 17 a | Mixed (**) | Fair |
| First Author (Year) | GA Age at MRI (Weeks) | Population | Incidence of Cerebral Injury in Fetus (%, n/N) | Cerebral Injury (Number of Observations) | MRI Sequence |
|---|---|---|---|---|---|
| Shinar (2022) [14] | 25.9 ± 4.2 weeks | sIUFD | 34.0 (16/47) | Ischemic injury (16) | T1- and T2-weighted MRI, SWI, and DWI |
| Rosen (2022) [15] | 28–32 weeks | TAPS | 13.0 (6/46) | Enlarged dural venous sinuses (1), subependymal blood lateral ventricles (1), severe cerebral ischemia (2), cerebellar hemorrhage (1), asymmetrical lateral ventricles (1) | T1- and T2-weighted MRI and DWI |
| Gebb (2022) [16] | NR | sIUFD | 30.0 (12/40) | Recipients: choroid plexus hemorrhage (2), GM-IVH grade I-II (6), cerebral malformation with PMG and schizencephaly (1); donors GM-IVH grade I (2), PMG (1) | T2-weighted MRI and DWI |
| Segev (2022) [8] | 30.9 ± 1.2 weeks | sIUFD ø | 3.5 (1/29) | Cerebral biometry Bilateral caudothalamic cystic changes (1) | T1- and T2-weighted MRI and DWI |
| Moradi (2022) [17] | 21.24 ± 2.29 weeks | sIUFD ø | 30.2 (13/43) | GM-IVH (10), extensive cerebral ischemia (2), mild ventriculomegaly (1) | T1- and T2-weighted MRI and DWI |
| Halevy (2021) [19] | 31.3 weeks (IQR 30–33) | Uncomplicated | N/A | Cerebral biometry | T2-weighted MRI |
| Hochberg (2021) [20] | 28–32 weeks | TTTS | 11.1 (17/153) | Ischemic brain injury (4), PVH (1), cerebellar hypoplasia (3), sinovenous thrombosis (1), porencephalic cyst (1), GM-IVH (5), ventriculomegaly (3) | T1- and T2-weighted MRI and DWI |
| Aertsen (2021) [21] | 28–32 weeks | TTTS | 4.8 (6/125) | Hemorrhagic injury (1), PMG (3), cortical atrophy (2) | T2-weighted MRI and DWI |
| Kocaoglu (2020) [7] | NR | TTTS | 10.4 (38/366) | Diffusion restriction (33), GM-IVH grade I-II (2), grade III-IV (3) | T1- and T2-weighted MRI and DWI |
| Stirnemann (2018) [22] | 30–32 weeks | TTTS | 2.9 (21/720) | Bilateral (2) or focal (1) leukomalacia (1), bilateral (1) or focal PMG, severe ischemic injury (8), unilateral schizencephaly (1), severe VM (4), VM with abnormal gyration (1), brain atrophy (1), unknown (1) | T1- and T2-weighted MRI |
| Conte (2018) [23] | 24 weeks (IQR 21–26 weeks) | sIUFD | 9.7 (42/430) | PVL (2), generalized (9), posterior (7) or bilateral para-sagittal and peri-Sylvian injury (3), encephalomalacia, focal non-hemorrhagic (14) and hemorrhagic (7) injury | T1- and T2-weighted MRI |
| Robinson (2017) [24] | 25 weeks (IQR 21–29 weeks) | Mixed | 27.1 (13/48) | IVH, delayed sulcation, BPD < 5th percentile and bilateral VM (1), bilateral occipital cortical infarction with PMG (1), decreased hemispheric size, asymmetrical ventricles < 10 mm (1), dural sinus thrombosis (1), cystic lesions (1), mildly delayed sulcation (2), encephalomalacia (2), severe abnormal sulcation (1), increased subarachnoid space (2), cerebral biometry <10th percentile (1) | T1- and T2-weighted MRI and DWI |
| van Klink (2015) [10] | 26.5 weeks (IQR 22.3–30.8) | sIUFD | 16/50 (32%) | NR | T1- and T2-weighted MRI |
| Jatzko (2015) [25] | 23.5 ± 2.3 weeks | sIUFD | 54.5 (6/11) | IVH grade I (2), IVH grade III (2), schizencephaly and several small parenchymal hemorrhages (1), cysts lateral to ganglionic eminence, mild VM (1) | T1- and T2-weighted MRI and DWI |
| Griffiths (2015) [26] | NR | sIUFD | 13.2 (9/68) | Demise with TTTS: mild VM (1), focal infarction with PMG (2), extensive encephalomalacia (1); demise with an unknown cause: mild VM (2), extensive encephalomalacia (3) | T1- and T2-weighted MRI and DWI |
| Weisz (2014) [27] | NR | TTTS | 17.3 ‡ (9/52) 3.8 ‡‡ (2/52) | Group after fetoscopic laser surgery: GMH (6), ischemia (3); follow-up at 30–32 weeks in same group: cerebral atrophy (1) and cerebral edema compatible with old infarct and ventricular dilation (1) | T1- and T2-weighted MRI and DWI |
| Hoffmann (2013) [28] | NR | sIUFD ø | 26.5 (9/34) | sIUFD with an unknown cause: ischemic injury (2), cerebral edema (1); sIUFD and TTTS: infarction (1), GM-IVH (1), bilateral PVH (1); selective reduction: bilateral cerebral ischemia (1), GM-IVH (2) | T1- and T2-weighted MRI and DWI |
| Fichera (2009) [29] | 20.6 weeks (IQR 19.1–31.5) | sIUFD | 0 (0/13) | No cerebral injury | T1- and T2-weighted MRI and DWI |
| O’Donoghue (2009) [13] | NR | sIUFD ø | 6.6 (5/76) | Selective reduction group: focal hemorrhage (1) and moderate VM with aqueduct stenosis (1); group with sIUFD with an unknown cause: MCA infarct (1), GMH (1), mild VM (1) | T1- and T2-weighted MRI |
| Jelin (2008) [30] | 24 weeks and 2 days | sIUFD | 33.3 (7/21) | sIUFD with TTTS: unilateral infarct with developing PMG (1), focal injury in left parietal lobe (1), severe destruction of supratentorial brain (1), choroid plexus and posterior fossa subarachnoid hemorrhage (1); TRAP group: bilateral germinolytic cysts, (1) Sylvian fissures slightly shallow (1); demise with an unknown cause: bilateral mild VM; delayed sulcation (1) | T1- and T2-weighted MRI |
| Kline-Fath (2007) [31] | NR | TTTS | 39.6 (19/48) | TTTS donor group: cerebral malformation (2) and cerebral sinovenous enlargement (12); TTTS recipient group: IVH grade I or II/ischemia (2) and cerebral sinus enlargement (3) | T1- and T2-weighted MRI |
| Hu (2006) [12] | NR | Mixed | 23.5 (4/17) | TTTS group: IVH grade IV (1); demise with an unknown cause: IVH grade I (2), porencephalic findings (1) | T1- and T2-weighted MRI |
| First Author (Year) | Age at MRI (Weeks) | Population | Incidence of Cerebral Injury in MC Twins (%, n/N) | Cerebral Injury (Number of Observations) | MRI Sequence |
|---|---|---|---|---|---|
| Anh (2022) [18] | At birth and 3 and 6 months after birth | TTTS | 0 (0/21) | Normal MRI | NR |
| van Klink (2015) [10] | NR | sIUFD | 23.4 (11/47) | Ischemic injury (3), IVH grade II (1), IVH grade III-IV (3), PVL grade III (3), severe cerebral atrophy (1) | T1- and T2-weighted MRI and DWI |
| O’Donoghue (2009) [13] | NR | sIUFD ø | 7.7 (8/104) | Selective reduction: focal hemorrhage (1), moderate VM with aqueduct stenosis; demise with an unknown cause: MCA infarct (1), focal hemorrhage (1), mild VM (1), bilateral ischemic injury basal ganglia (1), widespread cystic lesions (1), injury of the periventricular white matter and basal ganglia and infarct in Sylvian fissure (1) | T1- and T2-weighted MRI |
| First Author (Year) | Population | Incidence of Severe Cerebral Injury (%, n/N) | Severe Cerebral Injury (Number of Observations) | Neurological Outcome |
|---|---|---|---|---|
| Shinar (2022) [14] | sIUFD | N/A | NR | NR |
| Rosen (2022) [15] | TAPS | 6.5 (3/46) | Focal restricted diffusion in right thalamus and chronic ischemic changes in frontoparietal areas (1, A). Chronic ischemic changes in frontoparietal areas accompanied by frontal lobe atrophy (1, B), cerebellar hemorrhage (1, C) | A and B: TOP |
| Gebb (2022) [16] | sIUFD | 5.0 (2/40) | Diffuse PMG with primitive sulcation and decreased parenchyma posteriorly (1, A), cerebral malformation with PMG, and schizencephaly (1, B) | A: normal development at 4 months B: global developmental delay, seizures C: epilepsy, developmental delay, and right hemiparesis |
| Segev (2022) [8] | sIUFD | 0.0 (0/29) | 0 | N/A |
| Moradi (2022) [17] | sIUFD | 4.65 (2/43) | Extensive cerebral ischemia (2) | NR |
| Anh (2022) [18] | TTTS | 0 (0/21) | 0 | N/A |
| Halevy (2021) [19] | Uncomplicated | N/A | NR | NR |
| Hochberg (2021) [20] | TTTS | 2.0 (3/153) | Porencephalic cyst (1), IVH grade III (1), unilateral ventriculomegaly with deviation of midline (1) | NR |
| Aertsen (2021) [21] | TTTS | 2.4 (3/125) | Focal PMG (3) | Cerebral palsy at 5 years (1), TOP (1), and unknown (1) |
| Kocaoglu (2020) [7] | TTTS | 0.8 (3/366) | GMH grade III (1) and GMH grade IV (2) | NR |
| Stirnemann (2018) [22] | TTTS | 2.2 (16/720) | Bilateral leukomalacia (2), bilateral polymicrogyria (1), severe ischemic injury (8), Unilateral schizencephaly (1), severe ventriculomegaly (4) | NR |
| Conte (2018) [23] | sIUFD | N/A | NR | NR |
| Robinson (2017) [24] | Mixed | 13.2 (5/38) | IVH, delayed sulcation, BPD< 5th percentile and bilateral VM (1, A), bilateral occipital cortical infarction with polymicrogyria, cystic lesions (1, B), encephalomalacia (2, C), severe abnormal sulcation (1, D) | A: TOP B: developmental delay C: TOP (1) and palliative care (1) D: developmental delay at 6 months and died at 12 months |
| Van Klink (2015) [10] | sIUFD | 4/50 (8%) * 8/28 (28.6%) ** | Fetal MRI: MCA infarction (1, A), bilateral MCA infarction (1, B), multicystic encephalopathy (1, C), severe cerebral atrophy (1, D) Neonatal MRI: cPVL grade III (1), unilateral IVH grade II with infarction caudate nucleus (1), multicystic encephalopathy (2), diffuse cortical necrosis (3), unknown (1) | Fetal MRI group: TOP (2) and survival (2) Neonatal MRI group: neonatal death (4) and survival (4) |
| Jatzko (2015) [25] | sIUFD | 36.4 (4/11) | IVH grade III (2, A), closed schizencephaly and several small parenchymal hemorrhages (1, B), cysts lateral to ganglionic eminence and mild VM (1, C) | A: TOP (1) and neonatal death 2 days after birth (1) B: cerebral palsy at 5 years C: normal clinical assessment at 1 year |
| Griffiths (2015) [26] | sIUFD | 8.8 (6/68) | Focal infarction with PMG (2, A), micrencephaly with extensive encephalomalacia (3, B), and extensive encephalomalacia (1, C) | A: live birth at 38 weeks (1) and stillbirth at 32 weeks (1) B: neonatal death (1), TOP (1), and live birth at 38 weeks (1) C: extensive encephalomalacia: stillbirth at 28 weeks |
| Weisz (2014) [27] | TTTS | N/A | NR | NR |
| Hoffmann (2013) [28] | sIUFD | 14.7 (5/34) | Severe ischemic injury (2, A), several temporal lobe and periventricular infarcts (1, B), bilateral periventricular hemorrhage (1, C), bilateral cerebral ischemia (1, D) | A: TOP (1) and motor deficiencies at 1 year (1) B, C, and D: TOP |
| Fichera (2009) [29] | sIUFD | 0.0 (0/13) | 0 | N/A |
| O’Donoghue (2009) [13] | sIUFD | 1.3 (1/76) * 5.3 (4/76) ** | Fetal MRI: MCA infarction (1, A) Neonatal MRI: MCA infarction (1, B), bilateral ischemic injury in basal ganglia (1, C), widespread cystic lesions (1,D), injury of the periventricular white matter and basal ganglia and infarct in Sylvian fissure (1, E) | A: mild hemiplegia B: mild hemiplegia C: mild bilateral dystonic hemiplegia D: severe neurodevelopmental abnormality E: normal at 1 year |
| Jelin (2008) [30] | sIUFD | 14.3 (3/21) | Unilateral infarct with developing PMG (1), severe destruction of supratentorial brain (1), hemorrhage bilateral choroid plexus and posterior fossa and subarachnoid hemorrhage adjacent to cerebellum (1) | NR |
| Kline-Fath (2007) [31] | TTTS | 4.2 (2/48) | Cerebral malformation (2) | NR |
| Hu (2006) [12] | Mixed | 11.8 (2/17) | IVH grade IV (1), porencephalic findings (1) | NR |
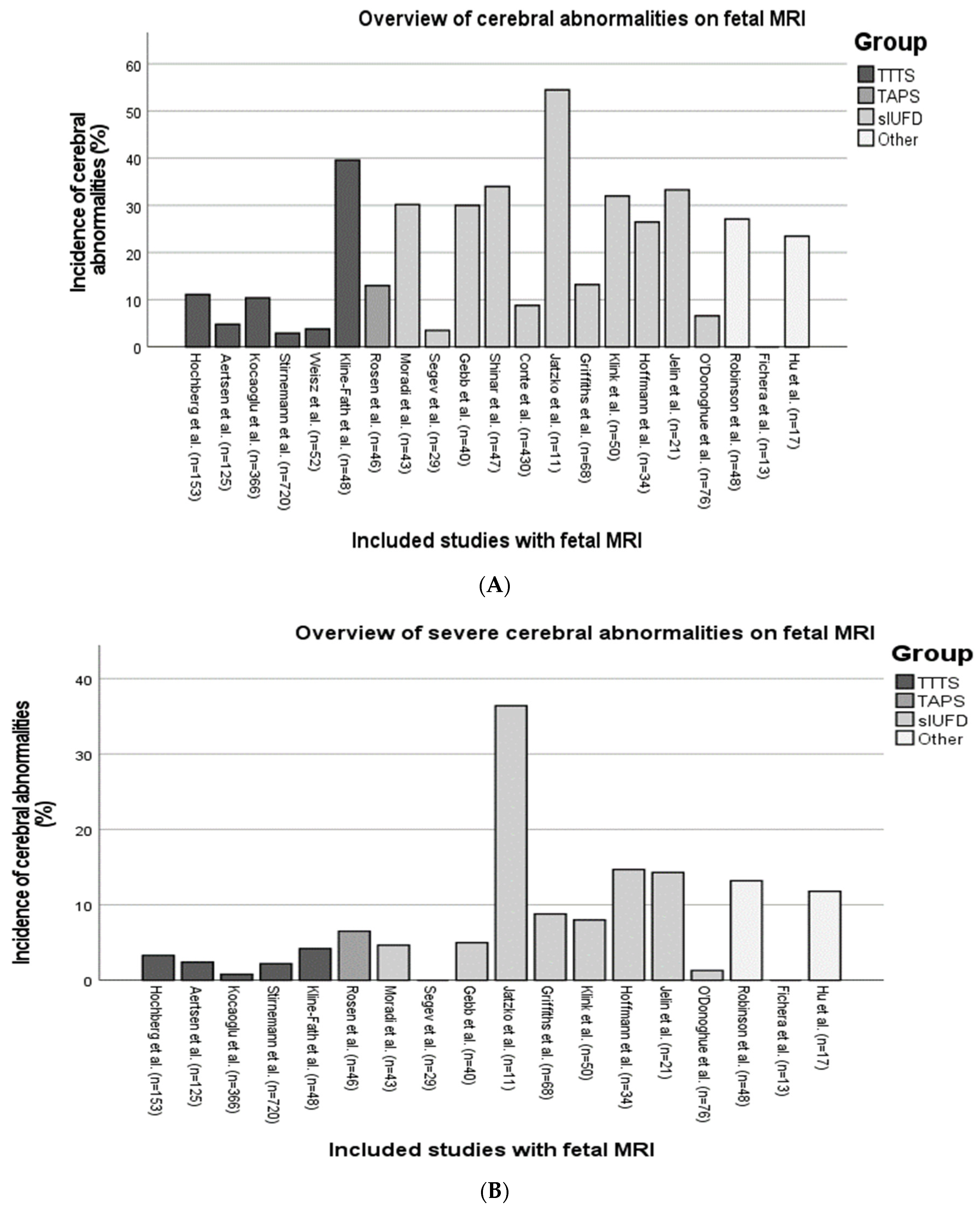
3.2. Fetal MRI Findings in MC Twins
3.2.1. Twin–Twin Transfusion Syndrome
3.2.2. Twin Anemia Polycythemia Sequence
3.2.3. Selective Fetal Growth Restriction
3.2.4. Single Intrauterine Fetal Demise
3.2.5. Other Complications
3.3. Neonatal MRI Findings in MC Twins
3.4. Structural Brain Development in MC Twins
3.5. Timing, Sequences Used, and Field Strength of the MRI
4. Discussion
4.1. Key Findings
4.2. Strengths and Limitations of This Study
4.3. Interpretation of Findings
4.3.1. Twin–Twin Transfusion Syndrome
4.3.2. Twin Anemia Polycythemia Sequence
4.3.3. Selective Fetal Growth Restriction
4.3.4. Single Intrauterine Fetal Demise
4.3.5. Structural Brain Development
4.4. Implications for Clinical Practice and Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Cochrane
- (“Monozygotic Twin” OR “Monozygotic Twins” OR “Identical Twin” OR “Identical Twins” OR “Monochorionic”):ti,ab,kw AND (“MRI” OR “Magnetic Resonance Imaging” OR “Diffusion-weighted imaging” OR “DWI MRI” OR “fMRI”):ti,ab,kw
- 07-03-2023: 7 results
- Embase
- (exp monozygotic twins/OR “Monozygotic Twin”.ti,ab. OR “Monozygotic Twins”.ti,ab. OR “Identical Twin”.ti,ab. OR “Identical Twins”.ti,ab. OR “Monochorionic”.ti,ab.) AND (exp Magnetic Resonance Imaging/OR “Magnetic Resonance Imaging”.ti,ab. OR “MRI”.ti,ab. OR “Diffusion-weighted imaging”.ti,ab. OR “DWI MRI”.ti,ab. OR “fMRI”.ti,ab.) NOT (conference OR conference abstract OR “conference review”).pt. AND 2000:2024.(sa_year).
- 07-03-2023: 940 results
- PubMed
- (“Twins, Monozygotic” [Mesh] OR “Monozygotic Twin” [tw] OR “Monozygotic Twins” [tw] OR “Identical Twin” [tw] OR “Identical Twins” [tw] OR “Monochorionic” [tw]) AND (“Magnetic Resonance Imaging” [Mesh] OR “Magnetic Resonance Imaging” [tw] OR “MRI” [tw] OR “Diffusion-weighted imaging” OR “DWI MRI” [tw] OR “fMRI” [tw]) AND (“2000”[Date-Publication]: “3000”[Date-Publication])
- 07-03-2023: 627 results
- Web of Science
- TS = (“Monozygotic Twin” OR “Monozygotic Twins” OR “Identical Twin” OR “Identical Twins” OR “Monochorionic”) AND TS = (“Magnetic Resonance Imaging” OR “MRI” OR “Diffusion-weighted imaging” OR “DWI MRI” OR “fMRI”) AND PY = (2000-2024)
- 07-03-2023: 479 results
Appendix B
- Newcastle–Ottawa Scale
- Selection
- (1)
- Representativeness of the exposed cohort
- (a)
- Truly representative (one star)
- (b)
- Somewhat representative (one star)
- (c)
- Selected group
- (d)
- No description of the derivation of the cohort
- (2)
- Selection of the non-exposed cohort
- (a)
- Drawn from the same community as the exposed cohort (one star)
- (b)
- Drawn from a different source
- (c)
- No description of the derivation of the non-exposed cohort
- (3)
- Ascertainment of exposure
- (a)
- Secure record (e.g., surgical record) (one star)
- (b)
- Structured interview (one star)
- (c)
- Written self-report
- (d)
- No description
- (e)
- Other
- (4)
- Demonstration that outcome of interest was not present at the start of the study
- (a)
- Yes (one star)
- (b)
- No
- Comparability (tick one or both boxes, as appropriate)
- (1)
- Comparability of cohorts on the basis of the design or analysis controlled for confounders
- (a)
- The study controls for age, sex, and marital status (one star)
- (b)
- The study controls for other factors (list) ___________ (one star)
- (c)
- Cohorts are not comparable on the basis of the design or analysis controlled for confounders
- Outcome
- (1)
- Assessment of outcome
- (a)
- Independent blind assessment (one star)
- (b)
- Record linkage (one star)
- (c)
- Self-report
- (d)
- No description
- (e)
- Other
- (2)
- Was follow-up long enough for outcomes to occur
- (a)
- Yes (one star)
- (b)
- No
- (3)
- Adequacy of follow-up of cohorts
- (a)
- Complete follow-up—all subjects accounted for (one star)
- (b)
- Subjects lost to follow-up, unlikely to introduce bias—number lost less than or equal to 20% or description of those lost suggested no different from those followed (one star)
- (c)
- Follow-up rate of less than 80% and no description of those lost
- (d)
- No statement
- Thresholds for converting the Newcastle–Ottawa scales to AHRQ standards (good, fair, and poor):
- Good quality: three or four stars in the selection domain AND one or two stars in the comparability domain AND two or three stars in the outcome/exposure domain.
- Fair quality: two stars in the selection domain AND one or two stars in the comparability domain AND two or three stars in the outcome/exposure domain.
- Poor quality: 0 or 1 star(s) in the selection domain OR 0 stars in the comparability domain OR 0 or 1 star(s) in the outcome/exposure domain.
Appendix C
| First Author (Year of Publication) | Selection | Comparability | Outcome | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness (*) | Non-Exposed Cohort (*) | Exposure (*) | Outcome of Interest (*) | Comparability of Cohorts (**) | Assessment (*) | Follow-Up (*) | Adequacy (*) | ||
| Shinar et al. (2022) [14] | * | * | * | - | ** | * | * | - | 7/9 |
| Rosen et al. (2022) [15] | * | * | * | * | ** | * | * | * | 9/9 |
| Gebb et al. (2022) [16] | * | * | * | - | ** | * | * | * | 8/9 |
| Segev et al. (2022) [6] | * | * | - | * | ** | * | * | * | 8/9 |
| Moradi et al. (2022) [17] | * | * | - | - | ** | * | * | * | 7/9 |
| Anh et al. (2022) [18] | * | * | * | * | ** | * | * | * | 9/9 |
| Halevy et al. (2021) [19] | * | * | * | * | ** | * | * | - | 8/9 |
| Hochberg et al. (2021) [20] | * | * | * | - | ** | * | * | * | 8/9 |
| Aertsen et al. (2021) [21] | * | * | * | * | ** | * | * | * | 9/9 |
| Kocaoglu et al. (2020) [7] | * | * | * | - | ** | * | - | * | 7/9 |
| Stirnemann et al. (2018) [22] | * | * | - | * | ** | * | * | * | 8/9 |
| Conte et al. (2018) [23] | * | * | * | - | ** | * | * | * | 8/9 |
| Robinson et al. (2017) [24] | - | * | * | - | ** | * | - | * | 6/9 |
| van Klink et al. (2015) [10] | * | * | * | - | ** | * | * | * | 8/9 |
| Jatzko et al. (2015) [25] | - | * | * | * | ** | * | * | - | 7/9 |
| Griffiths et al. (2015) [26] | * | - | * | - | * | * | * | * | 6/9 |
| Weisz et al. (2014) [27] | * | * | * | - | ** | * | * | * | 8/9 |
| Hoffmann et al. (2013) [28] | * | * | - | * | * | * | * | * | 7/9 |
| O’Donoghue et al. (2009) [13] | * | * | * | - | ** | * | * | - | 7/9 |
| Fichera et al. (2009) [29] | * | * | * | - | ** | * | - | * | 7/9 |
| Jelin et al. (2008) [30] | * | * | * | - | * | * | - | * | 6/9 |
| Kline-Fath et al. (2007) [31] | * | * | * | - | ** | * | - | * | 7/9 |
| Hu et al. (2006) [12] | - | * | * | - | * | * | - | * | 5/9 |
References
- Lopriore, E.; Lewi, L.; Khalil, A. Monochorionic Twins: A Delicate Balance. J. Clin. Med. 2019, 8, 1711. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, N.C.; Sparks, T.N.; Gosnell, K.A.; Rand, L.; Gonzalez, J.M.; Feldstein, V.A. Outcomes of Monochorionic, Diamniotic Twin Pregnancies with Prenatally Diagnosed Intertwin Weight Discordance. Am. J. Perinatol. 2021, 38, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Tollenaar, L.S.A.; Slaghekke, F.; van Klink, J.M.M.; Groene, S.G.; Middeldorp, J.M.; Haak, M.C.; Klumper, F.; Oepkes, D.; Lopriore, E. Twin-Twin Transfusion Syndrome with Anemia-Polycythemia: Prevalence, Characteristics, and Outcome. J. Clin. Med. 2019, 8, 1129. [Google Scholar] [CrossRef]
- Chambon, E.; Hachem, T.; Salvador, E.; Rigourd, V.; Bellanger, C.; Stirnemann, J.; Kermorvant-Duchemin, E.; Tissieres, P.; Ville, Y.; Lapillonne, A. Neonatal Hemodynamic Characteristics of the Recipient Twin of Twin-To-Twin Transfusion Syndrome Not Treated with Fetoscopic Laser Surgery. Children 2022, 9, 1766. [Google Scholar] [CrossRef] [PubMed]
- Hecher, K. Intrauterine surgery: How far we have come in 30 years. Ultrasound Obstet. Gynecol. 2021, 57, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Van Der Veeken, L.; Couck, I.; Van Der Merwe, J.; De Catte, L.; Devlieger, R.; Deprest, J.; Lewi, L. Laser for twin-to-twin transfusion syndrome: A guide for endoscopic surgeons. Facts Views Vis. Obgyn 2019, 11, 197–205. [Google Scholar] [PubMed]
- Kocaoglu, M.; Kline-Fath, B.M.; Calvo-Garcia, M.A.; Zhang, B.; Nagaraj, U.D. Magnetic resonance imaging of the fetal brain in monochorionic diamniotic twin gestation: Correlation of cerebral injury with ultrasound staging and survival outcomes. Pediatr. Radiol. 2020, 50, 1131–1138. [Google Scholar] [CrossRef]
- Segev, M.; Djurabayev, B.; Hadi, E.; Yinon, Y.; Rabinowicz, S.; Hoffmann, C.; Shrot, S. Third Trimester Structural and Diffusion Brain Imaging after Single Intrauterine Fetal Death in Monochorionic Twins: MRI-Based Cohort Study. AJNR Am. J. Neuroradiol. 2022, 43, 620–626. [Google Scholar] [CrossRef]
- Jarvis, D.; Mooney, C.; Cohen, J.; Papaioannou, D.; Bradburn, M.; Sutton, A.; Griffiths, P.D. A systematic review and meta-analysis to determine the contribution of mr imaging to the diagnosis of foetal brain abnormalities In Utero. Eur. Radiol. 2017, 27, 2367–2380. [Google Scholar] [CrossRef]
- van Klink, J.M.; van Steenis, A.; Steggerda, S.J.; Genova, L.; Sueters, M.; Oepkes, D.; Lopriore, E. Single fetal demise in monochorionic pregnancies: Incidence and patterns of cerebral injury. Ultrasound Obstet. Gynecol. 2015, 45, 294–300. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2013. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 7 March 2023).
- Hu, L.S.; Caire, J.; Twickler, D.M. MR findings of complicated multifetal gestations. Pediatr. Radiol. 2006, 36, 76–81. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, K.; Rutherford, M.A.; Engineer, N.; Wimalasundera, R.C.; Cowan, F.M.; Fisk, N.M. Transfusional fetal complications after single intrauterine death in monochorionic multiple pregnancy are reduced but not prevented by vascular occlusion. BJOG 2009, 116, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Shinar, S.; Harris, K.; Van Mieghem, T.; Lewi, L.; Morency, A.M.; Blaser, S.; Ryan, G. Early imaging predictors of fetal cerebral ischemic injury in monochorionic twin pregnancy complicated by spontaneous single intrauterine death. Ultrasound Obstet. Gynecol. 2022, 59, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.; Silber, R.; Schwartz, A.; Avnet, H.; Lipitz, S.; Shrot, S.; Hoffmann, C.; Weisz, B.; Yinon, Y. Fetal and neonatal brain injury in twins complicated by twin anemia polycythemia sequence. Prenat. Diagn. 2022, 42, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Gebb, J.; Hwang, R.; Paidas Teefey, C.; Soni, S.; Coleman, B.G.; Zarnow, D.M.; Moldenhauer, J.S.; Khalek, N. Magnetic resonance neuroimaging after laser for twin-twin transfusion syndrome with single fetal demise. Am. J. Obstet. Gynecol. 2022, 226, 728.e1–728.e8. [Google Scholar] [CrossRef]
- Moradi, B.; Badraqe, N.; Rahimi Sharbaf, F.; Firouznia, K.; Shirazi, M.; Kazemi, M.A.; Rahimi, R. Early detection of ischemic brain injuries by diffusion-weighted imaging after radiofrequency ablation for fetal reduction in monochorionic pregnancies. J. Clin. Ultrasound 2022, 50, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Anh, N.D.; Hung, H.S.; Sim, N.T.; Ha, N.T.T.; Nguyen, D.L.; Bac, N.D.; Tong, H.V.; Ville, Y.; Thuong, P.T.H. Fetoscopic Laser Ablation for the Selective Fetal Reduction in Twin-Twin Transfusion Syndrome Stage II-IV: The Experience of a New Fetal Medicine Center. Int. J. Womens Health 2022, 14, 555–563. [Google Scholar] [CrossRef]
- Halevy, T.; Nezer, M.; Halevy, J.; Ziv-Baran, T.; Barzilay, E.; Katorza, E. Twin discordance: A study of volumetric fetal brain MRI and neurodevelopmental outcome. Eur. Radiol. 2021, 31, 6676–6685. [Google Scholar] [CrossRef]
- Hochberg, A.; Silber, R.; Avnet, H.; Rosen, H.; Katorza, E.; Hoffmann, C.; Mazkereth, R.; Lipitz, S.; Weisz, B.; Yinon, Y. Fetal and neonatal brain lesions following laser ablation for twin-to-twin-transfusion-syndrome as detected by pre- and post-natal brain imaging. Prenat. Diagn. 2021, 41, 1531–1540. [Google Scholar] [CrossRef]
- Aertsen, M.; Berghe, C.V.T.D.T.; Deneckere, S.; Couck, I.; De Catte, L.; Lewi, L. The prevalence of brain lesions after in utero surgery for twin-to-twin transfusion syndrome on third-trimester MRI: A retrospective cohort study. Eur. Radiol. 2021, 31, 4097–4103. [Google Scholar] [CrossRef]
- Stirnemann, J.; Chalouhi, G.; Essaoui, M.; Bahi-Buisson, N.; Sonigo, P.; Millischer, A.E.; Lapillonne, A.; Guigue, V.; Salomon, L.J.; Ville, Y. Fetal brain imaging following laser surgery in twin-to-twin surgery. BJOG 2018, 125, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Conte, G.; Righini, A.; Griffiths, P.D.; Rustico, M.; Lanna, M.; Mackie, F.L.; Pinelli, L.; Prefumo, F.; Persico, N.; Igra, M.S.; et al. Brain-injured Survivors of Monochorionic Twin Pregnancies Complicated by Single Intrauterine Death: MR Findings in a Multicenter Study. Radiology 2018, 288, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Teoh, M.; Edwards, A.; Fahey, M.; Goergen, S. Fetal brain injury in complicated monochorionic pregnancies: Diagnostic yield of prenatal MRI following surveillance ultrasound and influence on prognostic counselling. Prenat. Diagn. 2017, 37, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Jatzko, B.; Rittenschober-Böhm, J.; Mailath-Pokorny, M.; Worda, C.; Prayer, D.; Kasprian, G.; Worda, K. Cerebral Lesions at Fetal Magnetic Resonance Imaging and Neurologic Outcome After Single Fetal Death in Monochorionic Twins. Twin Res. Hum. Genet. 2015, 18, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.D.; Sharrack, S.; Chan, K.L.; Bamfo, J.; Williams, F.; Kilby, M.D. Fetal brain injury in survivors of twin pregnancies complicated by demise of one twin as assessed by in utero MR imaging. Prenat. Diagn. 2015, 35, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Weisz, B.; Hoffmann, C.; Ben-Baruch, S.; Yinon, Y.; Gindes, L.; Katorza, E.; Shrim, A.; Bar Yosef, O.; Schiff, E.; Lipitz, S. Early detection by diffusion-weighted sequence magnetic resonance imaging of severe brain lesions after fetoscopic laser coagulation for twin-twin transfusion syndrome. Ultrasound Obstet. Gynecol. 2014, 44, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Weisz, B.; Yinon, Y.; Hogen, L.; Gindes, L.; Shrim, A.; Sivan, E.; Schiff, E.; Lipitz, S. Diffusion MRI findings in monochorionic twin pregnancies after intrauterine fetal death. AJNR Am. J. Neuroradiol. 2013, 34, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Fichera, A.; Zambolo, C.; Accorsi, P.; Martelli, P.; Ambrosi, C.; Frusca, T. Perinatal outcome and neurological follow up of the cotwins in twin pregnancies complicated by single intrauterine death. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 147, 37–40. [Google Scholar] [CrossRef]
- Jelin, A.C.; Norton, M.E.; Bartha, A.I.; Fick, A.L.; Glenn, O.A. Intracranial magnetic resonance imaging findings in the surviving fetus after spontaneous monochorionic cotwin demise. Am. J. Obstet. Gynecol. 2008, 199, 398.e1–398.e5. [Google Scholar] [CrossRef]
- Kline-Fath, B.M.; Calvo-Garcia, M.A.; O’Hara, S.M.; Crombleholme, T.M.; Racadio, J.M. Twin-twin transfusion syndrome: Cerebral ischemia is not the only fetal MR imaging finding. Pediatr. Radiol. 2007, 37, 47–56. [Google Scholar] [CrossRef]
- Stirnemann, J.; Slaghekke, F.; Khalek, N.; Winer, N.; Johnson, A.; Lewi, L.; Massoud, M.; Bussieres, L.; Aegerter, P.; Hecher, K.; et al. Intrauterine fetoscopic laser surgery versus expectant management in stage 1 twin-to-twin transfusion syndrome: An international randomized trial. Am. J. Obstet. Gynecol. 2021, 224, 528.e1–528.e12. [Google Scholar] [CrossRef] [PubMed]
- Spruijt, M.S.; Lopriore, E.; Tan, R.; Slaghekke, F.; Klumper, F.; Middeldorp, J.M.; Haak, M.C.; Oepkes, D.; Rijken, M.; van Klink, J.M.M. Long-Term Neurodevelopmental Outcome in Twin-to-Twin Transfusion Syndrome: Is there still Room for Improvement? J. Clin. Med. 2019, 8, 1226. [Google Scholar] [CrossRef] [PubMed]
- Gijtenbeek, M.; Haak, M.C.; Huberts, T.J.P.; Middeldorp, J.M.; Klumper, F.; Slaghekke, F.; Lopriore, E.; Oepkes, D.; van Klink, J.M.M. Perioperative fetal hemodynamic changes in twin-twin transfusion syndrome and neurodevelopmental outcome at two years of age. Prenat. Diagn. 2020, 40, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Tollenaar, L.S.A.; Lopriore, E.; Slaghekke, F.; Oepkes, D.; Middeldorp, J.M.; Haak, M.C.; Klumper, F.; Tan, R.; Rijken, M.; Van Klink, J.M.M. High risk of long-term neurodevelopmental impairment in donor twins with spontaneous twin anemia-polycythemia sequence. Ultrasound Obstet. Gynecol. 2020, 55, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Soni, A.; Kanaujia, S.K.; Kaushik, S. Brainstem Evoked Response Audiometry (BERA) in Neonates with Hyperbillirubinemia. Indian J. Otolaryngol. Head Neck Surg. 2016, 68, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Inklaar, M.J.; van Klink, J.M.M.; Stolk, T.T.; van Zwet, E.W.; Oepkes, D.; Lopriore, E. Cerebral injury in monochorionic twins with selective intrauterine growth restriction: A systematic review. Prenat. Diagn. 2014, 34, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Gratacós, E.; Lewi, L.; Muñoz, B.; Acosta-Rojas, R.; Hernandez-Andrade, E.; Martinez, J.M.; Carreras, E.; Deprest, J. A classification system for selective intrauterine growth restriction in monochorionic pregnancies according to umbilical artery Doppler flow in the smaller twin. Ultrasound Obstet. Gynecol. 2007, 30, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Groene, S.G.; de Vries, L.S.; Slaghekke, F.; Haak, M.C.; Heijmans, B.T.; de Bruin, C.; Roest, A.A.W.; Lopriore, E.; van Klink, J.M.M.; Steggerda, S.J. Changes in structural brain development after selective fetal growth restriction in monochorionic twins. Ultrasound Obstet. Gynecol. 2022, 59, 747–755. [Google Scholar] [CrossRef]
- Hill, K.M.; Masoudian, P.; Fung-Kee-Fung, K.; El Demellawy, D. Intrauterine Interventions for the Treatment of Twin Anemia-Polycythemia Sequence: A Systematic Review. J. Obstet. Gynaecol. Can. 2019, 41, 981–991. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondagh, M.; Lopriore, E.; de Vries, L.S.; Slaghekke, F.; Tollenaar, L.S.A.; van Klink, J.M.M.; Groene, S.G.; Steggerda, S.J. The Current Knowledge of Cerebral Magnetic Resonance Imaging in Monochorionic Twins: A Systematic Review of the Last 20 Years. J. Clin. Med. 2023, 12, 7211. https://doi.org/10.3390/jcm12237211
Rondagh M, Lopriore E, de Vries LS, Slaghekke F, Tollenaar LSA, van Klink JMM, Groene SG, Steggerda SJ. The Current Knowledge of Cerebral Magnetic Resonance Imaging in Monochorionic Twins: A Systematic Review of the Last 20 Years. Journal of Clinical Medicine. 2023; 12(23):7211. https://doi.org/10.3390/jcm12237211
Chicago/Turabian StyleRondagh, Mathies, Enrico Lopriore, Linda S. de Vries, Femke Slaghekke, Lisanne S. A. Tollenaar, Jeanine M. M. van Klink, Sophie G. Groene, and Sylke J. Steggerda. 2023. "The Current Knowledge of Cerebral Magnetic Resonance Imaging in Monochorionic Twins: A Systematic Review of the Last 20 Years" Journal of Clinical Medicine 12, no. 23: 7211. https://doi.org/10.3390/jcm12237211
APA StyleRondagh, M., Lopriore, E., de Vries, L. S., Slaghekke, F., Tollenaar, L. S. A., van Klink, J. M. M., Groene, S. G., & Steggerda, S. J. (2023). The Current Knowledge of Cerebral Magnetic Resonance Imaging in Monochorionic Twins: A Systematic Review of the Last 20 Years. Journal of Clinical Medicine, 12(23), 7211. https://doi.org/10.3390/jcm12237211







