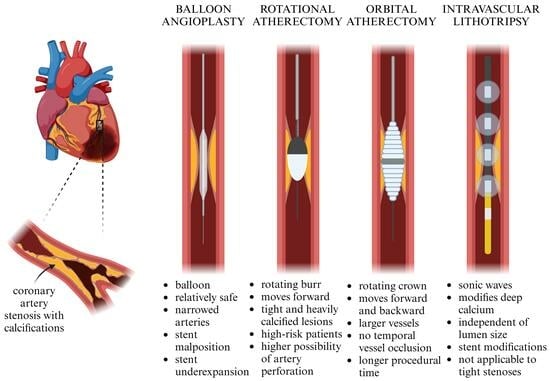
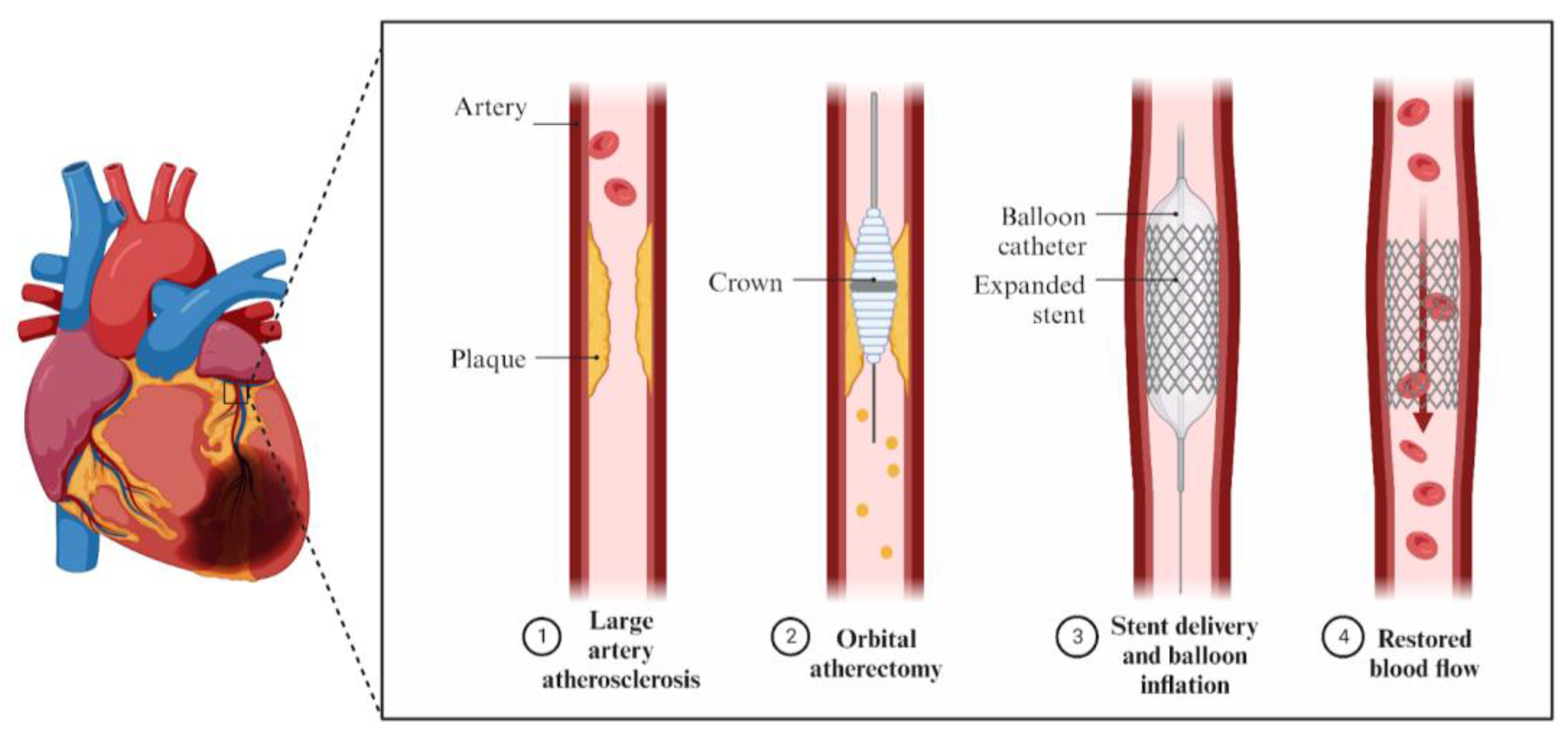
Rotational Atherectomy, Orbital Atherectomy, and Intravascular Lithotripsy Comparison for Calcified Coronary Lesions
Abstract
:1. Introduction
2. Mechanism of Action
3. Intravascular Imaging in RA, OA, and IVL
3.1. OCT Assessment in RA, OA, and IVL
3.2. OCT vs. IVUS in RA, OA, and IVL
4. Effectiveness
5. Periprocedural Complications and Short-Term Outcomes
6. Long-Term Outcomes
Double Antiplatlet Therapy in Complex PCI
7. Cost-Effectiveness
8. Conclusions
9. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madhavan, M.V.; Tarigopula, M.; Mintz, G.S.; Maehara, A.; Stone, G.W.; Généreux, P. Coronary Artery Calcification: Pathogenesis and Prognostic Implications. J. Am. Coll. Cardiol. 2014, 63, 1703–1714. [Google Scholar] [CrossRef]
- Kumar, P.; Bhatia, M. Coronary Artery Calcium Data and Reporting System (CAC-DRS): A Primer. J. Cardiovasc. Imaging 2023, 31, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zimoch, W.J.; Kubler, P.; Kosowski, M.; Tomasiewicz, B.; Krzysztofik, J.; Langner, A.; Jankowska, E.A.; Reczuch, K. Patients with Acute Myocardial Infarction and Severe Target Lesion Calcifications Undergoing Percutaneous Coronary Intervention Have Poor Long-Term Prognosis. Kardiol. Pol. 2017, 75, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Dweck, M.R.; Narula, N.; Pisapia, D.; Narula, J.; Strauss, H.W. Coronary Artery Calcification: From Mechanism to Molecular Imaging. JACC Cardiovasc. Imaging 2017, 10, 582–593. [Google Scholar] [CrossRef]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and Its Progression. JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef]
- Bass, T.A. High-Risk Percutaneous Coronary Interventions in Modern Day Clinical Practice. Circ. Cardiovasc. Interv. 2015, 8, e003405. [Google Scholar] [CrossRef]
- Vescovo, G.M.; Zivelonghi, C.; Scott, B.; Agostoni, P. Percutaneous Coronary Intervention for Chronic Total Occlusion. US Cardiol. Rev. 2020, 14, e11. [Google Scholar] [CrossRef]
- Aziz, S.; Ramsdale, D.R. Chronic Total Occlusions—The Challenge Continues. Eur. Cardiol. Rev. 2006, 2, 1–5. [Google Scholar] [CrossRef]
- Sakakura, K.; Ito, Y.; Shibata, Y.; Okamura, A.; Kashima, Y.; Nakamura, S.; Hamazaki, Y.; Ako, J.; Yokoi, H.; Kobayashi, Y.; et al. Clinical Expert Consensus Document on Rotational Atherectomy from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc. Interv. Ther. 2021, 36, 1–18. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Shlofmitz, R.; Lee, M.S. Orbital Atherectomy: A Comprehensive Review. Interv. Cardiol. Clin. 2019, 8, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Pursnani, S.; Korley, F.; Gopaul, R.; Kanade, P.; Chandra, N.; Shaw, R.E.; Bangalore, S. Percutaneous Coronary Intervention Versus Optimal Medical Therapy in Stable Coronary Artery Disease. Circ. Cardiovasc. Interv. 2012, 5, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Cuenza, L.R.; Jayme, A.C.; Sui, J.H.K. Clinical Outcomes of Patients Undergoing Rotational Atherectomy Followed by Drug-Eluting Stent Implantation: A Single-Center Real-World Experience. Heart Views 2017, 18, 115. [Google Scholar] [CrossRef]
- Ali, Z.A.; Brinton, T.J.; Hill, J.M.; Maehara, A.; Matsumura, M.; Karimi Galougahi, K.; Illindala, U.; Götberg, M.; Whitbourn, R.; Van Mieghem, N.; et al. Optical Coherence Tomography Characterization of Coronary Lithoplasty for Treatment of Calcified Lesions: First Description. JACC Cardiovasc. Imaging 2017, 10, 897–906. [Google Scholar] [CrossRef]
- Bhatt, P.; Parikh, P.; Patel, A.; Chag, M.; Chandarana, A.; Parikh, R.; Parikh, K. Orbital Atherectomy System in Treating Calcified Coronary Lesions: 3-Year Follow-up in First Human Use Study (ORBIT I Trial). Cardiovasc. Revascularization Med. 2014, 15, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Rotational vs. Orbital Atherectomy: How to Choose?|SCAI. Available online: https://scai.org/rotational-vs-orbital-atherectomy-how-choose?fbclid=IwAR2ctK1nNyVGILINLBXe6pSjS3XYaKgTNG3vTX4RtdemSIamBJJAdxqbSXc (accessed on 25 February 2023).
- Pietzsch, J.B.; Geisler, B.P.; Ikeno, F. Cost-Effectiveness of Orbital Atherectomy Compared to Rotational Atherectomy in Treating Patients with Severely Calcified Coronary Artery Lesions in Japan. Cardiovasc. Interv. Ther. 2018, 33, 328. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Kereiakes, D.J.; Shlofmitz, R.A.; Klein, A.J.; Riley, R.F.; Price, M.J.; Herrmann, H.C.; Bachinsky, W.; Waksman, R.; Stone, G.W. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 76, 2635–2646. [Google Scholar] [CrossRef]
- Chiang, C.S.M.; Alan Chan, K.C.; Lee, M.; Chan, K.T. Orbital-Tripsy: Novel Combination of Orbital-Atherectomy and Intravascular-Lithotripsy, in Calcified Coronaries after Failed Intravascular-Lithotripsy. JACC Case Rep. 2020, 2, 2437–2444. [Google Scholar] [CrossRef]
- Chen, G.; Zrenner, B.; Pyxaras, S.A. Combined Rotational Atherectomy and Intravascular Lithotripsy for the Treatment of Severely Calcified In-Stent Neoatherosclerosis: A Mini-Review. Cardiovasc. Revascularization Med. 2019, 20, 819–821. [Google Scholar] [CrossRef]
- Sharma, S.K.; Tomey, M.I.; Teirstein, P.S.; Kini, A.S.; Reitman, A.B.; Lee, A.C.; Généreux, P.; Chambers, J.W.; Grines, C.L.; Himmelstein, S.I.; et al. North American Expert Review of Rotational Atherectomy. Circ. Cardiovasc. Interv. 2019, 12, e007448. [Google Scholar] [CrossRef]
- Tomasiewicz, B.; Kubler, P.; Zimoch, W.; Kosowski, M.; Wańha, W.; Ładziński, S.; Rakotoarison, O.; Ochała, A.; Wojakowski, W.; Reczuch, K. Acute Angulation and Sequential Lesion Increase the Risk of Rotational Atherectomy Failure. Circ. J. 2021, 85, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Kübler, P.; Zimoch, W.; Kosowski, M.; Tomasiewicz, B.; Telichowski, A.; Reczuch, K. Acute Coronary Syndrome—Still a Valid Contraindication to Perform Rotational Atherectomy? Early and One-Year Outcomes. J. Cardiol. 2018, 71, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Reisman, M.; Shuman, B.J.; Harms, V. Analysis of Heat Generation during Rotational Atherectomy Using Different Operational Techniques. Cathet. Cardiovasc. Diagn. 1998, 44, 453–455. [Google Scholar] [CrossRef]
- Dhall, A.; Koshy, S.; Chouhan, N. Percutaneous Intervention of Calcific Coronary Stenosis. Available online: https://www.researchgate.net/publication/278786408_Percutaneous_Intervention_of_Calcific_Coronary_Stenosis (accessed on 2 October 2023).
- Gupta, T.; Weinreich, M.; Greenberg, M.; Colombo, A.; Latib, A. Rotational Atherectomy: A Contemporary Appraisal. Interv. Cardiol. Rev. 2019, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Barbato, E.; Carrié, D.; Dardas, P.; Fajadet, J.; Gaul, G.; Haude, M.; Khashaba, A.; Koch, K.; Meyer-Gessner, M.; Palazuelos, J.; et al. European Expert Consensus on Rotational Atherectomy. EuroIntervention 2015, 11, 30–36. [Google Scholar] [CrossRef]
- Sotomi, Y.; Shlofmitz, R.A.; Colombo, A.; Serruys, P.W.; Onuma, Y. Patient Selection and Procedural Considerations for Coronary Orbital Atherectomy System. Interv. Cardiol. Rev. 2016, 11, 33. [Google Scholar] [CrossRef]
- Dini, C.S.; Nardi, G.; Ristalli, F.; Mattesini, A.; Hamiti, B.; Di Mario, C. Contemporary Approach to Heavily Calcified Coronary Lesions. Interv. Cardiol. Rev. 2019, 14, 154–163. [Google Scholar] [CrossRef]
- Redfors, B.; Sharma, S.K.; Saito, S.; Kini, A.S.; Lee, A.C.; Moses, J.W.; Ali, Z.A.; Feldman, R.L.; Bhatheja, R.; Stone, G.W. Novel Micro Crown Orbital Atherectomy for Severe Lesion Calcification. Circ. Cardiovasc. Interv. 2020, 13, e008993. [Google Scholar] [CrossRef]
- Rola, P.; Kulczycki, J.J.; Barycki, M.; Włodarczak, S.; Furtan, Ł.; Kędzierska, M.; Giniewicz, K.; Doroszko, A.; Lesiak, M.; Włodarczak, A. Comparison of Orbital Atherectomy and Rotational Atherectomy in Calcified Left Main Disease: Short-Term Outcomes. J. Clin. Med. 2023, 12, 4025. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Martinsen, B.J.; Lee, M.; Rao, S.V.; Généreux, P.; Higgins, J.; Chambers, J.W.; Kirtane, A.J.; Brilakis, E.S.; Kandzari, D.E.; et al. Orbital Atherectomy for the Treatment of Severely Calcified Coronary Lesions: Evidence, Technique, and Best Practices. Expert Rev. Med. Devices 2017, 14, 867–879. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Chambers, J.; Moses, J.W.; Martinsen, B.; Meraj, P.; Jauhar, R.; Shlofmitz, R. TCT-389 Temporary Pacemaker Placement Incidence with the Diamondback 360® Coronary Orbital Atherectomy System Compared to Rotational Atherectomy. J. Am. Coll. Cardiol. 2015, 66, B157. [Google Scholar] [CrossRef]
- Wong, J.J.; Umapathy, S.; Keh, Y.S.; Lau, Y.H.; Yap, J.; Idu, M.; Chin, C.Y.; Fam, J.M.; Liew, B.W.; Chin, C.T.; et al. Coronary Intravascular Lithotripsy Versus Rotational Atherectomy in an Asian Population: Clinical Outcomes in Real-World Patients. Korean Circ. J. 2022, 52, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Egami, Y.; Nohara, H.; Kawanami, S.; Sugae, H.; Kawamura, A.; Ukita, K.; Matsuhiro, Y.; Nakamura, H.; Yasumoto, K.; et al. Direct Comparison of Rotational vs Orbital Atherectomy for Calcified Lesions Guided by Optical Coherence Tomography. JACC Cardiovasc. Interv. 2023, 16, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Wańha, W.; Tomaniak, M.; Wańczura, P.; Bil, J.; Januszek, R.; Wolny, R.; Opolski, M.P.; Kuźma, Ł.; Janas, A.; Figatowski, T.; et al. Intravascular Lithotripsy for the Treatment of Stent Underexpansion: The Multicenter IVL-DRAGON Registry. J. Clin. Med. 2022, 11, 1779. [Google Scholar] [CrossRef] [PubMed]
- Honton, B.; Monsegu, J. Best Practice in Intravascular Lithotripsy. Interv. Cardiol. Rev. Res. Resour. 2022, 17, e02. [Google Scholar] [CrossRef] [PubMed]
- Kimball, B.P.; Bui, S.; Cohen, E.A.; Carere, R.G.; Adelman, A.G. Early Experience with Directional Coronary Atherectomy: Documentation of the Learning Curve. Can. J. Cardiol. 1993, 9, 177–185. [Google Scholar]
- Ali, Z.A.; Maehara, A.; Généreux, P.; Shlofmitz, R.A.; Fabbiocchi, F.; Nazif, T.M.; Guagliumi, G.; Meraj, P.M.; Alfonso, F.; Samady, H.; et al. Optical Coherence Tomography Compared with Intravascular Ultrasound and with Angiography to Guide Coronary Stent Implantation (ILUMIEN III: OPTIMIZE PCI): A Randomised Controlled Trial. Lancet 2016, 388, 2618–2628. [Google Scholar] [CrossRef]
- Kaul, A.; Dhalla, P.S.; Bapatla, A.; Khalid, R.; Garcia, J.; Armenta-Quiroga, A.S.; Khan, S. Current Treatment Modalities for Calcified Coronary Artery Disease: A Review Article Comparing Novel Intravascular Lithotripsy and Traditional Rotational Atherectomy. Cureus 2020, 12, e10922. [Google Scholar] [CrossRef]
- Mattesini, A.; Nardi, G.; Martellini, A.; Sorini Dini, C.; Hamiti, B.; Stolcova, M.; Meucci, F.; Di Mario, C. Intravascular Imaging to Guide Lithotripsy in Concentric and Eccentric Calcific Coronary Lesions. Cardiovasc. Revascularization Med. 2020, 21, 1099–1105. [Google Scholar] [CrossRef]
- Shlofmitz, R.A.; Galougahi, K.K.; Jeremias, A.; Shlofmitz, E.; Thomas, S.V.; Ali, Z.A. Calcium Modification in Percutaneous Coronary Interventions. Interv. Cardiol. Clin. 2022, 11, 373–381. [Google Scholar] [CrossRef]
- Lee, M.S.; Shlofmitz, E.; Kong, J.; Lluri, G.; Srivastava, P.K.; Shlofmitz, R. Impact of the Use of Intravascular Imaging on Patients Who Underwent Orbital Atherectomy. J. Invasive Cardiol. 2018, 30, 77–80. [Google Scholar] [PubMed]
- Sandhyavenu, H.; Ullah, W.; Badu, I.; Zghouzi, M.; Baqal, O.; Ali, M.; Mir, T.; Minhas, A.M.K.; Johnson, D.; Virani, S.S.; et al. Outcomes of Intravascular Imaging in Orbital Atherectomy; Insight from the National Readmissions Database. Curr. Probl. Cardiol. 2023, 48, 101475. [Google Scholar] [CrossRef] [PubMed]
- Maehara, A.; Matsumura, M.; Ali, Z.A.; Mintz, G.S.; Stone, G.W. IVUS-Guided Versus OCT-Guided Coronary Stent Implantation. JACC Cardiovasc. Imaging 2017, 10, 1487–1503. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wu, H.; Kim, S.; Dai, X.; Jiang, X. Recent Advances in Transducers for Intravascular Ultrasound (IVUS) Imaging. Sensors 2021, 21, 3540. [Google Scholar] [CrossRef]
- de Donato, G.; Pasqui, E.; Alba, G.; Giannace, G.; Panzano, C.; Cappelli, A.; Setacci, C.; Palasciano, G. Clinical Considerations and Recommendations for OCT-Guided Carotid Artery Stenting. Expert Rev. Cardiovasc. Ther. 2020, 18, 219–229. [Google Scholar] [CrossRef]
- Aumann, S.; Donner, S.; Fischer, J.; Müller, F. Optical Coherence Tomography (OCT): Principle and Technical Realization. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 9783030166373. [Google Scholar]
- Cortese, B.; Piraino, D.; Gentile, D.; Onea, H.; Lazar, L. Intravascular Imaging for Left Main Stem Assessment: An Update on the Most Recent Clinical Data. Catheter. Cardiovasc. Interv. 2022, 100, 1220–1228. [Google Scholar] [CrossRef]
- Moulias, A.; Koros, R.; Papageorgiou, A.; Patrinos, P.; Spyropoulou, P.; Vakka, A.; Bozika, M.; Vasilagkos, G.; Apostolos, A.; Nastouli, K.-M.; et al. OCT Guidance in Bifurcation Percutaneous Coronary Intervention. Rev. Cardiovasc. Med. 2023, 24, 88. [Google Scholar] [CrossRef]
- Pawlowski, T.; Legutko, J.; Kochman, J.; Roleder, T.; Pregowski, J.; Chmielak, Z.; Kubica, J.; Ochala, A.; Parma, R.; Grygier, M.; et al. Clinical Use of Intracoronary Imaging Modalities in Poland. Expert Opinion of the Association of Cardiovascular Interventions of the Polish Cardiac Society. Kardiol. Pol. 2022, 80, 509–519. [Google Scholar] [CrossRef]
- Kini, A.S.; Vengrenyuk, Y.; Pena, J.; Motoyama, S.; Feig, J.E.; Meelu, O.A.; Rajamanickam, A.; Bhat, A.M.; Panwar, S.; Baber, U.; et al. Optical Coherence Tomography Assessment of the Mechanistic Effects of Rotational and Orbital Atherectomy in Severely Calcified Coronary Lesions. Catheter. Cardiovasc. Interv. 2015, 86, 1024–1032. [Google Scholar] [CrossRef]
- Blachutzik, F.; Meier, S.; Weissner, M.; Schlattner, S.; Gori, T.; Ullrich-Daub, H.; Gaede, L.; Achenbach, S.; Möllmann, H.; Chitic, B.; et al. Comparison of Coronary Intravascular Lithotripsy and Rotational Atherectomy in the Modification of Severely Calcified Stenoses. Am. J. Cardiol. 2023, 197, 93–100. [Google Scholar] [CrossRef]
- Nef, H.; Schlattner, S.; Weissner, M.; Gori, T.; Ullrich, H.; Gaede, L.; Achenbach, S.; Möllmann, H.; Blumenstein, J.; Aksoy, A.; et al. TCT-176 Randomized Comparison of Intracoronary Lithotripsy and Rotational Atherectomy for the Treatment of Severely Calcified Vessels—ROTA.Shock Trial. J. Am. Coll. Cardiol. 2022, 80, B71. [Google Scholar] [CrossRef]
- Januszek, R.; Siudak, Z.; Malinowski, K.P.; Wańha, W.; Surowiec, S.; Heba, G.; Pawlik, A.; Kameczura, T.; Wojakowski, W.; Jaguszewski, M.; et al. Factors Determining the Frequency of Optical Coherence Tomography and Intravascular Ultrasound Use in Patients Treated with Percutaneous Coronary Interventions in Recent Years: Analysis Based on a Large National Registry. Kardiol. Pol. 2023, 81, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Ito, Y.; Yamawaki, M.; Araki, M.; Obokata, M.; Sakamoto, Y.; Mori, S.; Tsutsumi, M.; Honda, Y.; Makino, K.; et al. Optical Coherence Tomography-Guided versus Intravascular Ultrasound-Guided Rotational Atherectomy in Patients with Calcified Coronary Lesions. EuroIntervention 2020, 16, e313–e321. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Li, Q.; Ma, Y.; Cao, C.; Liu, J.; Zhao, H.; Lu, M.; Hou, C.; Wang, W. Comparison of Optical Coherence Tomography-Guided and Intravascular Ultrasound-Guided Rotational Atherectomy for Calcified Coronary Lesions. BMC Cardiovasc. Disord. 2021, 21, 290. [Google Scholar] [CrossRef] [PubMed]
- Kurogi, K.; Ishii, M.; Ikebe, S.; Kaichi, R.; Mori, T.; Komaki, S.; Yamamoto, N.; Yamanaga, K.; Arima, Y.; Yamamoto, E.; et al. Optical Coherence Tomography—Versus Intravascular Ultrasound-Guided Stent Expansion in Calcified Lesions. Cardiovasc. Interv. Ther. 2022, 37, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Kirtane, A.J.; Kandzari, D.E.; Armstrong, E.J.; Krucoff, M.W.; Redfors, B.; Ben-Yehuda, O.; Lerew, D.R.; Ali, Z.A.; Maehara, A.; et al. Randomized Evaluation of Vessel Preparation with Orbital Atherectomy Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Artery Lesions: Design and Rationale of the ECLIPSE Trial. Am. Heart J. 2022, 249, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kereiakes, D.J.; Hill, J.M.; Shlofmitz, R.A.; Klein, A.J.; Riley, R.F.; Price, M.J.; Herrmann, H.C.; Bachinsky, W.; Waksman, R.; Stone, G.W. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Lesions: 1-Year Results from the Disrupt CAD III Study. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Mintz, G.S. Intravascular Imaging of Coronary Calcification and Its Clinical Implications. JACC Cardiovasc. Imaging 2015, 8, 461–471. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Richardt, G.; Joachim Büttner, H.; Toelg, R.; Geist, V.; Meinertz, T.; Schofer, J.; King, L.; Neumann, F.-J.; Khattab, A.A. High-Speed Rotational Atherectomy before Paclitaxel-Eluting Stent Implantation in Complex Calcified Coronary Lesions. JACC Cardiovasc. Interv. 2013, 6, 10–19. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Toelg, R.; Byrne, R.A.; Geist, V.; El-Mawardy, M.; Allali, A.; Rheude, T.; Robinson, D.R.; Abdelghani, M.; Sulimov, D.S.; et al. High-Speed Rotational Atherectomy Versus Modified Balloons Prior to Drug-Eluting Stent Implantation in Severely Calcified Coronary Lesions. Circ. Cardiovasc. Interv. 2018, 11, e007415. [Google Scholar] [CrossRef]
- Allali, A.; Abdel-Wahab, M.; Elbasha, K.; Mankerious, N.; Traboulsi, H.; Kastrati, A.; El-Mawardy, M.; Hemetsberger, R.; Sulimov, D.S.; Neumann, F.-J.; et al. Rotational Atherectomy of Calcified Coronary Lesions: Current Practice and Insights from Two Randomized Trials. Clin. Res. Cardiol. 2023, 112, 1143–1163. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.W.; Feldman, R.L.; Himmelstein, S.I.; Bhatheja, R.; Villa, A.E.; Strickman, N.E.; Shlofmitz, R.A.; Dulas, D.D.; Arab, D.; Khanna, P.K.; et al. Pivotal Trial to Evaluate the Safety and Efficacy of the Orbital Atherectomy System in Treating De Novo, Severely Calcified Coronary Lesions (ORBIT II). JACC Cardiovasc. Interv. 2014, 7, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Yamazaki, S.; Takahashi, A.; Namiki, A.; Kawasaki, T.; Otsuji, S.; Nakamura, S.; Shibata, Y. Intravascular Lithotripsy for Vessel Preparation in Calcified Coronary Arteries Prior to Stent Placement―Japanese Disrupt CAD IV Study 1-Year Results. Circ. Rep. 2022, 4, CR-22-0068. [Google Scholar] [CrossRef] [PubMed]
- Kereiakes, D.J.; Di Mario, C.; Riley, R.F.; Fajadet, J.; Shlofmitz, R.A.; Saito, S.; Ali, Z.A.; Klein, A.J.; Price, M.J.; Hill, J.M.; et al. Intravascular Lithotripsy for Treatment of Calcified Coronary Lesions. JACC Cardiovasc. Interv. 2021, 14, 1337–1348. [Google Scholar] [CrossRef]
- Matsuo, H.; Watanabe, S.; Watanabe, T.; Warita, S.; Kojima, T.; Hirose, T.; Iwama, M.; Ono, K.; Takahashi, H.; Segawa, T.; et al. Prevention of No-Reflow/Slow-Flow Phenomenon during Rotational Atherectomy—A Prospective Randomized Study Comparing Intracoronary Continuous Infusion of Verapamil and Nicorandil. Am. Heart J. 2007, 154, 994.e1–994.e6. [Google Scholar] [CrossRef]
- Goel, S.; Pasam, R.T.; Chava, S.; Gotesman, J.; Sharma, A.; Malik, B.A.; Frankel, R.; Shani, J.; Gidwani, U.; Latib, A. Orbital Atherectomy versus Rotational Atherectomy: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2020, 303, 16–21. [Google Scholar] [CrossRef]
- Brinton, T.J.; Ali, Z.A.; Hill, J.M.; Meredith, I.T.; Maehara, A.; Illindala, U.; Lansky, A.; Götberg, M.; Van Mieghem, N.M.; Whitbourn, R.; et al. Feasibility of Shockwave Coronary Intravascular Lithotripsy for the Treatment of Calcified Coronary Stenoses. Circulation 2019, 139, 834–836. [Google Scholar] [CrossRef]
- Derntl, M.; Weidinger, F. Managing No-Reflow during Percutaneous Coronary Intervention. Interv. Cardiol. 2012, 4, 461–472. [Google Scholar] [CrossRef]
- Kübler, P.; Zimoch, W.; Kosowski, M.; Tomasiewicz, B.; Rakotoarison, O.; Telichowski, A.; Reczuch, K. The Use of Rotational Atherectomy in High-Risk Patients: Results from a High-Volume Centre. Kardiol. Pol. 2018, 76, 1360–1368. [Google Scholar] [CrossRef]
- Valdes, P.J.; Nagalli, S.; Diaz, M.A. Rotational Atherectomy. In StatPearls; NCBI: Bethesda, MD, USA, 2023. [Google Scholar]
- Gorol, L.J.; Katedra, I.; Kardiologii, O.K.; Gorol, J.; Tajstra, M.; Gąsior, M.; Lekston, A. Rotational Atherectomy-Renaissance of the Technique. Chor. Serca I Naczyń 2018, 15, 29–35. [Google Scholar]
- Sakakura, K.; Ako, J.; Wada, H.; Naito, R.; Funayama, H.; Arao, K.; Kubo, N.; Momomura, S. Comparison of Frequency of Complications With On-Label Versus Off-Label Use of Rotational Atherectomy. Am. J. Cardiol. 2012, 110, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Latib, A.; Ruparelia, N.; Ielasi, A.; D’Ascenzo, F.; Pennacchi, M.; Sardella, G.; Garbo, R.; Meliga, E.; Moretti, C.; et al. In-Hospital and Midterm Clinical Outcomes of Rotational Atherectomy Followed by Stent Implantation: The ROTATE Multicentre Registry. EuroIntervention 2016, 12, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, M.; Baev, R.; Dieker, P.; Kassner, G.; Khattab, A.A.; Toelg, R.; Sulimov, D.; Geist, V.; Richardt, G. Long-Term Clinical Outcome of Rotational Atherectomy Followed by Drug-Eluting Stent Implantation in Complex Calcified Coronary Lesions. Catheter. Cardiovasc. Interv. 2013, 81, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Whitlow, P.L.; Bass, T.A.; Kipperman, R.M.; Sharaf, B.L.; Ho, K.K.L.; Cutlip, D.E.; Zhang, Y.; Kuntz, R.E.; Williams, D.O.; Lasorda, D.M.; et al. Results of the Study to Determine Rotablator and Transluminal Angioplasty Strategy (STRATAS). Am. J. Cardiol. 2001, 87, 699–705. [Google Scholar] [CrossRef]
- Safian, R.D.; Feldman, T.; Muller, D.W.M.; Mason, D.; Schreiber, T.; Haik, B.; Mooney, M.; O’Neill, W.W. Coronary Angioplasty and Rotablator Atherectomy Trial (CARAT): Immediate and Late Results of a Prospective Multicenter Randomized Trial. Catheter. Cardiovasc. Interv. 2001, 53, 213–220. [Google Scholar] [CrossRef]
- Kawamura, A.; Egami, Y.; Nishino, M.; Tanouchi, J. The C-CAT Sign May Predict Coronary Artery Perforation in Severe Calcified Lesions during Coronary Intervention: A Case Series. Eur. Heart J. Case Rep. 2023, 7, ytad075. [Google Scholar] [CrossRef]
- Kiernan, T.J.; Yan, B.P.; Ruggiero, N.; Eisenberg, J.D.; Bernal, J.; Cubeddu, R.J.; Witzke, C.; Don, C.; Cruz-Gonzalez, I.; Rosenfield, K.; et al. Coronary Artery Perforations in the Contemporary Interventional Era. J. Interv. Cardiol. 2009, 22, 350–353. [Google Scholar] [CrossRef]
- Javaid, A.; Buch, A.N.; Satler, L.F.; Kent, K.M.; Suddath, W.O.; Lindsay, J.; Pichard, A.D.; Waksman, R. Management and Outcomes of Coronary Artery Perforation During Percutaneous Coronary Intervention. Am. J. Cardiol. 2006, 98, 911–914. [Google Scholar] [CrossRef]
- Ajluni, S.C.; Glazier, S.; Blankenship, L.; O’Neill, W.W.; Safian, R.D. Perforations after Percutaneous Coronary Interventions: Clinical, Angiographic, and Therapeutic Observations. Cathet. Cardiovasc. Diagn. 1994, 32, 206–212. [Google Scholar] [CrossRef]
- Doll, J.A.; Hira, R.S.; Kearney, K.E.; Kandzari, D.E.; Riley, R.F.; Marso, S.P.; Grantham, J.A.; Thompson, C.A.; McCabe, J.M.; Karmpaliotis, D.; et al. Management of Percutaneous Coronary Intervention Complications. Circ. Cardiovasc. Interv. 2020, 13, e008962. [Google Scholar] [CrossRef]
- Mousa, M.A.A.; Bingen, B.O.; Al Amri, I.; Mertens, B.J.A.; Taha, S.; Tohamy, A.; Youssef, A.; Jukema, J.W.; Montero-Cabezas, J.M. Efficacy and Safety of Intravascular Lithotripsy Versus Rotational Atherectomy in Balloon-Crossable Heavily Calcified Coronary Lesions. Cardiovasc. Revascularization Med. 2023, 48, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.; Thakkar, S.; Patel, K.; Majmundar, M.; Shlofmitz, E.; Kumar, A.; Gupta, N.; Adalja, D.; Patel, H.P.; Jauhar, R.; et al. Short Term Outcomes of Rotational Atherectomy versus Orbital Atherectomy in Patients Undergoing Complex Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Scand. Cardiovasc. J. 2021, 55, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.A.; Nef, H.; Escaned, J.; Werner, N.; Banning, A.P.; Hill, J.M.; De Bruyne, B.; Montorfano, M.; Lefevre, T.; Stone, G.W.; et al. Safety and Effectiveness of Coronary Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Stenoses. Circ. Cardiovasc. Interv. 2019, 12, e008434. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, A.; Salazar, C.; Becher, M.U.; Tiyerili, V.; Weber, M.; Jansen, F.; Sedaghat, A.; Zimmer, S.; Leick, J.; Grube, E.; et al. Intravascular Lithotripsy in Calcified Coronary Lesions. Circ. Cardiovasc. Interv. 2019, 12, e008154. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.; El-Jack, S.; Newcombe, R.; Glenie, T.; Armstrong, G.; Khan, A. Shockwave Intravascular Lithotripsy for Calcified Coronary Lesions: First Real-World Experience. J. Invasive Cardiol. 2019, 31, 46–48. [Google Scholar] [CrossRef]
- Soriano, F.; Veas, N.; Piccinelli, E.; Oreglia, J. Coronary Dissection Due to Intravascular Lithoplasty Balloon Rupture. EuroIntervention 2019, 15, e558–e559. [Google Scholar] [CrossRef]
- Khattab, A.A.; Richardt, G. Rotational Atherectomy Followed by Drug-Eluting Stent Implantation (Rota-DES): A Rational Approach for Complex Calcified Coronary Lesions. Minerva Cardioangiol. 2008, 56, 107–115. [Google Scholar]
- Khattab, A.A.; Otto, A.; Hochadel, M.; Toelg, R.; Geist, V.; Richardt, G. Drug-Eluting Stents Versus Bare Metal Stents Following Rotational Atherectomy for Heavily Calcified Coronary Lesions: Late Angiographic and Clinical Follow-Up Results. J. Interv. Cardiol. 2007, 20, 100–106. [Google Scholar] [CrossRef]
- Bouisset, F.; Barbato, E.; Reczuch, K.; Dobrzycki, S.; Meyer-Gessner, M.; Bressollette, E.; Cayla, G.; Lhermusier, T.; Zajdel, W.; Palazuelos Molinero, J.; et al. Clinical Outcomes of PCI with Rotational Atherectomy: The European Multicentre Euro4C Registry. EuroIntervention 2020, 16, e305–e312. [Google Scholar] [CrossRef]
- Hajj, M.E.; Hill, A.; El Hajj, S.; Staub, S.; Fernandes, V.; Maran, A. One Year Outcomes of Orbital versus Rotational Atherectomy for the Treatment of Heavily Calcified Coronary Disease. Interv. Cardiol. 2020, 12, 11–16. [Google Scholar] [CrossRef]
- Généreux, P.; Lee, A.C.; Kim, C.Y.; Lee, M.; Shlofmitz, R.; Moses, J.W.; Stone, G.W.; Chambers, J.W. Orbital Atherectomy for Treating De Novo Severely Calcified Coronary Narrowing (1-Year Results from the Pivotal ORBIT II Trial). Am. J. Cardiol. 2015, 115, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Shlofmitz, E.; Goldberg, A.; Shlofmitz, R. Multicenter Registry of Real-World Patients with Severely Calcified Coronary Lesions Undergoing Orbital Atherectomy: 1-Year Outcomes. J. Invasive Cardiol. 2018, 30, 121–124. [Google Scholar] [PubMed]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.-P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease Developed in Collaboration with EACTS. Eur. J. Cardio-Thorac. Surg. 2018, 53, 34–78. [Google Scholar] [CrossRef] [PubMed]
- Apostolos, A.; Chlorogiannis, D.; Vasilagkos, G.; Katsanos, K.; Toutouzas, K.; Aminian, A.; Alexopoulos, D.; Davlouros, P.; Tsigkas, G. Safety and Efficacy of Shortened Dual Antiplatelet Therapy after Complex Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Hell. J. Cardiol. 2023, 71, 33–41. [Google Scholar] [CrossRef]
- Dangas, G.; Baber, U.; Sharma, S.; Giustino, G.; Mehta, S.; Cohen, D.J.; Angiolillo, D.J.; Sartori, S.; Chandiramani, R.; Briguori, C.; et al. Ticagrelor with or without Aspirin after Complex PCI. J. Am. Coll. Cardiol. 2020, 75, 2414–2424. [Google Scholar] [CrossRef]
- Rishad, S.; McEntegart, M.; Ford, T.J.; Di Mario, C.; Fajadet, J.; Lindsay, M.; Watkins, S.; Eteiba, H.; Brogan, R.; Good, R.; et al. Comparative Study of Costs and Resource Utilization of Rotational Atherectomy versus Intravascular Lithotripsy for Percutaneous Coronary Intervention. Minerva Cardiol. Angiol. 2022, 70, 332–340. [Google Scholar] [CrossRef]
- Taqi, H.; Eltaib, A.; Vanezis, A. A Comparative Study of the Economics and Safety of Intravascular Lithotripsy versus Rotational Atherectomy for Calcium Modulation in Percutaneous Coronary Intervention: A Uk Tertiary Centre Experience. In Proceedings of the British Cardiovascular Society Annual Conference, ‘100 years of Cardiology’, Manchester, UK, 6–8 June 2022; p. A50. [Google Scholar]





| IVUS | Diagnosis of intermediate stenosis of the left coronary artery trunk |
| IVUS/OCT | Optimization of stent implantation procedures in native arteries |
| Coronary artery recanalization procedures (guidewire position assessment, true/false lumen navigation) | |
| Studies on the progression/regression of atherosclerosis | |
| IVUS > OCT | Optimizing the left coronary artery trunk angioplasty procedure |
| Imaging for spontaneous coronary artery dissection | |
| Vasculopathy after heart transplantation | |
| OCT > IVUS | Optimizing revascularization in patients with current coronary artery calcifications |
| Intracoronary imaging for suspected acute coronary syndrome | |
| Diagnosis of the causes of stent implantation failure | |
| Diagnosis of neo-atherosclerosis |
| Category | Parameter | RA | OA | IVL |
|---|---|---|---|---|
| Mechanism of action | Device | Rotating burr (140,000–160,000 rpm) | Rotating crown (80,000–120,000 rpm) | Emits sonic waves (80–120 impulses) |
| Independent of lumen size | − | − | + | |
| Modifies deep calcium | − | +/− | + | |
| Temporal vessel occlusion | + | − | ++ | |
| Tight stenosis | + | + | − | |
| Wire bias | + | + | − | |
| Modifies noncalcified lesions | − | + | − | |
| Treatment of in-stent restenosis | − | − | + | |
| Periprocedural complications | Wire entrapment | ++ | + | − |
| No-/slow-flow risk | 6–15% | 0.9% | None reported | |
| Dissection | Lower risk | Higher risk | Rare | |
| Perforation | Lower risk | Higher risk | Rare | |
| Effectiveness | Procedural success (stent delivery) | 92.5%, 98% | 97.7% | 92.4% |
| Short-term outcomes | 30-day MACE | 5% | 10.4% | 7.2% |
| Long-term outcomes | 1-year MACE | 15% | 14.4% | 13.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florek, K.; Bartoszewska, E.; Biegała, S.; Klimek, O.; Malcharczyk, B.; Kübler, P. Rotational Atherectomy, Orbital Atherectomy, and Intravascular Lithotripsy Comparison for Calcified Coronary Lesions. J. Clin. Med. 2023, 12, 7246. https://doi.org/10.3390/jcm12237246
Florek K, Bartoszewska E, Biegała S, Klimek O, Malcharczyk B, Kübler P. Rotational Atherectomy, Orbital Atherectomy, and Intravascular Lithotripsy Comparison for Calcified Coronary Lesions. Journal of Clinical Medicine. 2023; 12(23):7246. https://doi.org/10.3390/jcm12237246
Chicago/Turabian StyleFlorek, Kamila, Elżbieta Bartoszewska, Szymon Biegała, Oliwia Klimek, Bernadeta Malcharczyk, and Piotr Kübler. 2023. "Rotational Atherectomy, Orbital Atherectomy, and Intravascular Lithotripsy Comparison for Calcified Coronary Lesions" Journal of Clinical Medicine 12, no. 23: 7246. https://doi.org/10.3390/jcm12237246
APA StyleFlorek, K., Bartoszewska, E., Biegała, S., Klimek, O., Malcharczyk, B., & Kübler, P. (2023). Rotational Atherectomy, Orbital Atherectomy, and Intravascular Lithotripsy Comparison for Calcified Coronary Lesions. Journal of Clinical Medicine, 12(23), 7246. https://doi.org/10.3390/jcm12237246