Research on Sleep Dynamics in Cleft Lip and Palate Patients Using Simple Sleep Testing
Abstract
:1. Introduction
2. Materials and Methods
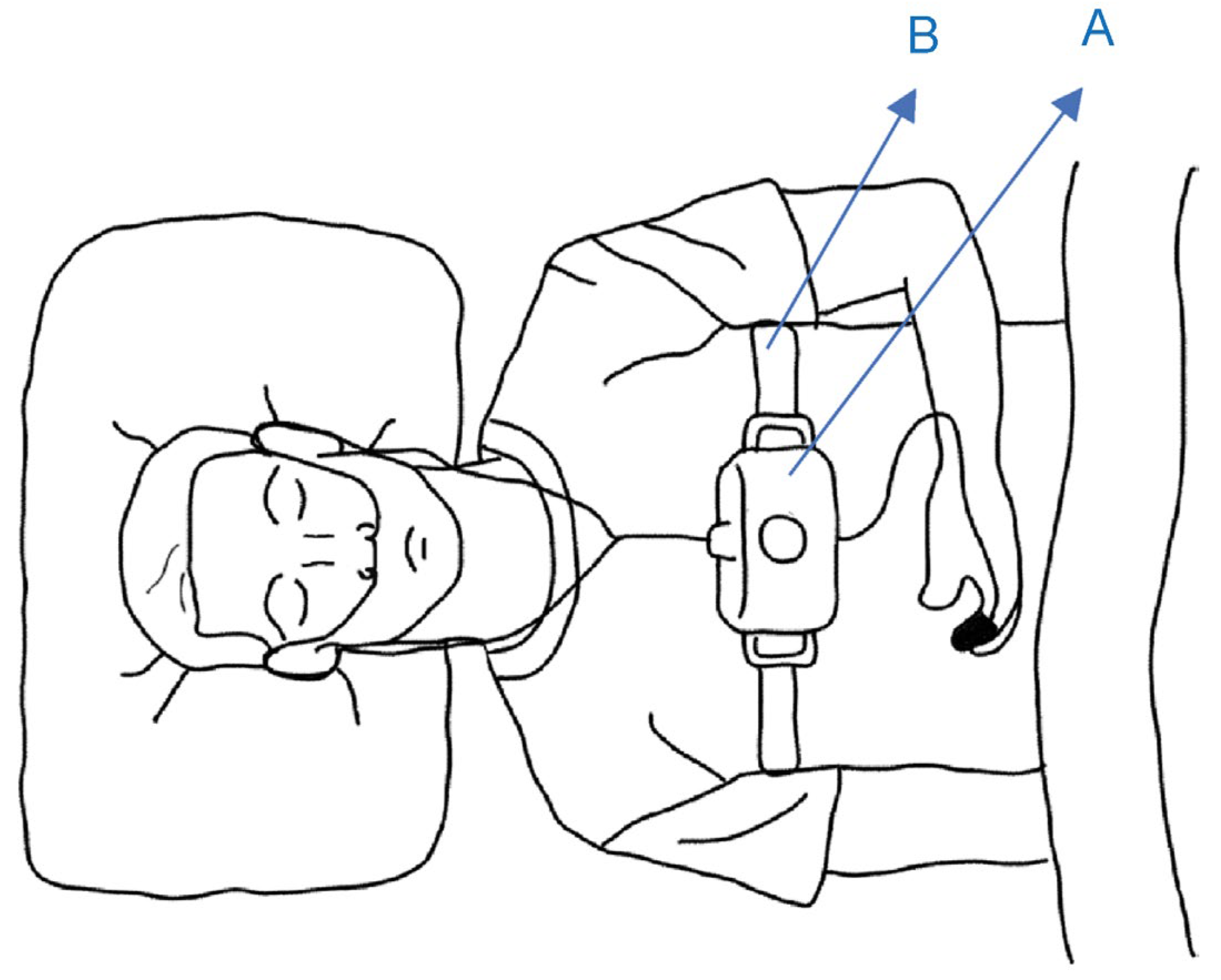
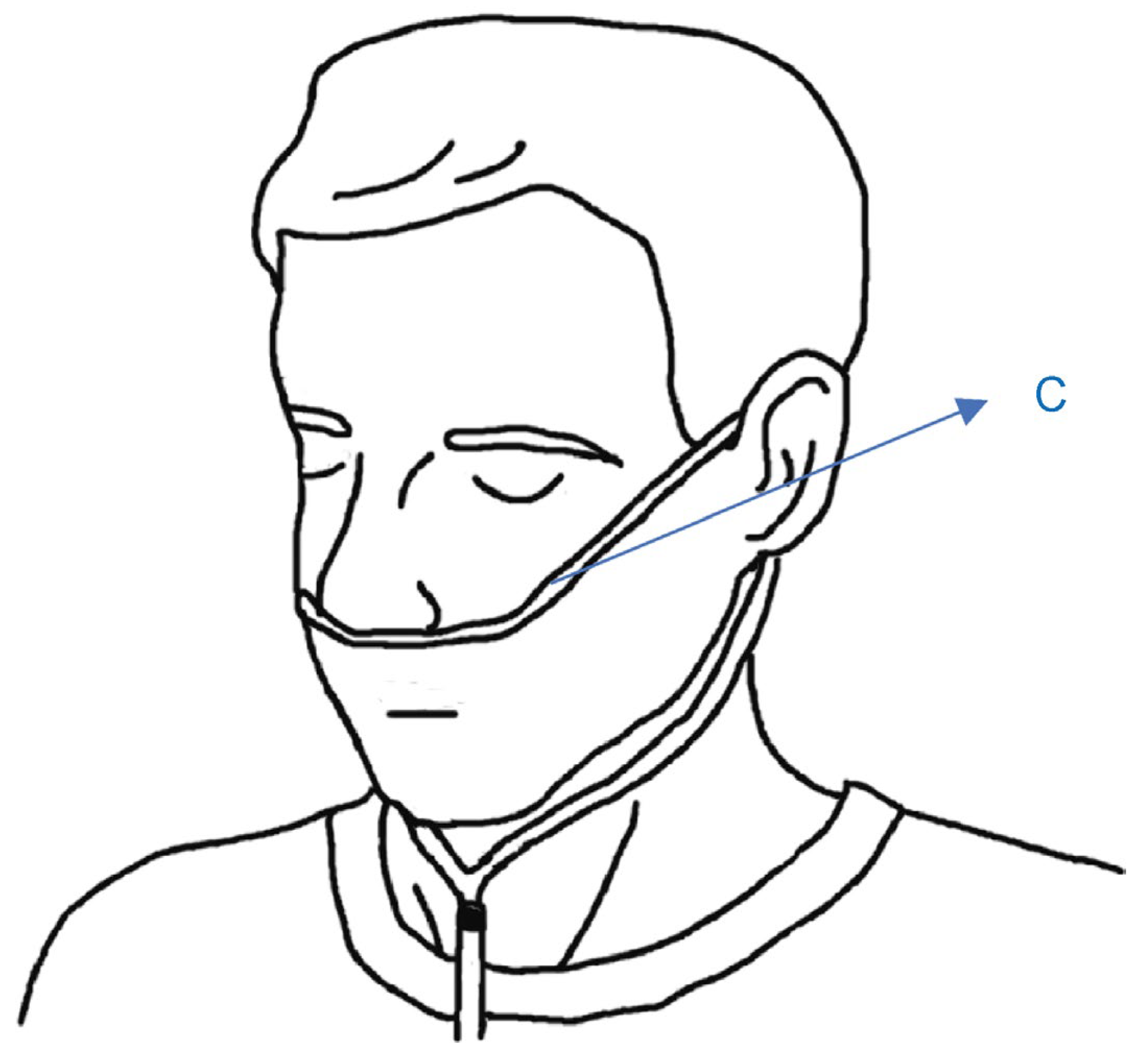
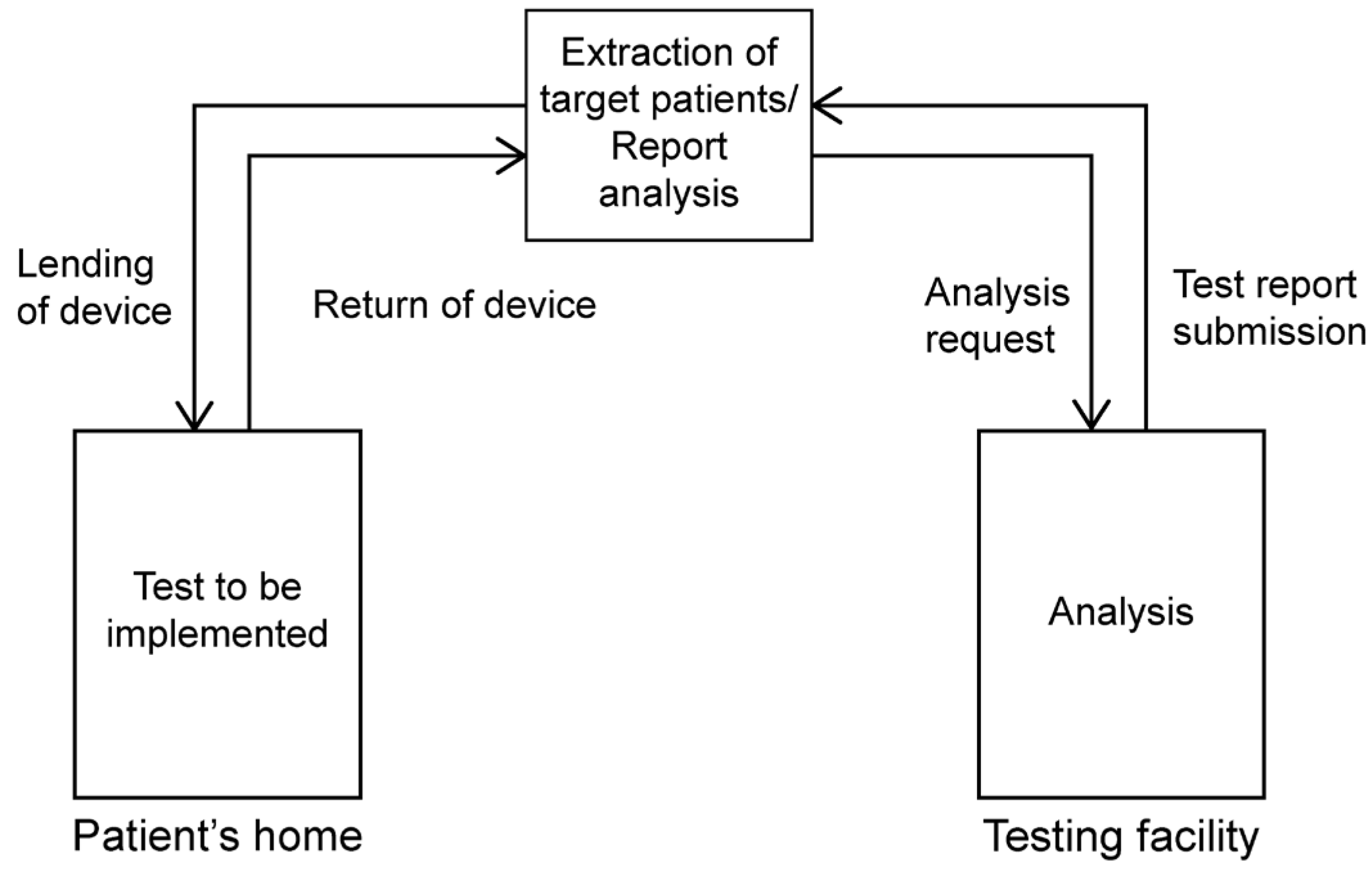
2.1. Simple Overnight Sleep Test
2.2. Cephalometric Radiograph Image
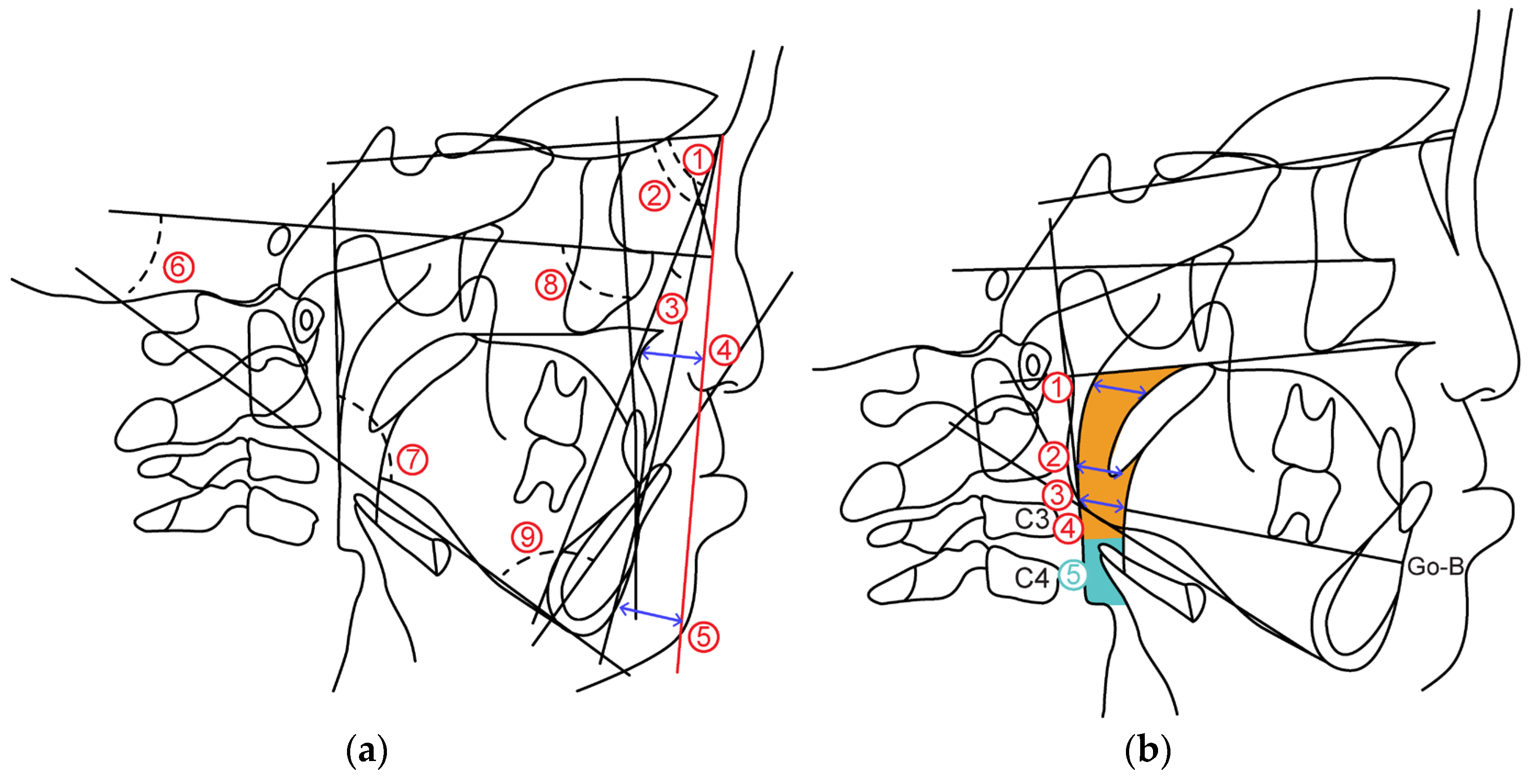
2.2.1. Cephalometric Analysis
- (1)
- Skeletal pattern
- ①
- SNA: SN angle between plane and straight-line NA
- ②
- SNB: SN angle between plane and straight-line NB
- ③
- ANB: Angle formed by straight line AN and straight line NB
- ④
- McNamara to A: Distance from McNamara line to point A
- ⑤
- McNamara to Pog: Distance from McNamara line to Pog
- ⑥
- FH plane to Mandibular plane angle: Angle between mandibular plane and Frankfurt plane
- ⑦
- Gonial Angle: Angle between mandibular plane and mandibular posterior marginal plane
- (2)
- Dental pattern
- ⑧
- U1 to FH plane angle: between maxillary central incisor tooth axis and Frankfurt plane Angle
- ⑨
- L1 to Mandibular plane angle: Mandibular central incisor tooth axis and mandibular plane Angle
2.2.2. Airway Distance, Area
- (1)
- Airway distance
- ①
- SPAS: Linear distance between the point where the axis parallel to the Go-B plane touches the posterior soft palate and the point where it touches the posterior pharyngeal wall.
- ②
- MAS: Linear distance from the point where a line parallel to the Go-B line passes through the lowest point of the soft palate and touches the posterior pharyngeal wall.
- ③
- IAS: Linear distance between two points extending the Go-B line and intersecting the posterior border of the tongue and posterior wall of the pharynx.
- (2)
- Airway area
- ④
- Orpharynx: Area surrounded by a straight line connecting the point where the palatal plane intersects with the posterior pharyngeal wall, a straight line passing through the tip of the epiglottis parallel to the palatal plane and the posterior pharyngeal wall, hyoid bone, and soft palate wall.
- ⑤
- Hypopharynx: Area bounded by two straight lines parallel to the Palatal plane passing through the hypopharyngeal area, tip of the epiglottis, anterior-inferior point of the fourth cervical vertebra, posterior pharyngeal wall, and posterior epiglottis wall.
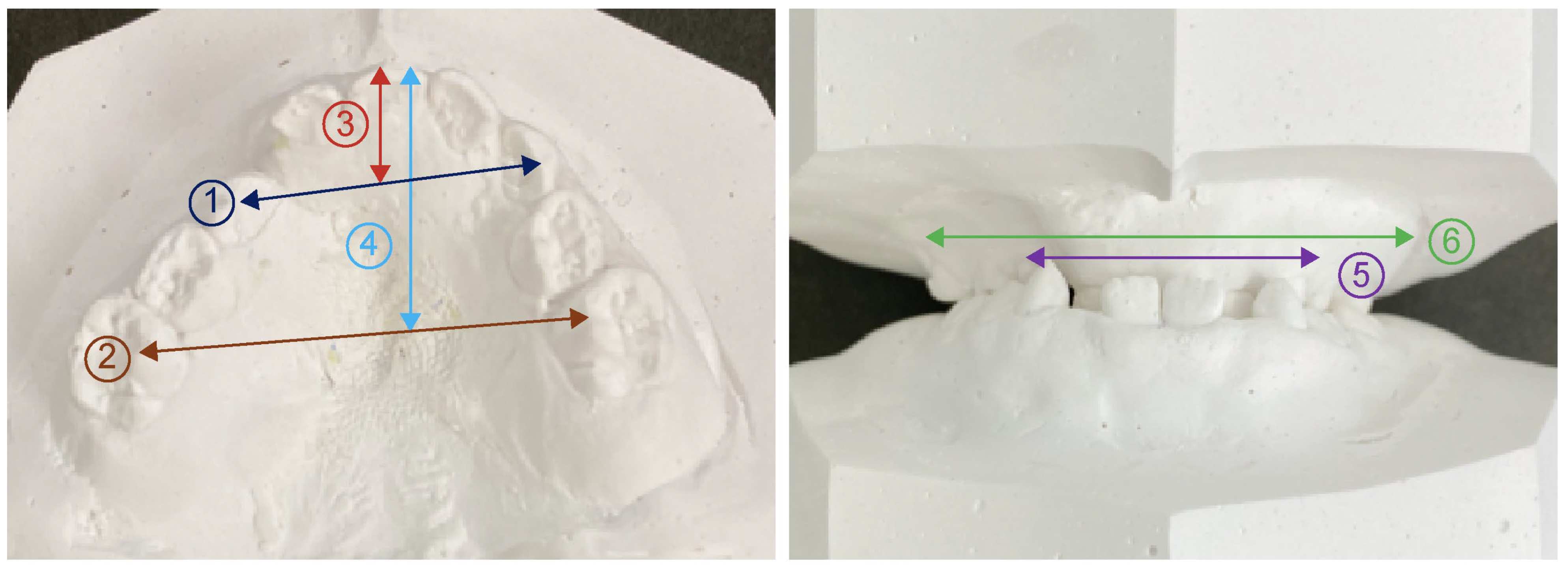
2.3. Model Analysis
3. Results
3.1. Simple Overnight Sleep Test
3.2. Cephalometric Radiograph Image
3.2.1. Cephalometric Analysis
3.2.2. Antero-Posterior Airway Distance and Area
3.3. Model Analysis
4. Discussion
General Orthodontic Treatment for Children with Cleft Lip and Palate
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guilleminault, C.; Hoed, J.; Muter, M.M. Clinical overview of the sleep apnea syndromes. In Sleep Apnea Syndromes; Guilleminault, C., Dement, W., Eds.; Alan R. Liss Inc.: New York, NY, USA, 1977; p. 1. [Google Scholar]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Bassiri, A. Clinical features and evalutation of obstructive sleep apnea-hyponea syndrome. In Principles and Practice of Sleep Medicine, 4th ed.; Elsevier Saunders Co: Philadelphia, PA, USA, 2005; pp. 1043–1052. [Google Scholar]
- Levin, H.S. A cephalometoric analysis of cleft palate deficiencies in the middle third of the face. Angle Orthod. 1963, 33, 186–194. [Google Scholar]
- Graber, T.M. Changing philosophies in cleft palate management. J. Pediatr. 1950, 37, 400–415. [Google Scholar] [CrossRef] [PubMed]
- Graber, T.M. Craniofacial morphology in cleft lip and plate deformities. Surg. Gynec. Obstet. 1949, 88, 359–369. [Google Scholar]
- Lowe, A.A.; Ono, T.; Ferguson, K.A.; Pae, E.K.; Ryan, C.F.; Fleetham, J.A. Cephalometric comparisons of craniofacial and upper airway structure by skeletal subtype and gender in patients with obstructive sleep apnea. Am. J. Orthod. Dentofac. Orthop. 1996, 110, 6. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Stolarz, A.; Mikulewicz, M.; Duś-Ilnicka, I. Current concepts and challenges in the treatment of cleft lip and palate patients—A comprehensive review. J. Pers Med. 2022, 12, 2089. [Google Scholar] [CrossRef] [PubMed]
- Kerstin, U.L.; Anne, C.B.; Welzenbach, J.; Ishorst, N.; Thonissen, M.; Galesloot, T.E.; Mangold, E. Non-syndromic cleft lip with or without cleft palate: Genome-wide association study in europeans identifies a suggestive risk locus at 16p12.1 and supports SH3PXD2A as a clefting susceptibility gene. Genes 2019, 10, 1023. [Google Scholar]
- Shi, B.; Loosee, J.A. The impact of cleft lip and palate on maxillofacial growth. Int. J. Oral. Sci. 2015, 7, 14–17. [Google Scholar] [CrossRef]
- Vyas, T.; Gupta, P.; Kumar, S.; Gupta, R.; Gupta, T.; Singh, H.P. Cleft of lip and palate: A review. J. Fam. Med. Prim. Care 2020, 9, 2621–2625. [Google Scholar] [CrossRef]
- Otsuki, K.; Yamanishi, T.; Tome, W.; Shintaku, Y.; Seikai, T.; Fujimoto, Y.; Kogo, M. Occlusion at 5 years of age following hard palate closure with vestibular flap. Cleft Palate-Craniofacial J. 2020, 57, 729–735. [Google Scholar] [CrossRef]
- Guilleminault, C.; Tilkian, A.; Dement, C.W. The sleep apnea syndromes. Ann. Rev. Med. 1976, 27, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Topaloglu-Ak, A.; Kurtulmus, H.; Basa, S.; Sabuncuoglu, O. Can sleeping habits be associated with sleep bruxism, temporomandibular disorders and dental caries among children? Dent. Med. Probl. 2022, 59, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Cerón, L.; Pacheco, M.; Delgado Gaete, A.; Bravo Torres, W.; Astudillo Rubio, D. Therapies for sleep bruxism in dentistry: A critical evaluation of systematic reviews. Dent. Med. Probl. 2023, 60, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Alqaderi, H.; Tavares, M.; Al-Mulla, F.; Al-Ozairi, E.; Goodson, J.M. Late bedtime and dental caries incidence in Kuwaiti children:A longitudinal multilevel analysis. Community Dent. Oral Epidemiol. 2020, 48, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, M.; Bogunia-Kubik, K.; Mazur, G.; Danel, D.; Smardz, J.; Wojakowska, A.; Poreba, R.; Dratwa, M.; Chaszczewska-Markowska, M.; Winocur, E.; et al. Genetic basis of sleep bruxism and sleep apnea—Response to a medical puzzle. Sci. Rep. 2020, 10, 7497. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.B.; Manfredini, D.; Tavares-Silva, C.; Costa, L.; Luiz, R.R.; Paiva, S.; Serra-Negra, J.M.; Fonseca-Gonçalves, A.; Maia, L.C. Association of possible sleep bruxism in children with different chronotype profiles and sleep characteristics. Chronobiol. Int. 2018, 35, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Martynowicz, H.; Gac, P.; Brzecka, A.; Poreba, R.; Wojakowska, A.; Mazur, G.; Smardz, J.; Wieckiewicz, M. The relationship between sleep bruxism and obstructive sleep apnea based on polysomnographic findings. J. Clin. Med. 2019, 8, 1653. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. The International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Westchester, NY, USA, 2014. [Google Scholar]
- Murakami, A.; Kawanabe, H.; Hosoya, H.; Fukui, K. Effect of sleep-disturbed breathing on maxillofacial growth and development in school-aged children. Orthod. Waves 2021, 80, 87–95. [Google Scholar] [CrossRef]
- Iwasaki, T.; Yanagisawa-Minami, A.; Suga, H.; Shirazawa, Y.; Tsujii, T.; Yamamoto, Y.; Ban, Y.; Sato-Hashiguchi, M.; Sato, H.; Kanomi, R.; et al. Rapid maxillary expansion effects of nasal airway in children with cleft lip and palate using computational fluid dynamics. Orthod. Craniofac. Res. 2019, 22, 201–207. [Google Scholar] [CrossRef]
- Drettner, B. The nasal airway and hearing in patients with cleft palate. Acta Otolaryng. 1960, 52, 131–142. [Google Scholar] [CrossRef]
- Graber, T.M. A cephalometric analysis of the developmental pattern and facial morphology in cleft palate. Angle Orthod. 1949, 19, 91–100. [Google Scholar]
- Riley, R.; Guilleminault, C.; Herran, J.; Powell, N. Cephalometric analyses and flow-volume loops in obstractive sleep apnea patients. Sleep 1983, 6, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K.; Alfawzan, A.A.; Akhter, F.; Alswairki, H.J.; Chaudhari, P.K. Evaluation of lip morphology and nasolabial angle in non-syndromic cleft lip and/palate and non-cleft individuals. Appl. Sci. 2021, 12, 357. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, N.; Li, J.; Zheng, Q.; Shi, B. Growth patterns of the nasolabial region following unilateral cleft lip primary. Front. Pediatr. 2023, 11, 1136467. [Google Scholar] [CrossRef] [PubMed]
- Okushi, T.; Tonogi, M.; Arisaka, T.; Kobayashi, S.; Tsukamoto, Y.; Morishita, H.; Sto, K.; Sano, C.; Chiba, S.; Yamane, G.Y.; et al. Effect of maxillomandibular advancement on morphology of velopharyngeal apace. J. Oral Maxillofac. Surg. 2011, 69, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, Y.; Ross, R.B. Facial growth in children with isolated cleft palate. Cleft Palate J. 1969, 6, 290–302. [Google Scholar]
- Reiley, R.; Guilleminault, C.; Powell, N.; Simmons, F.B. Palatopharyngoplasty failure, cephalometric roentgenograms, and obstructive sleep apnea. Otolaryngol. Head Neck Surg. 1985, 93, 240–243. [Google Scholar] [CrossRef]
- Lyberg, T.; Krogstad, O.; Djupesland, G. Cephalometric analysis in patients with obstructive sleep apnea syndrome: II. Soft tissue morphology. J. Laryngol. Otol. 1989, 103, 293–297. [Google Scholar] [CrossRef]
- Hairfield, W.M.; Warren, D.W. Dimensions of the cleft nasal airway in adult: A comparison with subjects without cleft. Cleft Palate J. 1989, 26, 9–13. [Google Scholar]
- Drake, A.F.; Davis, J.U.; Warren, D.W. Nasal Airway Size in Cleft and Noncleft Children. Laryngoscope 1993, 103, 915–917. [Google Scholar] [CrossRef]
- Mazaheri, M.; Harding, R.L.; Nanda, S. The effect of surgery onmaxillary growth and cleft width. Plast. Reconstr. Surg. 1967, 40, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Subtelny, J.D. Width of the nasopharynx and related anatomic structures in normal and unoperated cleft palate children. Am. J. Orthod. 1955, 41, 889–909. [Google Scholar] [CrossRef]
- Brodie, A.G. Anatomy and physiology of head and neck musculature. Am. J. Orthod. 1950, 36, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Pruzansky, S. Description, classification, and analysis of unoperated clefts of the lip and palate. Am. J. Orthod. 1953, 39, 590–611. [Google Scholar] [CrossRef]
- Hama, K. Morphological study of the craniofacial skeleton within a profile in cleft lip and plate. J. Osaka Univ. 1964, 4, 41. [Google Scholar]
- Steegman, R.; Schoeman, A.; Dieters, A.; Jongsma, B.; Jansma, J.; van der Meer, J.; Ren, Y. Three-dimensional volumetric changes in the airway of growing unilateral complete cleft lip and palate patients after bone-anchored maxillary protraction. Am. J. Orthod. Dentofac. Orthop. 2022, 162, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Nelson, F.O. A new extra-oral orthodontic appliance. Int. J. Orthod. 1968, 6, 24–27. [Google Scholar]
- Friede, H.; Lennartsson, B. Forward traction of the maxilla in cleft lip and palate patients. Eur. J. Orthod. 1981, 3, 21–39. [Google Scholar] [CrossRef]
- Ranta, R. Protraction of the cleft maxilla. Eur. J. Orthod. 1988, 10, 215–222. [Google Scholar] [CrossRef]
- Tindlund, R.S.; Rygh, P.; Boe, O.E. Orthopedic protraction of the upper jaw in cleft lip and palate patients during the deciduous and mixed dentition periods in comparison with normal growth and devel- opment. Cleft Palate-Craniofacial J. 1993, 30, 182–194. [Google Scholar] [CrossRef]
- Buschang, P.H.; Porter, C.; Genecov, E.; Genecov, D.; Sayler, K.E. Face mask therapy of preadolescents with unilateral cleft lip and palate. Angle Orthod. 1994, 64, 145–150. [Google Scholar]

| Cleft lip and Palate Group | Control Group | Significant Difference | |
|---|---|---|---|
| REI | 4.4 ± 2.9 | 1.8 ± 0.7 | 0.015 * |
| SP02 | 96.3 ± 0.8 | 96.0 ± 0.6 | 0.446 |
| Cleft lip and Palate Group | Control Group | Significant Difference | |
|---|---|---|---|
| SNA | 76.8 ± 4.2 | 80.2 ± 2.8 | 0.049 * |
| SNB | 77.6 ± 2.7 | 77.6 ± 2.1 | 0.648 |
| ANB | −0.7 ± 4.0 | 2.7 ± 1.9 | 0.003 ** |
| McNamara to A | −2.2 ± 4.6 | −1.3 ± 2.5 | 0.483 |
| McNamara to Pog | −6.0 ± 4.8 | −7.4 ± 3.8 | 0.483 |
| FH plane to Mandibular plane angle | 28.8 ± 4.8 | 30.5 ± 6.4 | 0.648 |
| Gonial Angle | 126 ± 7.1 | 127.0 ± 7.2 | 0.879 |
| U1 to FH | 93.6 ± 10.2 | 111.0 ± 5.2 | 0.000 ** |
| L1 to Mandibular plane angle | 80.1 ± 6.6 | 93.2 ± 8.7 | 0.000 ** |
| APAS | 7.8 ± 1.7 | 12.2 ± 3.4 | 0.011 * |
| MAS | 8.2 ± 1.6 | 12.3 ± 3.4 | 0.010 ** |
| IAS | 9.5 ± 4.3 | 13.4 ± 3.7 | 0.006 ** |
| Orpharynx | 411.0 ± 105.3 | 564.6 ± 130.6 | 0.005 ** |
| Hypopharynx | 164.0 ± 44.2 | 195.7 ± 62.8 | 0.313 |
| Cleft lip and Palate Group | Control Group | Significant Difference | |
|---|---|---|---|
| Anterior width of maxillary alveolar basal arch | 36.4 ± 2.5 | 40.3 ± 2.6 | 0.000 ** |
| Posterior width of maxillary alveolar basal arch | 53.6 ± 3.8 | 59.4 ± 2.6 | 0.002 ** |
| Anterior width of maxillary coronal arch | 28.6 ± 2.3 | 33.0 ± 2.5 | 0.001 ** |
| Posterior width of maxillary coronal arch | 38.9 ± 3.2 | 47.2 ± 2.0 | 0.000 ** |
| Anterior length of maxillary coronal arch | 11.0 ± 1.7 | 12.9 ± 2.1 | 0.030 * |
| Posterior length of maxillary coronal arch | 22.8 ± 3.6 | 32.4 ± 1.8 | 0.000 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemoto, N.; Kawanabe, H.; Fukui, K.; Oyama, A.; Okamoto, T.; Shimamura, K. Research on Sleep Dynamics in Cleft Lip and Palate Patients Using Simple Sleep Testing. J. Clin. Med. 2023, 12, 7254. https://doi.org/10.3390/jcm12237254
Nemoto N, Kawanabe H, Fukui K, Oyama A, Okamoto T, Shimamura K. Research on Sleep Dynamics in Cleft Lip and Palate Patients Using Simple Sleep Testing. Journal of Clinical Medicine. 2023; 12(23):7254. https://doi.org/10.3390/jcm12237254
Chicago/Turabian StyleNemoto, Naoko, Hitoshi Kawanabe, Kazunori Fukui, Akihiko Oyama, Toru Okamoto, and Kazuhiro Shimamura. 2023. "Research on Sleep Dynamics in Cleft Lip and Palate Patients Using Simple Sleep Testing" Journal of Clinical Medicine 12, no. 23: 7254. https://doi.org/10.3390/jcm12237254







