Effect of Antidiabetic Drugs on Bone Health in Patients with Normal Renal Function and in Chronic Kidney Disease (CKD): Insight into Clinical Challenges in the Treatment of Type 2 Diabetes
Abstract
:1. Introduction
2. Search Strategy
3. Epidemiology and Pathophysiology of Type 2 Diabetes and Diabetic Kidney Disease
4. Bone Health in Type 2 Diabetes and CKD
5. Antidiabetics and Bone Health in Patients with Normal Renal Function and in CKD
5.1. Experimental Studies
5.2. Clinical Studies
| Drug Class | Experimental Studies | Clinical Studies | Meta-Analyses on Fracture Risk |
|---|---|---|---|
| Metformin | -Stimulation of RUNX-2, BMP-2, osteocalcin, OSTERIX, OPG, differentiation of MSC toward osteoblasts -CKD: prevents increase in Cr, P, PTH, FGF23, vascular calcification, and decline in Ca | -Potential positive effects on BMD and bone properties -CKD: potential positive effects on BMD | -Neutral (potential positive) effect |
| GLP-1RA | -Stimulation of Wnt/β-catenin signaling, RUNX-2, osteocalcin, OPG, differentiation of MSC toward osteoblasts, increase in femoral BMD and blood flow -CKD: renoprotective effects | -No effect on BTMs and BMD (may prevent weight-loss-associated BMD reduction) -CKD: renoprotective effects, lower all-cause mortality | -Potential positive effect |
| DDP-4i | -Reduction in RANKL action (sitagliptin), RUNX2, osteocalcin, collagen, mineralization (saxagliptin); different effects through IL, cytokines, T- and B-cells -CKD: renoprotective effects | -Neutral and positive effects on BMTs and BMD; increase in TBS | -Neutral effect -CKD: neutral effect |
| SGLT-2i | -Increase in bone resorption markers, perturbation of microarchitecture (canagliflozin); stimulation of RANKL, inflammation, BMP2 (empagliflozin), -CKD: renoprotective effects | -Increase in β-CTX and osteocalcin, BMD reduction (canagliflozin); no effects on BTMs and BMD (other molecules); no effects on bone quality -CKD: reduced risk of dialysis, transplantation, AKI, and mortality | -Neutral effect -CKD: neutral effect |
| SU | -Stimulation of bone formation (glimepiride) through eNOS and PI3K/AKT | -Inconsistent data on effects on BTMs, neutral effects on BMD | -Neutral effect |
| Insulin | -Promotes osteoblasts proliferation, ALK and collagen production | -No effect on BTMs, increase in BMD | -Negative effect |
| TZD | -Stimulation of RANKL, MSC differentiation toward osteoblasts adipocytes through PPAR-γ | -Possible negative effects on BTMs, decrease in BMD | -Negative effect |
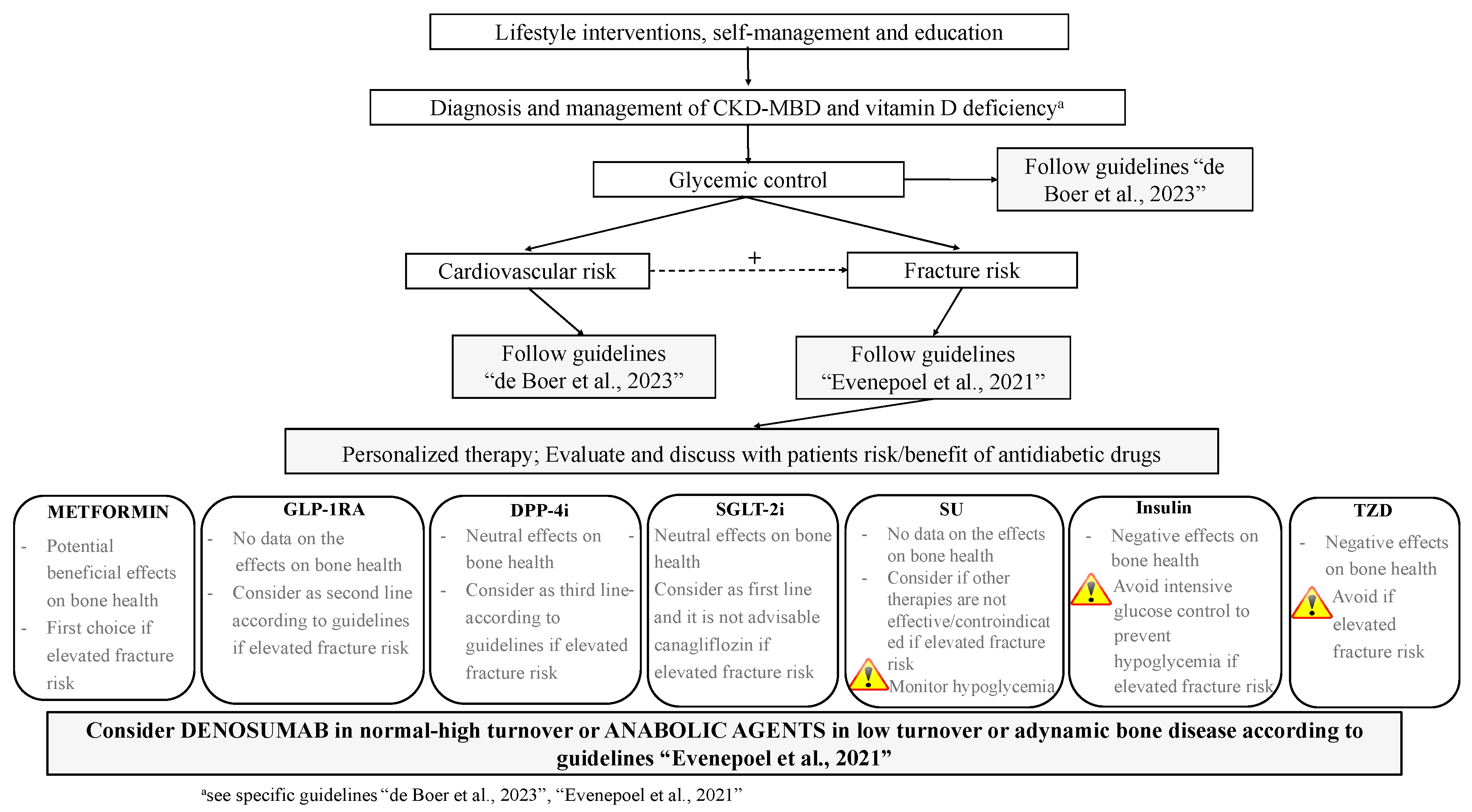
6. The Use of Antidiabetics in T2DM with and without CKD: From Standards of Care to Strategies for Supporting Bone Health
7. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Sayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 9. Pharmacologic approaches to glycemic treatment: Standards of care in diabetes—2023. Diabetes Care 2023, 46, S140–S157. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.L.; Abrahamsen, B.; Napoli, N.; Akesson, K.; Chandran, M.; Eastell, R.; El-Hajj Fuleihan, G.; Josse, R.; Kendler, D.L.; Kraenzlin, M.; et al. Diagnosis and management of bone fragility in diabetes: An emerging challenge. Osteoporos. Int. 2018, 29, 2585–2596. [Google Scholar] [CrossRef]
- Eastell, R.; Vittinghoff, E.; Lui, L.; Ewing, S.K.; Schwartz, A.V.; Bauer, D.C.; Black, D.M.; Bouxsein, M.L. Diabetes mellitus and the benefit of antiresorptive therapy on fracture risk. J. Bone Miner. Res. 2022, 37, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.L.; Silverman, S.; Fujiwara, S.; Saag, K.; Napoli, N.; Soen, S.; Enomoto, H.; Melby, T.E.; Disch, D.P.; Marin, F.; et al. Real-world effectiveness of teriparatide on fracture reduction in patients with osteoporosis and comorbidities or risk factors for fractures: Integrated analysis of 4 prospective observational studies. Bone 2018, 116, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, R.; Hans, D.; Hattersley, G.; Mitlak, B.; Fitzpatrick, L.A.; Wang, Y.; Schwartz, A.V.; Miller, P.D.; Josse, R.G. Abaloparatide in postmenopausal women with osteoporosis and type 2 diabetes: A post hoc analysis of the ACTIVE Study. JBMR Plus 2020, 4, e10346. [Google Scholar] [CrossRef] [PubMed]
- Kheniser, K.G.; Polanco Santos, C.M.; Kashyap, S.R. The effects of diabetes therapy on bone: A clinical perspective. J. Diabetes Complicat. 2018, 32, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, C.; Colangelo, L.; Santori, R.; Renella, M.; Mastrantonio, M.; Minisola, S.; Pepe, J. The interplay between bone and glucose metabolism. Front. Endocrinol. 2020, 11, 122. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Diabetes. Available online: http://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 3 October 2022).
- Afkarian, M.; Zelnick, L.R.; Hall, Y.N.; Heagerty, P.J.; Tuttle, K.; Weiss, N.S.; De Boer, I.H. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA 2016, 316, 602–610. [Google Scholar] [CrossRef]
- Annual Data Report. Available online: https://adr.usrds.org/ (accessed on 18 October 2022).
- De Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Rosas, S.E.; Rossing, P.; Bakris, G. Diabetes management in chronic kidney disease: A consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2022, 102, 974–989. [Google Scholar] [CrossRef]
- Gembillo, G.; Ingrasciotta, Y.; Crisafulli, S.; Luxi, N.; Siligato, R.; Santoro, D.; Trifirò, G. Kidney disease in diabetic patients: From Pathophysiology to Pharmacological Aspects with a Focus on Therapeutic Inertia. Int. J. Mol. Sci. 2021, 22, 4824. [Google Scholar] [CrossRef]
- Pugliese, G.; Solini, A.; Bonora, E.; Fondelli, C.; Orsi, E.; Nicolucci, A.; Penno, G.; RIACE Study Group. Chronic kidney disease in type 2 diabetes: Lessons from the Renal Insufficiency And Cardiovascular Events (RIACE) Italian Multicentre Stud. Nutr. Metab. Cardiovac. Dis. 2014, 24, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Nawroth, P.P.; Herzig, S.; Üstünel, B.E. Emerging Targets in Type 2 Diabetes and Diabetic Complications. Adv. Sci. 2021, 8, 2100275. [Google Scholar] [CrossRef] [PubMed]
- Premaratne, E.; Verma, S.; Ekinci, E.I.; Theverkalam, G.; Jerums, G.; MacIsaac, R.J. The impact of hyperfiltration on the diabetic kidney. Diabetes Metab. 2015, 41, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Porrini, E.; Ruggenenti, P.; Mogensen, C.E.; Barlovic, D.P.; Praga, M.; Cruzado, J.M.; Hojs, R.; Abbate, M.; de Vries, A.P.J.; ERA-EDTA Diabesity Working Group. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2015, 3, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. CJASN 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.-H.; Hsu, Y.-C.; Chen, T.-H.; Lin, C.-L. Recent Advances in Diabetic Kidney Diseases: From Kidney Injury to Kidney Fibrosis. Int. J. Mol. Sci. 2021, 22, 11857. [Google Scholar] [CrossRef]
- Cianferotti, L.; Cipriani, C.; Corbetta, S.; Corona, G.; Defeudis, G.; Lania, A.G.; Messina, C.; Napoli, N.; Mazziotti, G. Bone quality in endocrine diseases: Determinants and clinical relevance. J. Endocrinol. Investig. 2023, 46, 1283–1304. [Google Scholar] [CrossRef]
- Walle, M.; Whittier, D.E.; Frost, M.; Müller, R.; Collins, C.J. Meta-analysis of diabetes mellitus-associated differences in bone structure assessed by High-resolution Peripheral Quantitative Computed Tomography. Curr. Osteoporos. Rep. 2022, 20, 398–409. [Google Scholar] [CrossRef]
- Weber, D.R.; Long, F.; Zemel, B.S.; Kindler, J.M. Glycemic Control and Bone in Diabetes. Curr. Osteoporos. Rep. 2022, 20, 379–388. [Google Scholar] [CrossRef]
- Kindler, J.M.; Laing, E.M.; Liu, W.; Dain, J.A.; Lewis, R.D. Pentosidine is associated with cortical bone geometry and insulin resistance in otherwise healthy children. J. Bone Miner. Res. 2019, 34, 1446–1450. [Google Scholar] [CrossRef]
- Karim, L.; Moulton, J.; Van Vliet, M.; Velie, K.; Robbins, A.; Malekipour, F.; Abdeen, A.; Ayres, D.; Bouxsein, M.L. Bone microarchitecture, biomechanical properties, and advanced glycation end-products in the proximal femur of adults with type 2 diabetes. Bone 2018, 114, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.B.; Torres, A.M.; Palomino, P.M.; Marty, E.; Saiyed, R.; Cohn, M.; Jo, J.; Warner, S.; Sroga, G.E.; King, K.B.; et al. Altered tissue composition, microarchitecture, and mechanical performance in cancellous bone from men with type 2 diabetes mellitus. J. Bone Miner. Res. 2019, 34, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Koromani, F.; Oei, L.; Shevroja, E.; Trajanoska, K.; Schoufour, J.; Muka, T.; Franco, O.H.; Ikram, M.A.; Zillikens, M.C.; Uitterlinden, A.G.; et al. Vertebral fractures in individuals with type 2 diabetes: More than skeletal complications alone. Diabetes Care 2020, 43, 137–144. [Google Scholar] [CrossRef]
- KDIGO. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Young, T.K.; Toussaint, N.D.; Di Tanna, G.L.; Arnott, C.; Hockham, C.; Kang, A.; Schutte, A.E.; Perkovic, V.; Mahaffey, K.W.; Agarwal, R.; et al. Risk Ffactors for fracture in patients with coexisting chronic kidney disease and type 2 diabetes: An observational analysis from the CREDENCE Trial. J. Diabetes Res. 2022, 2022, 9998891. [Google Scholar] [CrossRef]
- Malluche, H.; Porter, D.S.; Monier-Faugere, M.C.; Mawad, H.; Pienkowski, D. Differences in bone quality in lower cancellous bone volume and reduced trabecular thickness. J. Am. Soc. Nephrol. 2012, 23, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Sartò, G.V.R.; Gallieni, M.; Cosmai, L.; Messa, P.; Rossini, M.; Chiodini, I.; Plebani, M.; Evenepoel, P.; Harvey, N.; et al. Time for Revival of Bone Biopsy with Histomorphometric Analysis in Chronic Kidney Disease (CKD): Moving from Skepticism to Pragmatism. Nutrients 2022, 14, 1742. [Google Scholar] [CrossRef]
- Khairallah, P.; Nickolas, T.L.; Fusaro, M. How and when to assess bone mineral density and bone quality in chronic kidney disease patients? Nephrol. Dial. Transplant. 2021, 36, 774–776. [Google Scholar] [CrossRef]
- Ramalhoa, J.; Marques, I.; Hans, D.; Dempster, D.; Zhoud, H.; Patele, P.; Pereira, R.M.R.; Jorgettia, V.; Moysesa, R.M.A.; Nickolas, T.L. The trabecular bone score: Relationships with trabecular and cortical microarchitecture measured by HR-pQCT and histomorphometry in patients with chronic kidney disease. Bone 2018, 116, 215–220. [Google Scholar] [CrossRef]
- El-Husseini, A.; Abdalbary, M.; Lima, F.; Issa, M.; Ahmed, M.-T.; Winkler, M.; Srour, H.; Davenport, D.; Wang, G.; Faugere, M.-C.; et al. Low turnover renal osteodystrophy with abnormal bone quality and vascular calcification in patients with mild-to-moderate CKD. Kidney Int. Rep. 2022, 7, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.A.; Cheung, A.M.; West, S.L.; Lok, C.E. Bone mineral density by DXA and HR pQCT can discriminate fracture status in men and women with stages 3 to 5 chronic kidney disease. Osteoporos. Int. 2012, 23, 2805–2813. [Google Scholar] [CrossRef]
- Hygum, K.; Starup-Linde, J.; Harsløf, T.; Vestergaard, P.; Langdahl, B.L. MECHANISMS IN ENDOCRINOLOGY: Diabetes mellitus, a state of low bone turnover—A systematic review and meta-analysis. Eur. J. Endocrinol. 2017, 176, R137–R157. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.L.; Li, D.D.; Zhang, J.J.; Hsu, Y.H.; Wang, T.S.; Zhai, S.D.; Song, Y.Q. Lack of evidence for a harmful effect of sodium-glucose co-transporter 2 (SGLT2) inhibitors on fracture risk among type 2 diabetes patients: A network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2016, 18, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Geng, N.; Niu, Y.; Zhao, H.; Fei, W.; Chen, S.; Ping Ren, L. Relationship between geriatric nutritional risk index and osteoporosis in type 2 diabetes in Northern China. BMC Endocr. Disord. 2022, 22, 308. [Google Scholar] [CrossRef]
- Tseng, M.Y.; Liang, J.; Wu, C.C.; Cheng, H.S.; Yang, C.T.; Chen, C.Y.; Shyu, Y.I.L. Better nutrition trajectory improves recovery following a hip fracture surgery for older persons with diabetes mellitus. Aging Clin. Exp. Res. 2022, 34, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Bahardoust, M.; Yarali, M.; Donyadideh, M.; Rahimi, E.; Naderi, D.; Tehrani, F.M.; Delpisheh, A. The use of metformin, su fonylurea compounds and insulin and the risk of hip fractures in diabetic patients: A systematic review and meta-analysis of observational studies. BMC Musculoskelet. Disord. 2023, 24, 367. [Google Scholar] [CrossRef] [PubMed]
- Ala, M.; Ala, M. Metformin for cardiovascular protection, inflammatory bowel disease, osteoporosis, periodontitis, polycystic ovarian syndrome, neurodegeneration, cancer, inflammation and senescence: What Is next? ACS Pharmacol. Transl. Sci. 2021, 4, 1747–1770. [Google Scholar] [CrossRef]
- Bornstein, S.; Moschetta, M.; Kawano, Y.; Sacco, A.; Huynh, D.; Brooks, D.; Manier, S.; Fairfield, H.; Falank, C.; Roccaro, A.M.; et al. Metformin affects cortical bone mass and marrow adiposity in diet-induced obesity in male mice. Endocrinology 2017, 158, 3369–3385. [Google Scholar] [CrossRef]
- Duan, W.; Zou, H.; Zang, N.; Ma, D.; Yang, B.; Zhu, L. Metformin increases bone marrow adipose tissue by promoting mesenchymal stromal cells apoptosis. Aging 2023, 15, 542–552. [Google Scholar] [CrossRef]
- De Broe, M.E.; Jouret, F. Does metformin do more benefit or harm in chronic kidney disease patients? Kidney Int. 2020, 98, 1098–1101. [Google Scholar] [CrossRef]
- Neven, E.; Vervaet, B.; Brand, K.; Gottwald-Hostalek, U.; Opdebeeck, B.; De Maré, A.; Verhulst, A.; Lalau, J.-D.; Kamel, S.; De Broe, M.E.; et al. Metformin prevents the development of severe chronic kidney disease and its associated mineral and bone disorder. Kidney Int. 2018, 94, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Mabilleau, G.; Pereira, M.; Chenu, C. Novel skeletal effects of glucagon-like peptide-1 (GLP-1) receptor agonists. J. Endocrinol. 2018, 236, R29–R42. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, S.; Xue, P.; Li, Y. Liraglutide, a glucagon-like peptide-1 receptor agonist, facilitates osteogenic proliferation and differentiation in MC3T3-E1 cells through phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), extracellular signal-related kinase (ERK)1/2, and cAMP/protein kinase A (PKA) signaling pathways involving beta-catenin. Exp. Cell Res. 2017, 360, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Daniilopoulou, I.; Vlachou, E.; Lambrou, G.I.; Ntikoudi, A.; Dokoutsidou, E.; Fasoi, G.; Govina, O.; Kavga, A.; Tsartsalis, A.N. The impact of GLP1 agonists on bone metabolism: A systematic review. Medicina 2022, 58, 224. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Tanabe, J.; Ogura, Y.; Nagai, Y.; Sugaya, T.; Ohata, K.; Natsuki, Y.; Ichikawa, D.; Watanabe, S.; Inoue, K.; et al. Renoprotective effect of GLP-1 receptor agonist, liraglutide, in early-phase diabetic kidney disease in spontaneously diabetic Torii fatty rats. Clin. Exp. Nephrol. 2021, 25, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, C.; Liang, J.; Yu, M.; Qu, X. Effect of Dipeptidyl Peptidase-4 Inhibitors on Bone Metabolism and the Possible Underlying Mechanisms. Front. Pharmacol. 2017, 8, 487. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, F.; Qu, X.; Zhai, Z.; Hu, G.; Chen, X.; Zhang, X. Sitagliptin, An Anti-diabetic Drug, Suppresses Estrogen Deficiency-Induced OsteoporosisIn Vivo and Inhibits RANKL-Induced Osteoclast Formation and Bone Resorption In Vitro. Front. Pharmacol. 2017, 8, 407. [Google Scholar] [CrossRef]
- Yang, Q.; Fu, B.; Luo, D.; Wang, H.; Cao, H.; Chen, X.; Tian, L.; Yu, X. The multiple biological functions of Dipeptidyl Peptidase-4 in bone metabolism. Front. Endocrinol. 2022, 13, 856954. [Google Scholar] [CrossRef]
- CoppoliCoppolino, G.; Leporini, C.; Rivoli, L.; Ursini, F.; di Paola, E.D.; Cernaro, V.; Arturi, F.; Bolignano, D.; Russo, E.; De Sarro, G.; et al. Exploring the effects of DPP-4 inhibitors on the kidney from the bench to clinical trials. Pharmacol. Res. 2018, 219, 274–294. [Google Scholar] [CrossRef]
- Dong, B.; Lv, R.; Wang, J.; Che, L.; Wang, Z.; Huai, Z.; Wang, Y.; Xu, L. The extraglycemic effect of SGLT-2is on mineral and bone metabolism and bone fracture. Front. Endocrinol. 2022, 13, 918350. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.E.; Yahya, G.; Popoviciu, M.S.; Cavalu, S.; Abd-Eldayem, M.A.; Saber, S. Unlocking the full potential of SGLT2 inhibitors: Expanding applications beyond glycemic control. Int. J. Mol. Sci. 2023, 24, 6039. [Google Scholar] [CrossRef] [PubMed]
- Neuen, B.L.; Young, T.; Heerspink, H.J.L.; Neal, B.; Perkovic, V.; Billot, L.; Mahaffey, K.W.; Charytan, D.M.; Wheeler, D.C.; Arnott, C.; et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Kitada, K. Possible renoprotective mechanisms of SGLT2 inhibitors. Front. Med. 2023, 10, 1115413. [Google Scholar] [CrossRef]
- Fronczek-Sokol, J.; Pytlik, M. Effect of glimepiride on the skeletal system of ovariectomized and non-ovariectomized rats. Pharmacol. Rep. 2014, 66, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Gu, B.; Xiong, W.; Tan, B.; Geng, W.; Li, J.; Liu, H. Glimepiride promotes osteogenic differentiation in rat osteoblasts via the PI3K/Akt/eNOS pathway in a high glucose microenvironment. PLoS ONE 2014, 9, e112243. [Google Scholar] [CrossRef]
- Conte, C.; Epstein, S.; Napoli, N. Insulin resistance and bone: A biological partnership. Acta Diabetol. 2018, 55, 305–314. [Google Scholar] [CrossRef]
- Thrailkill, K.M.; Lumpkin, C.K.; Bunn, R.C.; Kemp, S.F.; Fowlkes, J.L.; Maor, G.; Vasiliver-Shamis, G.; Hazan-Brill, R.; Wertheimer, E.; Karnieli, E.; et al. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E735–E745. [Google Scholar] [CrossRef]
- Lazarenko, O.P.; Rzonca, S.O.; Hogue, W.R.; Swain, F.L.; Suva, L.J.; Lecka-Czernik, B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology 2007, 148, 2669–2680. [Google Scholar] [CrossRef]
- Pop, L.M.; Lingvay, I.; Yuan, Q.; Li, X.; Adams-Huet, B.; Maalouf, N.M. Impact of pioglitazone on bone mineral density and bone marrow fat content. Osteoporos. Int. 2017, 28, 3261–3269. [Google Scholar] [CrossRef]
- Nordklint, A.K.; Almdal, T.P.; Vestergaard, P.; Lundby-Christensen, L.; Boesgaard, T.W.; Breum, L.; Gade-Rasmussen, B.; Sneppen, S.B.; Gluud, C.; Hemmingsen, B.; et al. The effect of metformin versus placebo in combination with insulin analogues on bone mineral density and trabecular bone score in patients with type 2 diabetes mellitus: A randomized placebo-controlled trial. Osteop Int. 2018, 29, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V.; Pan, Q.; Aroda, V.R.; Crandall, J.P.; Kriska, A.; Piromalli, C.; Wallia, A.; Temprosa, M.; Florez, H.; Diabetes Prevention Program Research Group. Long-term effects of lifestyle and metformin interventions in DPP on bone density. Osteoporos. Int. 2021, 32, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Q.; He, H.; Jiang, L.; Lee, K.O.; Li, D.; Ma, J. Metformin treatment is associated with an increase in bone mineral density in type 2 diabetes mellitus patients in China: A retrospective single center study. Diabetes Metab. 2022, 48, 101350. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Liang, G.; Feng, Y.; Jiang, Y.; Qu, F. The potential therapeutic role of metformin in diabetic and non-diabetic bone impairment. Pharmaceuticals 2022, 15, 1274. [Google Scholar] [CrossRef] [PubMed]
- Lekkala, S.; Sacher, S.E.; Taylor, E.A.; Williams, R.M.; Moseley, K.F.; Donnelly, E. Increased advanced glycation endproducts, stiffness, and hardness in iliac crest bone from postmenopausal women with type 2 diabetes mellitus on insulin. J. Bone Miner. Res. 2023, 38, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Du, X.; Wu, M.J.; Shi, B.M. The use of metformin, insulin, sulphonylureas, and thiazolidinediones and the risk of fracture: Systematic review and meta-analysis of observational studies. Obes. Rev. 2019, 20, 1494–1503. [Google Scholar] [CrossRef]
- Salari-Moghaddam, A.; Sadeghi, O.; Keshteli, A.H.; Larijani, B.; Esmaillzadeh, A. Metformin use and risk of fracture: A systematic review and meta-analysis of observational studies. Osteoporos. Int. 2019, 30, 1167–1173. [Google Scholar] [CrossRef]
- Tsai, W.-H.; Kong, S.-K.; Lin, C.-L.; Cheng, K.-H.; Cheng, Y.-T.; Chien, M.-N.; Lee, C.-C.; Tsai, M.-C. Risk of fracture caused by anti-diabetic drugs in individuals with type 2 diabetes: A network meta-analysis. Diabetes Res. Clin. Pract. 2022, 192, 110082. [Google Scholar] [CrossRef]
- Cheng, L.; Hu, Y.; Li, Y.Y.; Cao, X.; Bai, N.; Lu, T.T.; Li, G.Q.; Li, N.; Wang, A.N.; Mao, X.M. Glucagon-like peptide-1 receptor agonists and risk of bone fracture in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetes Metab. Res. Rev. 2019, 35, e3168. [Google Scholar] [CrossRef]
- Driessen, J.H.; de Vries, F.; van Onzenoort, H.; Harvey, N.C.; Neef, C.; van den Bergh, J.P.; Vestergaard, P.; Henry, R.M. The use of incretins and fractures—A meta-analysis on population-based real life data. Br. J. Clin. Pharmacol. 2017, 83, 923–926. [Google Scholar] [CrossRef]
- Bunck, M.C.; Poelma, M.; Eekhoff, E.M.; Schweizer, A.; Heine, R.J.; Nijpels, G.; Foley, J.E.; Diamant, M. Effects of vildagliptin on postprandial markers of bone resorption and calcium homeostasis in recently diagnosed, well-controlled type 2 diabetes patients. J. Diabetes 2012, 4, 181–185. [Google Scholar] [CrossRef]
- Hegazy, S.K. Evaluation of the anti-osteoporotic effects of metformin and sitagliptin in postmenopausal diabetic women. J. Bone Miner. Metab. 2015, 33, 207–212. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kim, K.Y.; Yoo, M.Y.; Moon, H.; Ku, E.J.; Oh, T.K.; Jeon, H.J. Effect of dipeptidyl peptidase-4 inhibitors on bone health in patients with tpe 2 diabetes mellitus. J. Clin. Med. 2021, 10, 4775. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, T.; Zhou, H.; Peng, H.; Yan, C. Risk of Fractures Associated with Dipeptidyl Peptidase-4 Inhibitor Treatment: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Ther. 2019, 10, 1879–1892. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhu, J.; Hao, Y.; Guo, C.; Zhou, Z. Dipeptidyl peptidase-4 inhibitors and fracture risk: An updated meta-analysis of randomized clinical trials. Sci. Rep. 2016, 6, 29104. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; Liu, F.; Yang, Z.; Yu, S.; Liu, Z.; Yang, Q.; Sun, F. Risk of fracture with dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes aellitus: A systematic review and network meta-analysis combining 177 randomized controlled trials with a median follow-up of 26 weeks. Front. Pharmacol. 2022, 13, 825417. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.; Jeyakumar, N.; Kang, Y.; Dixon, S.; Garg, A.; Naylor, K.; Weir, M.; Clemens, K. Fracture risk of sodium-glucose cotransporter-2 inhibitors in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2022, 17, 835–842. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Watts, N.B.; Usiskin, K.; Polidori, D.; Fung, A.; Sullivan, D.; Rosenthal, N. Evaluation of Bone Mineral Density and Bone Biomarkers in Patients with Type 2 Diabetes Treated with Canagliflozin. J. Clin. Endocrinol. Metab. 2016, 101, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ljunggren, O.; Bolinder, J.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sjöström, C.D.; Sugg, J.; Parikh, S. No effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes. Metab. 2012, 14, 990–999. [Google Scholar] [CrossRef]
- van Dalem, J.; Werkman, N.C.; Bergh, J.P.v.D.; Rossi, B.; Viggers, R.; Eastell, R.; Burden, A.M.; Stehouwer, C.D.; Klungel, O.H.; Brouwers, M.C.; et al. Use of sodium-glucose co-transporter 2 inhibitors, changes in body mass index and risk of fracture: A population-based cohort study. Diabetes Res. Clin. Pract. 2022, 190, 109993. [Google Scholar] [CrossRef]
- Watts, N.B.; Bilezikian, J.P.; Usiskin, K.; Edwards, R.; Desai, M.; Law, G.; Meininger, G. Effects of Canagliflozin on Fracture Risk in Patients with Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2016, 101, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, T.; Cheng, Y.; Lu, Y.; Xue, M.; Xu, L.; Liu, X.; Yu, X.; Sun, B.; Chen, L. Effects of SGLT2 inhibitors on fractures and bone mineral density in type 2 diabetes: An updated meta-analysis. Diabetes Metab. Res. Rev. 2019, 35, e3170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Jardine, M.; Perkovic, V.; Matthews, D.R.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Desai, M.; Oh, R.; Simpson, R.; et al. Canagliflozin and fracture risk in individuals with type 2 diabetes: Results from the CANVAS Program. Diabetologia 2019, 62, 1854–1867. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Ding, L.-L.; Zhang, M.; Zhou, H.-R. Safety of four SGLT2 inhibitors in three chronic diseases: A meta-analysis of large randomized trials of SGLT2 inhibitors. Diabetes Vascul. Dis. Res. 2021, 18, 14791641211011016. [Google Scholar] [CrossRef]
- Zinman, B.; Haffner, S.M.; Herman, W.H.; Holman, R.R.; Lachin, J.M.; Kravitz, B.G.; Paul, G.; Jones, N.P.; Aftring, R.P.; Viberti, G.; et al. Effect of rosiglitazone, metformin, and glyburide on bone biomarkers in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2010, 95, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Vianna, A.G.D.; de Lacerda, C.S.; Pechmann, L.M.; Polesel, M.G.; Marino, E.C.; Borba, V.Z.C.; Barreto, F.d.C. Vildagliptin has the same safety profile as a sulfonylurea on bone metabolism and bone mineral density in post-menopausal women with type 2 diabetes: A randomized controlled trial. Diabetol. Metab. Syndr. 2017, 9, 35. [Google Scholar] [CrossRef]
- Gilbert, M.P.; Pratley, R.E. The impact of diabetes and diabetes medications on bone health. Endocr. Rev. 2015, 36, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.P.; Marre, M.; Holst, J.J.; Garber, A.; Baeres, F.M.; Thomsen, H.; Pratley, R.E. Comparison of the Long-Term Effects of Liraglutide and Glimepiride Monotherapy on Bone Mineral Density in Patients with Type 2 Diabetes. Endocr. Pract. 2016, 22, 406–411. [Google Scholar] [CrossRef]
- Tao, Y.; Meng, E.; Shi, J.; Zhang, Z. Sulfonylureas use and fractures risk in elderly patients with type 2 diabetes mellitus: A meta-analysis study. Aging Clin. Experim Res. 2021, 33, 2133–2139. [Google Scholar] [CrossRef]
- Liu, D.; Bai, J.-J.; Yao, J.-J.; Wang, Y.-B.; Chen, T.; Xing, Q.; Bai, R. Association of insulin glargine treatment with bone mineral density in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2021, 14, 1909–1917. [Google Scholar] [CrossRef]
- Stage, T.B.; Christensen, M.H.; Jorgensen, N.R.; Beck-Nielsen, H.; Brosen, K.; Gram, J.; Frost, M. Effects of metformin, rosiglitazone and insulin on bone metabolism in patients with type 2 diabetes. Bone 2018, 112, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, N.H.; Vestergaard, P. Diabetes and osteoporosis e treating two entities: A challenge or cause for concern? Best. Pract. Res. Clin. Rheumatol. 2022, 36, 101779. [Google Scholar] [CrossRef] [PubMed]
- Billington, E.O.; Grey, A.; Bolland, M.J. The effect of thiazolidinediones on bone mineral density and bone turnover: Systematic review and meta-analysis. Diabetologia 2015, 58, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Cairoli, E.; Grassi, G.; Gaudio, A.; Palermo, A.; Vescini, F.; Falchetti, A.; Merlotti, D.; Eller-Vainicher, C.; Carnevale, V.; Scillitani, A.; et al. Validation of the clinical consensus recommendations on the management of fracture risk in postmenopausal women with type 2 diabetes. Nutr. Metab. Cardiov. Dis. 2023, 33, 158–167. [Google Scholar] [CrossRef]
- Evenepoel, P.; Cunningham, J.; Ferrari, S.; Haarhaus, M.; Javaid, M.K.; Lafage-Proust, M.-H.; Prieto-Alhambra, D.; Torres, P.U.; Cannata-Andia, J.; Vervloet, M.; et al. European Consensus Statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4-G5D. Nephrol. Dial. Transplant. 2021, 36, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Farsani, M.A.; Banitalebi, E.; Faramarzi, M.; Bakhtiari, N.; Rahimi, M.; Duque, G. Bone–muscle crosstalk following exercise plus Ursolic acid by myomiR-133a/Cx43-Runx2 axis in aged type 2 diabetes rat models. Chem. Biol. Interact. 2023, 370, 110315. [Google Scholar] [CrossRef]
- Chapman, A.; Meyer, C.; Renehan, E.; Hill, K.D.; Browning, C.J. Exercise interventions for the improvement of falls-related outcomes among older adults with diabetes mellitus: A systematic review and meta-analyses. J. Diabetes Compl. 2017, 31, 631–645. [Google Scholar] [CrossRef]
- Abildgaard, J.; Johansen, M.Y.; Skov-Jeppesen, K.; Andersen, L.B.; Karstoft, K.; Hansen, K.B.; Hartmann, B.; Holst, J.J.; Pedersen, B.K.; Ried-Larsen, M. Effects of a lifestyle intervention on bone turnover in persons with type 2 diabetes: A post hoc analysis of the U-TURN trial. Med. Sci. Sports Exerc. 2021, 54, 38–46. [Google Scholar] [CrossRef]
- Hidayat, K.; Fang, Q.-L.; Shi, B.-M.; Qin, L.-Q. Influence of glycemic control and hypoglycemia on the risk of fracture in patients with diabetes mellitus: A systematic review and meta-analysis of observational studies. Osteoporos. Int. 2021, 32, 1693–1704. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cipriani, C.; Lauriero, G.; Tripepi, G.; Ferrari, S.; Bover, J.; Ravera, M.; Barbuto, S.; Cianciolo, G.; De Nicola, L.; Brandi, M.L.; et al. Effect of Antidiabetic Drugs on Bone Health in Patients with Normal Renal Function and in Chronic Kidney Disease (CKD): Insight into Clinical Challenges in the Treatment of Type 2 Diabetes. J. Clin. Med. 2023, 12, 7260. https://doi.org/10.3390/jcm12237260
Cipriani C, Lauriero G, Tripepi G, Ferrari S, Bover J, Ravera M, Barbuto S, Cianciolo G, De Nicola L, Brandi ML, et al. Effect of Antidiabetic Drugs on Bone Health in Patients with Normal Renal Function and in Chronic Kidney Disease (CKD): Insight into Clinical Challenges in the Treatment of Type 2 Diabetes. Journal of Clinical Medicine. 2023; 12(23):7260. https://doi.org/10.3390/jcm12237260
Chicago/Turabian StyleCipriani, Cristiana, Gabriella Lauriero, Giovanni Tripepi, Serge Ferrari, Jordi Bover, Maura Ravera, Simona Barbuto, Giuseppe Cianciolo, Luca De Nicola, Maria Luisa Brandi, and et al. 2023. "Effect of Antidiabetic Drugs on Bone Health in Patients with Normal Renal Function and in Chronic Kidney Disease (CKD): Insight into Clinical Challenges in the Treatment of Type 2 Diabetes" Journal of Clinical Medicine 12, no. 23: 7260. https://doi.org/10.3390/jcm12237260
APA StyleCipriani, C., Lauriero, G., Tripepi, G., Ferrari, S., Bover, J., Ravera, M., Barbuto, S., Cianciolo, G., De Nicola, L., Brandi, M. L., Minisola, S., Mereu, M. C., Corrao, G., Del Vecchio, L., & Fusaro, M. (2023). Effect of Antidiabetic Drugs on Bone Health in Patients with Normal Renal Function and in Chronic Kidney Disease (CKD): Insight into Clinical Challenges in the Treatment of Type 2 Diabetes. Journal of Clinical Medicine, 12(23), 7260. https://doi.org/10.3390/jcm12237260








