Exploring the Pressure Characteristics of the PRESERFLO MicroShunt in In Vitro Studies and Effects of Sclera on Device Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
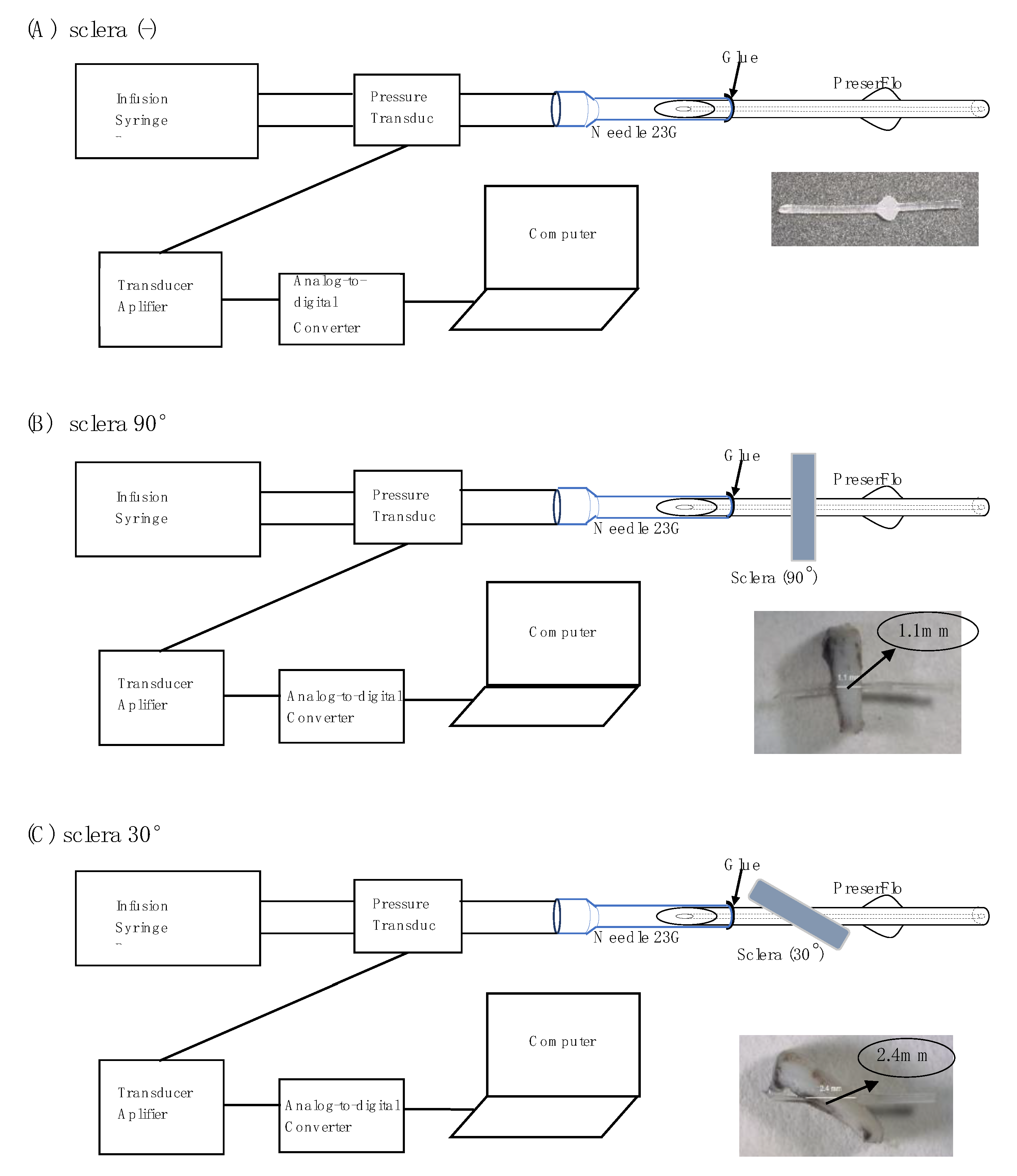
2.2. Experimental Setting
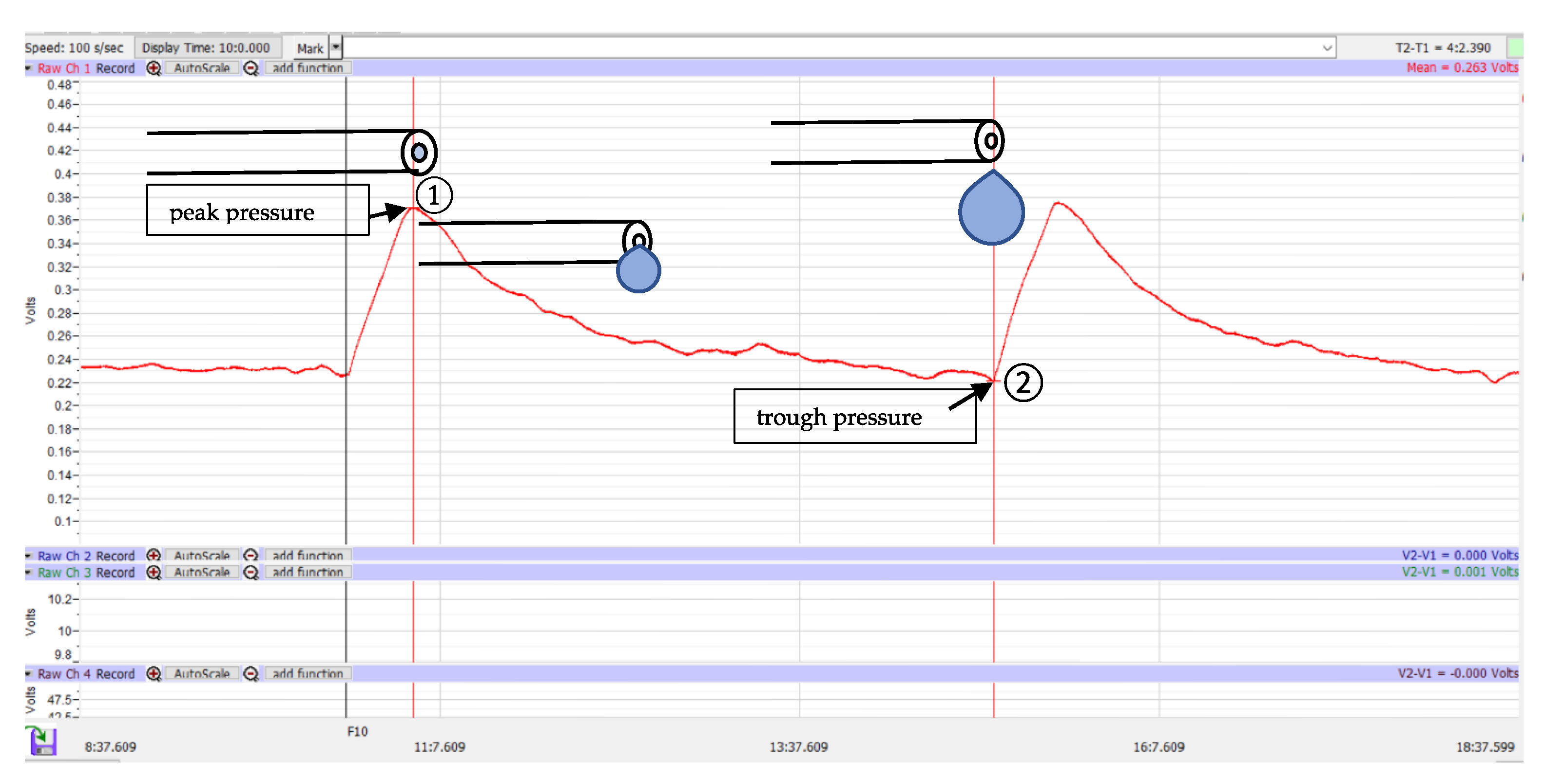
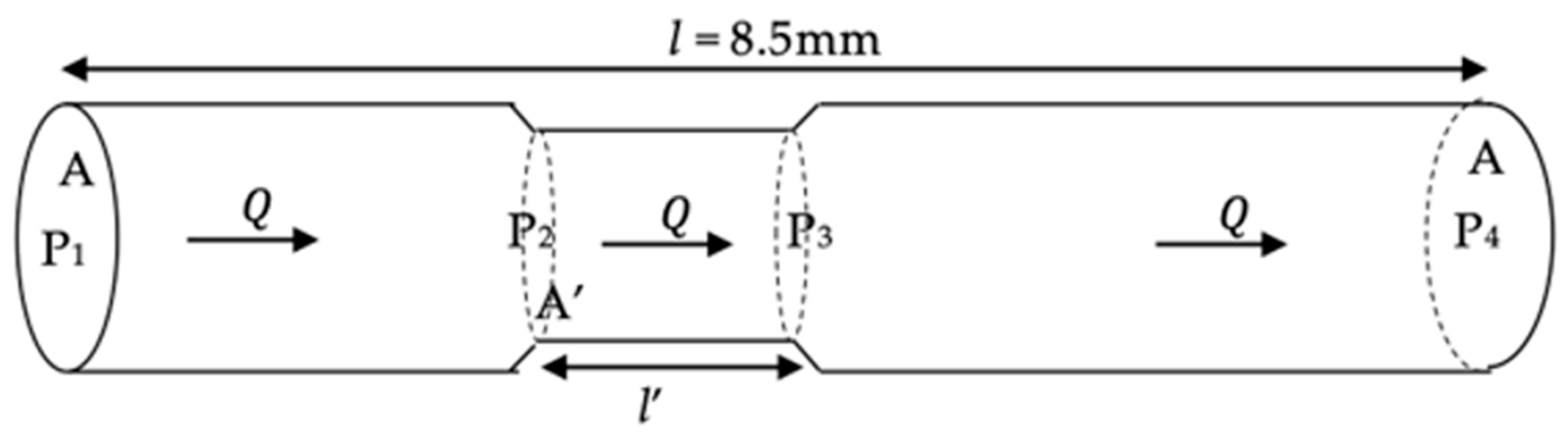
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Bluwol, E. Glaucoma treatment. Rev. Prat. 2016, 66, 508–513. [Google Scholar] [PubMed]
- Pereira, I.C.F.; van de Wijdeven, R.; Wyss, H.M.; Beckers, H.J.M.; den Toonder, J.M.J. Conventional glaucoma implants and the new MIGS devices: A comprehensive review of current options and future directions. Eye 2021, 35, 3202–3221. [Google Scholar] [CrossRef] [PubMed]
- Rowson, A.C.; Hogarty, D.T.; Maher, D.; Liu, L. Minimally Invasive Glaucoma Surgery: Safety of Individual Devices. J. Clin. Med. 2022, 11, 6833. [Google Scholar] [CrossRef] [PubMed]
- Costagliola, C.; Sbordone, M.; Gandolfi, S.; Cesari, L.; Furneri, G.; Fea, A.M. Minimally Invasive Surgery in Mild-to-Moderate Glaucoma Patients in Italy: Is It Time to Change? Clin. Ophthalmol. 2020, 14, 2639–2655. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, F.A.; Neeson, C.; Solá-Del Valle, D. Microinvasive Glaucoma Surgery: An Evidence-Based Review. Semin. Ophthalmol. 2021, 36, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Wagner, I.V.; Stewart, M.W.; Dorairaj, S.K. Updates on the Diagnosis and Management of Glaucoma. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 618–635. [Google Scholar] [CrossRef] [PubMed]
- Gambini, G.; Carlà, M.M.; Giannuzzi, F.; Caporossi, T.; De Vico, U.; Savastano, A.; Baldascino, A.; Rizzo, C.; Kilian, R.; Caporossi, A.; et al. PreserFlo® MicroShunt: An Overview of This Minimally Invasive Device for Open-Angle Glaucoma. Vision 2022, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Pawiroredjo, S.S.M.; Bramer, W.M.; Pawiroredjo, N.D.; Pals, J.; Poelman, H.J.; de Vries, V.A.; Wolfs, R.C.W.; Ramdas, W.D. Efficacy of the PRESERFLO MicroShunt and a Meta-Analysis of the Literature. J. Clin. Med. 2022, 11, 7149. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, L.; Riss, I.; Batlle, J.F.; Kato, Y.P.; Martin, J.B.; Arrieta, E.; Palmberg, P.; Parrish, R.K., 2nd; Weber, B.A.; Kwon, Y.; et al. The development of a micro-shunt made from poly(styrene-block-isobutylene-block-styrene) to treat glaucoma. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, L.; Wilson, G.J.; Barry, J.J.; Schoephoerster, R.T.; Parel, J.M.; Kennedy, J.P. Medical applications of poly(styrene-block-isobutylene-block-styrene) (“SIBS”). Biomaterials 2008, 29, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, B.; Tripathy, K. Minimally Invasive Glaucoma Surgery. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ibarz Barberá, M.; Hernández-Verdejo, J.L.; Bragard, J.; Burguete, J.; Fernández, L.M.; Rivero, P.T.; de Liaño, R.G.; Teus, M.A. Evaluation of the Ultrastructural and In Vitro Flow Properties of the PRESERFLO MicroShunt. Transl. Vis. Sci. Technol. 2021, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Masdipa, A.; Kaidzu, S.; Tanito, M. Flow Pressure Characteristics of the Ahmed Glaucoma Valve and Possible Effect of Entrapped Air in the Tube. Transl. Vis. Sci. Technol. 2023, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, K. A study on the change of droplet volume by the increase of flow rate. SN Appl. Sci. 2020, 2, 1603. [Google Scholar] [CrossRef]
- Lüke, J.N.; Reinking, N.; Dietlein, T.S.; Haendel, A.; Enders, P.; Lappas, A. Intraoperative primary partial occlusion of the PreserFlo MicroShunt to prevent initial postoperative hypotony. Int. Ophthalmol. 2023, 43, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Batlle, J.F.; Fantes, F.; Riss, I.; Pinchuk, L.; Alburquerque, R.; Kato, Y.P.; Arrieta, E.; Peralta, A.C.; Palmberg, P.; Parrish, R.K., 2nd; et al. Three-Year Follow-up of a Novel Aqueous Humor MicroShunt. J. Glaucoma. 2016, 25, e58–e65. [Google Scholar] [CrossRef]
- Schlenker, M.B.; Durr, G.M.; Michaelov, E.; Ahmed, I.I.K. Intermediate Outcomes of a Novel Standalone Ab Externo SIBS Microshunt with Mitomycin C. Am. J. Ophthalmol. 2020, 215, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.D.; Barnebey, H.S.; Moster, M.R.; Stiles, M.C.; Vold, S.D.; Khatana, A.K.; Flowers, B.E.; Grover, D.S.; Strouthidis, N.G.; Panarelli, J.F. Ab-Externo MicroShunt versus Trabeculectomy in Primary Open-Angle Glaucoma: One-Year Results from a 2-Year Randomized, Multicenter Study. Ophthalmology 2021, 128, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.I.K.; Sadruddin, O.; Panarelli, J.F. Subconjunctival filtration in evolution: Current evidence on MicroShunt implantation for treating patients with glaucoma. Eye Vis. 2023, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Lupardi, E.; Laffi, G.L.; Moramarco, A.; Barboni, P.; Fontana, L. Systematic Preserflo MicroShunt Intraluminal Stenting for Hypotony Prevention in Highly Myopic Patients: A Comparative Study. J. Clin. Med. 2023, 12, 1677. [Google Scholar] [CrossRef] [PubMed]




| Pressure, | Median (IQR) | p Value | ||||
|---|---|---|---|---|---|---|
| mmHg | (-) | 90° | 30° | (-) vs. 90° | (-) vs. 30° | 30° vs. 90° |
| Peak | 6.76 (6.10–7.14) | 7.81 (7.06–8.16) | 7.96 (6.71–8.55) | 0.0020 | 0.0039 | 0.77 |
| Trough | 4.74 (4.07–5.45) | 6.25 (5.02–7.11) | 5.76 (5.07–6.07) | 0.0039 | 0.037 | 0.57 |
| p value | 0.0020 | 0.0020 | 0.0020 | |||
| Area, μm2 | Sclera (-) | Sclera 90° | p Value |
|---|---|---|---|
| Median (IQR) | 3927 (3804–4108) | 3515 (3342–3900) | 0.0078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masdipa, A.; Kaidzu, S.; Tanito, M. Exploring the Pressure Characteristics of the PRESERFLO MicroShunt in In Vitro Studies and Effects of Sclera on Device Performance. J. Clin. Med. 2023, 12, 7266. https://doi.org/10.3390/jcm12237266
Masdipa A, Kaidzu S, Tanito M. Exploring the Pressure Characteristics of the PRESERFLO MicroShunt in In Vitro Studies and Effects of Sclera on Device Performance. Journal of Clinical Medicine. 2023; 12(23):7266. https://doi.org/10.3390/jcm12237266
Chicago/Turabian StyleMasdipa, Andi, Sachiko Kaidzu, and Masaki Tanito. 2023. "Exploring the Pressure Characteristics of the PRESERFLO MicroShunt in In Vitro Studies and Effects of Sclera on Device Performance" Journal of Clinical Medicine 12, no. 23: 7266. https://doi.org/10.3390/jcm12237266






