Simultaneous Bilateral Video–Endoscopic Inguinal Lymphadenectomy for Penile Carcinoma: Surgical Setting, Feasibility, Safety, and Preliminary Oncological Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Variables Definition
2.2. Outcome Measurements and Statistical Analysis
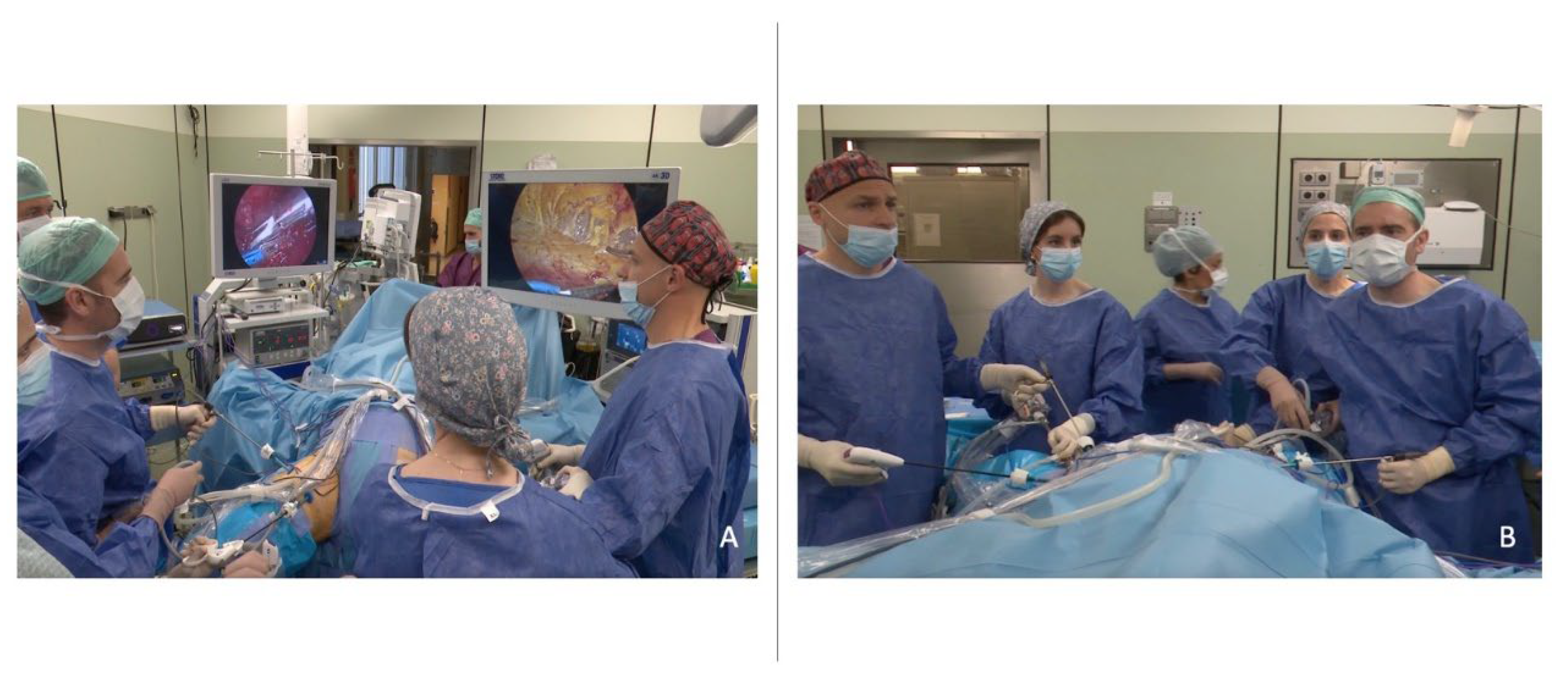
2.3. Patient Positioning, Trocar Placement and Surgical Setting
2.4. Surgical Technique and Postoperative Care
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ayres, B.; Crook, J.; van der Heijden, M.; Johnstone, P.; Necchi, A.; Oliveira, P.; Spiess, P.E.; Johnstone, P.A.S.; Crook, J.; Pettaway, C.A.; et al. EAU-ASCO Collaborative Guidelines on 2023; American Society of Clinical Oncology Publications: Alexandria, VA, USA, 2023. [Google Scholar]
- Wood, H.M.; Angermeier, K.W. Anatomic considerations of the penis, lymphatic drainage, and biopsy of the sentinel node. Urol. Clin. N. Am. 2010, 37, 327–334. [Google Scholar] [CrossRef]
- Woldu, S.L.; Ci, B.; Hutchinson, R.C.; Krabbe, L.-M.; Singla, N.; Passoni, N.M.; Clinton, T.N.; Raj, G.V.; Miller, D.S.; Sagalowsky, A.I.; et al. Usage and survival implications of surgical staging of inguinal lymph nodes in intermediate- to high-risk, clinical localized penile cancer: A propensity-score matched analysis. Urol. Oncol. 2018, 36, 159.e7–159.e17. [Google Scholar] [CrossRef] [PubMed]
- Fallara, G.; Pozzi, E.; Cakir, O.O.; Tandogdu, Z.; Castiglione, F.; Salonia, A.; Alnajjar, H.M.; Muneer, A. Diagnostic Accuracy of Dynamic Sentinel Lymph Node Biopsy for Penile Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2023, 9, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Ercole, C.E.; Pow-Sang, J.M.; Spiess, P.E. Update in the surgical principles and therapeutic outcomes of inguinal lymph node dissection for penile cancer. Urol. Oncol. 2013, 31, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Tobias-Machado, M.; Tavares, A.; Molina, W.R.; Forseto, P.H.; Juliano, R.V.; Wroclawski, E.R. Video endoscopic inguinal lymphadenectomy (VEIL): Minimally invasive resection of inguinal lymph nodes. Int. Braz. J. Urol. 2006, 32, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.S.; Tomar, V.; Bhattar, R.; Jha, A.K.; Priyadarshi, S. Video Endoscopic Inguinal Lymphadenectomy vs. Open Inguinal Lymphadenectomy for Carcinoma Penis: Expanding Role and Comparison of Outcomes. Urology 2018, 113, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sethia, K.K. Prospective study comparing video-endoscopic radical inguinal lymph node dissection (VEILND) with open radical ILND (OILND) for penile cancer over an 8-year period. BJU Int. 2017, 119, 530–534. [Google Scholar] [CrossRef]
- Tobias-Machado, M.; Ornellas, A.A.; Hidaka, A.K.; Medina, L.G.; Mattos, P.A.L.; Besio, R.S.; Abreu, D.; Castro, P.R.; Nishimoto, R.H.; Astigueta, J.; et al. Long-term oncological and surgical outcomes after Video Endoscopic Inguinal Lymphadenectomy (VEIL) in patients with penile cancer. Int. Braz. J. Urol. 2023, 49, 580–589. [Google Scholar] [CrossRef]
- Catalona, W.J. Modified inguinal lymphadenectomy for carcinoma of the penis with preservation of saphenous veins: Technique and preliminary results. J. Urol. 1988, 140, 306–310. [Google Scholar] [CrossRef]
- Patel, K.N.; Salunke, A.; Bakshi, G.; Jayaprakash, D.; Pandya, S.J. Robotic-Assisted Video-Endoscopic Inguinal Lymphadenectomy (RAVEIL) and Video-Endoscopic Inguinal Lymphadenectomy (VEIL) versus Open Inguinal Lymph-Node Dissection (OILND) in carcinoma of penis: Comparison of perioperative outcomes, complications and oncological outcomes. A systematic review and meta-analysis. Urol. Oncol. 2022, 40, 112.e11–112.e22. [Google Scholar] [CrossRef]
- Nabavizadeh, R.; Petrinec, B.; Nabavizadeh, B.; Singh, A.; Rawal, S.; Master, V. Inguinal lymph node dissection in the era of minimally invasive surgical technology. Urol. Oncol. 2023, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, K.; Li, R.; Lu, J.; Wu, T.; Liu, Z.; Fu, X.; Tang, Q.; Ma, J. Bilateral inguinal lymphadenectomy using simultaneous double laparoscopies for penile cancer: A retrospective study. Urol. Oncol. 2022, 40, 112.e1–112.e9. [Google Scholar] [CrossRef] [PubMed]
- Pompeo, A.; Tobias-Machado, M.; Molina, W.R.; Ii, J.L.; Sehrt, D.; Pompeo, A.C.L.; Kim, F.J. Extending boundaries in minimally invasive procedures with simultaneous bilateral video endoscopic inguinal lymphadenectomy (veil) for penile cancer: Initial Denver health medical center and ABC school of medicine experience and surgical considerations. Int. Braz. J. Urol. 2013, 39, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Herrel, L.A.; Butterworth, R.M.; Jafri, S.M.; Ying, C.; Delman, K.A.; Kooby, D.A.; Ogan, K.E.; Canter, D.J.; Maste, V.A. Bilateral Endoscopic Inguinofemoral Lymphadenectomy Using Simultaneous Carbon Dioxide Insufflation: An Initial Report of a Novel Approach. Can. J. Urol. 2012, 19, 6306–6309. Available online: https://pubmed.ncbi.nlm.nih.gov/22704321/ (accessed on 25 July 2023). [PubMed]
- Horvath, B.; Kloesel, B.; Todd, M.M.; Cole, D.J.; Prielipp, R.C. The Evolution, Current Value, and Future of the American Society of Anesthesiologists Physical Status Classification System. Anesthesiology 2021, 135, 904–919. [Google Scholar] [CrossRef] [PubMed]
- Chipollini, J.; Azizi, M.; Vullo, S.L.; Mariani, L.; Zhu, Y.; Ye, D.W.; Ornellas, A.A.; Watkin, N.; Ager, M.; Hakenberg, O.; et al. Identifying an optimal lymph node yield for penile squamous cell carcinoma: Prognostic impact of surgical dissection. BJU Int. 2020, 125, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Soodana-Prakash, N.; Koru-Sengul, T.; Miao, F.; Lopategui, D.M.; Savio, L.F.; Moore, K.J.; Johnson, T.A.; Alameddine, M.; Barboza, M.P.; Parekh, D.J.; et al. Lymph node yield as a predictor of overall survival following inguinal lymphadenectomy for penile cancer. Urol. Oncol. 2018, 36, 471.e19–471.e27. [Google Scholar] [CrossRef]
- Daseler, E.H. Radical excision of the inguinal and iliac lymph glands. Univ. Hosp. Bull. 1949, 15, 70–74. [Google Scholar]
- Nabavizadeh, R.; Petrinec, B.; Necchi, A.; Tsaur, I.; Albersen, M.; Master, V. Utility of Minimally Invasive Technology for Inguinal Lymph Node Dissection in Penile Cancer. J. Clin. Med. 2020, 9, 2501. [Google Scholar] [CrossRef]
- Bandini, M.; Ahmed, M.; Basile, G.; Watkin, N.; Master, V.; Zhu, Y.; Prakash, G.; Rodriguez, A.; Ssebakumba, M.K.; Leni, R.; et al. A global approach to improving penile cancer care. Nat. Rev. Urol. 2022, 19, 231–239. [Google Scholar] [CrossRef]
- Prakash, G.; Arora, A.; Bandini, M.; Basile, G.; Pal, M.; Griffiths, G.; Cornes, R.; Zhu, Y.; Rodriguez, A.; Alberson, M.; et al. Variations in Penile Cancer Management: Results from the Global Society of Rare Genitourinary Tumors Survey. Clin. Genitourin. Cancer 2023, 21, 376–382. [Google Scholar] [CrossRef]
- Glascock, J.M.; Winfield, H.N.; Lund, G.O.; Donovan, J.F.; Ping, S.T.S.; Griffiths, D.L. Carbon dioxide homeostasis during transperitoneal or extraperitoneal laparoscopic pelvic lymphadenectomy: A real-time intraoperative comparison. J. Endourol. 1996, 10, 319–323. [Google Scholar] [CrossRef]
- Moro, F.D.; Crestani, A.; Valotto, C.; Guttilla, A.; Soncin, R.; Mangano, A.; Zattoni, F. Anesthesiologic effects of transperitoneal versus extraperitoneal approach during robot-assisted radical prostatectomy: Results of a prospective randomized study. Int. Braz. J. Urol. 2015, 41, 466–472. [Google Scholar] [CrossRef]


| Variable | cB-VEIL (n = 20) | sB-VEIL (n = 10) | p-Value |
|---|---|---|---|
| Age, yrs | |||
| Median (IQR) | 69 (62.8–76.2) | 76.5 (71–79) | 0.18 |
| BMI, kg/m | |||
| Median (IQR) | 28.1 (27.6–33.7) | 26.2 (25–28.5) | 0.08 |
| ASA score, n (%) | 0.6 | ||
| 1 | 1 (5) | 0 (0) | |
| 2 | 8 (40) | 6 (60) | |
| 3 | 11 (55) | 4 (40) | |
| Diabetes, n (%) | 0.9 | ||
| No | 14 (70) | 6 (60) | |
| Yes | 6 (30) | 4 (40) | |
| Smoking status, n (%) | 0.8 | ||
| Never | 11 (55) | 5 (50) | |
| Current | 5 (25) | 3 (30) | |
| Former | 4 (20) | 2 (20) | |
| Primary surgery type, n (%) | 0.9 | ||
| Glandectomy | 8 (45) | 4 (40) | |
| Partial penectomy | 7 (35) | 4 (40) | |
| Total penectomy | 4 (20) | 2 (20) | |
| Primary pT stage, n (%) | 0.03 | ||
| pT1 | 0 (0) | 4 (40) | |
| pT2 | 10 (50) | 3 (30) | |
| pT3 | 10 (50) | 3 (30) | |
| Primary tumor grade, n (%) | 0.1 | ||
| G1 | 3 (15) | 1 (10) | |
| G2 | 10 (50) | 4 (40) | |
| G3 | 7 (35) | 5 (50) | |
| cN stage, n (%) | 0.7 | ||
| cN0 | 4 (20) | 3 (30) | |
| cN1-2 | 16 (80) | 7 (70) | |
| LVI, n (%) | 0.9 | ||
| No | 16 (80) | 7 (70) | |
| Yes | 4 (20) | 3 (30) | |
| pN stage, n (%) | 0.6 | ||
| pN0 | 14 (70) | 6 (60) | |
| pN1 | 3 (15) | 3 (30) | |
| pN2 | 3 (15) | 1 (10) |
| Variable | cB-VEIL (n = 20) | sB-VEIL (n = 10) | p-Value |
|---|---|---|---|
| Operative time, min | |||
| Median (IQR) | 240 (184–300) | 170 (164–180) | <0.01 |
| Total n. LN removed | |||
| Median (IQR) | 13.5 (10.8–18) | 14 (11.8–15.2) | 0.7 |
| Total n. positive LN | |||
| Median (IQR) | 0 (0–1.25) | 0 (0–1) | 0.9 |
| N. LN right | |||
| Median (IQR) | 7 (5.75–10.2) | 7 (5–8) | 0.5 |
| N. positive LN right, | |||
| Median (IQR) | 0 (0–1) | 0 (0–1) | 0.09 |
| N. LN left | |||
| Median (IQR) | 7 (5.75–7.5) | 7 (6.5–8) | 0.7 |
| N. positive LN left, | |||
| Median (IQR) | 0 (0–0) | 0 (0–1) | 0.2 |
| EBL, mL | |||
| Median (IQR) | 10 (10–25) | 10 (10–25) | 0.9 |
| LOS, days | |||
| Median (IQR) | 6 (5–7.25) | 7 (6.75–8.5) | 0.2 |
| Drainage removal, days | |||
| Median (IQR) | 28.5 (21.5–50.5) | 25.5 (19.2–32.8) | 0.2 |
| Wound complication, n (%) | 0.7 | ||
| No | 17 (85) | 9 (90) | |
| Yes | 3 (15) | 1 (10) | |
| Lymphocele, n (%) | 0.9 | ||
| No | 17 (80) | 8 (80) | |
| Yes | 4 (20) | 2 (20) | |
| Lymphedema, n (%) | 0.9 | ||
| No | 14 (70) | 7 (70) | |
| Yes | 6 (30) | 3 (30) | |
| DVT, n (%) | 0.8 | ||
| No | 19 (95) | 10 (100) | |
| Yes | 1 (5) | 0 (0) | |
| Clavien–Dindo classification, n (%) * | 0.6 | ||
| 0 | 6 (30) | 4 (40) | |
| 1 | 6 (30) | 2 (20) | |
| 2 | 5 (25) | 3 (30) | |
| 3a | 3 (15) | 1 (10) | |
| 3b | 0 (0) | 0 (0) | |
| 90-d readmission, days | 0.9 | ||
| No | 16 (80) | 8 (80) | |
| Yes | 4 (20) | 2 (20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaya, J.M.; Basile, G.; Gavrilov, P.; Gallioli, A.; Territo, A.; Robalino, J.; Hernandez, P.; Sanchez-Molina, R.; Bravo, A.; Algaba, F.; et al. Simultaneous Bilateral Video–Endoscopic Inguinal Lymphadenectomy for Penile Carcinoma: Surgical Setting, Feasibility, Safety, and Preliminary Oncological Outcomes. J. Clin. Med. 2023, 12, 7272. https://doi.org/10.3390/jcm12237272
Gaya JM, Basile G, Gavrilov P, Gallioli A, Territo A, Robalino J, Hernandez P, Sanchez-Molina R, Bravo A, Algaba F, et al. Simultaneous Bilateral Video–Endoscopic Inguinal Lymphadenectomy for Penile Carcinoma: Surgical Setting, Feasibility, Safety, and Preliminary Oncological Outcomes. Journal of Clinical Medicine. 2023; 12(23):7272. https://doi.org/10.3390/jcm12237272
Chicago/Turabian StyleGaya, Josep M., Giuseppe Basile, Pavel Gavrilov, Andrea Gallioli, Angelo Territo, Jorge Robalino, Pedro Hernandez, Raul Sanchez-Molina, Alejandra Bravo, Ferran Algaba, and et al. 2023. "Simultaneous Bilateral Video–Endoscopic Inguinal Lymphadenectomy for Penile Carcinoma: Surgical Setting, Feasibility, Safety, and Preliminary Oncological Outcomes" Journal of Clinical Medicine 12, no. 23: 7272. https://doi.org/10.3390/jcm12237272
APA StyleGaya, J. M., Basile, G., Gavrilov, P., Gallioli, A., Territo, A., Robalino, J., Hernandez, P., Sanchez-Molina, R., Bravo, A., Algaba, F., Huguet, J., Sanguedolce, F., Palou, J., Rosales, A., & Breda, A. (2023). Simultaneous Bilateral Video–Endoscopic Inguinal Lymphadenectomy for Penile Carcinoma: Surgical Setting, Feasibility, Safety, and Preliminary Oncological Outcomes. Journal of Clinical Medicine, 12(23), 7272. https://doi.org/10.3390/jcm12237272







