Percutaneous Left Atrial Appendage Occlusion—Current Evidence and Future Directions
Abstract
:1. Introduction
2. Patient Selection
2.1. Patients Who Should Be Considered
2.2. Patients Who Should Not Be Considered
3. Imaging
3.1. Anatomy and Function of the LAA
3.2. Baseline Imaging
3.3. Periprocedural Imaging
3.4. Postprocedural Imaging and Device Surveillance
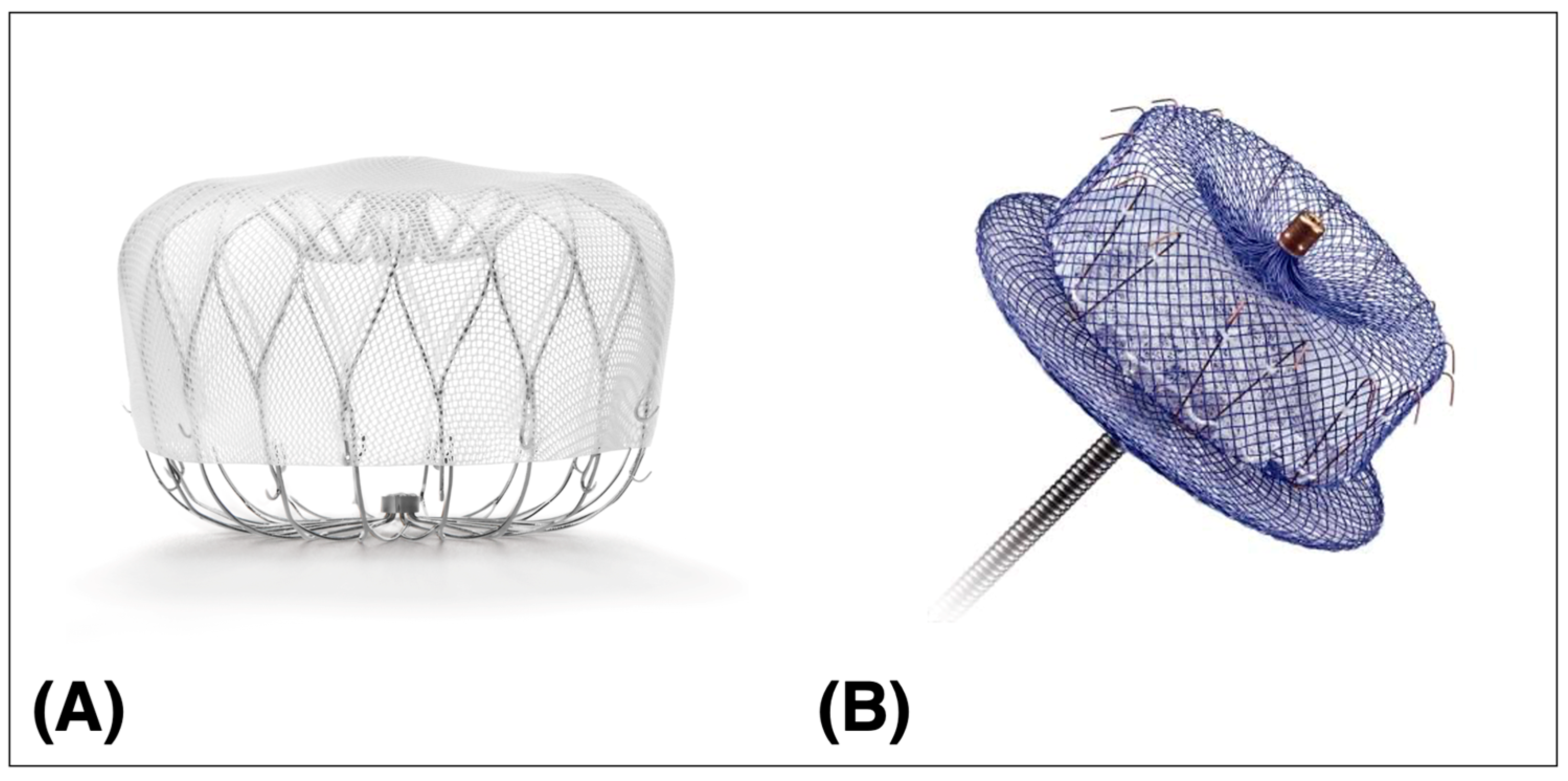
4. Procedural Aspects
- The widely implemented TEE guidance for LAAO is the main reason for the utilization of general anesthesia in the US. As a feasible and safe alternative, many European centers routinely perform LAAO with a moderate conscious sedation protocol resulting in a reduction in procedural time, hospital stay, and costs [37,38].
- Vascular access is preferably achieved by ultrasound-guided puncture of the right femoral vein.
- Therapeutic anticoagulation is attained by administration of unfractionated heparin. Activated clotting time (ACT) should range between 250 and 300 s. Periodical ACT monitoring is obligatory. After completion of the intervention, administration of protamine may be considered if hemostasis is not achieved by other means (e.g., manual compression, suture, etc.).
- Coaxial orientation of the access sheath with the LAA is the purpose of a correct TSP. Typically, an inferior and posterior septal position on the interatrial septum is chosen. Specific anatomy may require a different TSP site; preprocedural imaging can help determine the location of the TSP. Passage through a patent foramen ovale should be avoided as it may not result in coaxiality of the device with the LAA. TEE guidance for TSP and sheath positioning is best achieved in a bicaval view.
- LAA dimensions vary depending on the hydration status. For correct device sizing, LAA measurements should be performed when mean LA pressure is ≥12 mmHg.
- Entry into the LAA should be as atraumatic as possible to reduce the risk of perforation/pericardial effusion. The use of a pigtail catheter is advocated.
- LAAO device deployment is performed according to the manufacturer’s instruction. Established release criteria for the two available devices should be considered (Table 2).
- Assessment of pericardial effusion should be performed at the beginning and end of the LAAO procedure.
- Given the risk of periprocedural stroke, a neurological assessment should be performed after anesthesia/sedation.
- Administration of appropriate endocarditis prophylaxis for 6 months after device implantation.
5. Intraprocedural Complications
5.1. Pericardial Effusion
5.2. Management of Pericardial Effusion/Tamponade
5.3. LAAO Device Embolization
5.4. Management of Device Embolization
5.5. Periprocedural Strokes
5.6. Management of Periprocedural Stroke
6. Postprocedural Complications
6.1. Device-Related Thrombosis
6.2. Peri-Device Leaks
7. Antithrombotic Regimen after LAAO
8. Further Directions
8.1. Patient Selection
8.2. Imaging
8.3. LAAO Devices
8.4. Antithrombotic Regimen
9. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ACT | Activated clotting time |
| AF | Atrial fibrillation |
| CT | Computed tomography |
| DAPT | Dual antiplatelet therapy |
| DOAC | Direct oral anticoagulant |
| DRT | Device-related thrombosis |
| ECG | Electrocardiogram |
| FDA | Food and Drug Administration |
| ICE | Intracardiac echocardiography |
| LA | Left atrium |
| LAA | Left atrial appendage |
| LAAO | Left atrial appendage occlusion |
| NCDR | National Cardiovascular Data Registry |
| OAC | Oral anticoagulation |
| PDL | Peri-device leak |
| PVI | Pulmonary vein isolation |
| RCT | Randomized controlled trial |
| TAVR | Transcatheter aortic valve replacement |
| TEE | Transesophageal echocardiography |
| TTE | Transthoracic echocardiography |
| TSP | Transseptal puncture |
| VKA | Vitamin K antagonist |
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation 2019, 139, e56–e528, Erratum in Circulation 2020, 141, e33. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498, Erratum in Eur. Heart J. 2021, 42, 507+546–547+4194. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T., Jr.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151, Erratum in Circulation 2019, 140, e285. [Google Scholar] [CrossRef] [PubMed]
- Blackshear, J.L.; Odell, J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 1996, 61, 755–759. [Google Scholar] [CrossRef]
- Mahajan, R.; Brooks, A.G.; Sullivan, T.; Lim, H.S.; Alasady, M.; Abed, H.S.; Ganesan, A.N.; Nayyar, S.; Lau, D.H.; Roberts-Thomson, K.C.; et al. Importance of the underlying substrate in determining thrombus location in atrial fibrillation: Implications for left atrial appendage closure. Heart 2012, 98, 1120–1126. [Google Scholar] [CrossRef]
- Collado, F.M.S.; von Buchwald, C.M.L.; Anderson, C.K.; Madan, N.; Suradi, H.S.; Huang, H.D.; Jneid, H.; Kavinsky, C.J. Left Atrial Appendage Occlusion for Stroke Prevention in Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e022274. [Google Scholar] [CrossRef] [PubMed]
- Dudzińska-Szczerba, K.; Kułakowski, P.; Michałowska, I.; Baran, J. Association Between Left Atrial Appendage Morphology and Function and the Risk of Ischaemic Stroke in Patients with Atrial Fibrillation. Arrhythmia Electrophysiol. Rev. 2022, 11, e09. [Google Scholar] [CrossRef]
- Hsu, J.C.; Maddox, T.M.; Kennedy, K.F.; Katz, D.F.; Marzec, L.N.; Lubitz, S.A.; Gehi, A.K.; Turakhia, M.P.; Marcus, G.M. Oral Anticoagulant Therapy Prescription in Patients with Atrial Fibrillation across the Spectrum of Stroke Risk: Insights from the NCDR PINNACLE Registry. JAMA Cardiol. 2016, 1, 55–62. [Google Scholar] [CrossRef]
- Salmasi, S.; Loewen, P.S.; Tandun, R.; Andrade, J.G.; De Vera, M.A. Adherence to oral anticoagulants among patients with atrial fibrillation: A systematic review and meta-analysis of observational studies. BMJ Open 2020, 10, e034778. [Google Scholar] [CrossRef]
- Chen, N.; Brooks, M.M.; Hernandez, I. Latent Classes of Adherence to Oral Anticoagulation Therapy Among Patients With a New Diagnosis of Atrial Fibrillation. JAMA Netw. Open 2020, 3, e1921357. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Sievert, H.; Halperin, J.; Doshi, S.K.; Buchbinder, M.; Neuzil, P.; Huber, K.; Whisenant, B.; Kar, S.; Swarup, V.; et al. Percutaneous Left Atrial Appendage Closure vs Warfarin for Atrial Fibrillation: A randomized clinical trial. JAMA 2014, 312, 1988–1998, Erratum in JAMA 2015, 313, 1061. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin Therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12, Erratum in J. Am. Coll. Cardiol. 2014, 64, 1186. [Google Scholar] [CrossRef] [PubMed]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef] [PubMed]
- Turagam, M.K.; Osmancik, P.; Neuzil, P.; Dukkipati, S.R.; Reddy, V.Y. Left Atrial Appendage Closure Versus Oral Anticoagulants in Atrial Fibrillation: A Meta-Analysis of Randomized Trials. J. Am. Coll. Cardiol. 2020, 76, 2795–2797. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Reddy, V.Y.; Gordon, N.T.; Delurgio, D.; Doshi, S.K.; Desai, A.J.; Stone, J.E., Jr.; Kar, S. Long-Term Safety and Efficacy in Continued Access Left Atrial Appendage Closure Registries. J. Am. Coll. Cardiol. 2019, 74, 2878–2889. [Google Scholar] [CrossRef]
- Boersma, L.V.; Schmidt, B.; Betts, T.R.; Sievert, H.; Tamburino, C.; Teiger, E.; Pokushalov, E.; Kische, S.; Schmitz, T.; Stein, K.M.; et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: Peri-procedural outcomes from the EWOLUTION registry. Eur. Heart J. 2016, 37, 2465–2474. [Google Scholar] [CrossRef]
- Freeman, J.V.; Varosy, P.; Price, M.J.; Slotwiner, D.; Kusumoto, F.M.; Rammohan, C.; Kavinsky, C.J.; Turi, Z.G.; Akar, J.; Koutras, C.; et al. The NCDR Left Atrial Appendage Occlusion Registry. J. Am. Coll. Cardiol. 2020, 75, 1503–1518. [Google Scholar] [CrossRef]
- Saw, J.; Bennell, M.C.; Singh, S.M.; Wijeysundera, H.C. Cost-Effectiveness of Left Atrial Appendage Closure for Stroke Prevention in Atrial Fibrillation Patients with Contraindications to Anticoagulation. Can. J. Cardiol. 2016, 32, 1355.e9–1355.e14. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Akehurst, R.L.; Gavaghan, M.B.; Amorosi, S.L.; Holmes, D.R., Jr. Cost-Effectiveness of Left Atrial Appendage Closure for Stroke Reduction in Atrial Fibrillation: Analysis of Pooled, 5-Year, Long-Term Data. J. Am. Heart Assoc. 2019, 8, e011577. [Google Scholar] [CrossRef]
- Labori, F.; Persson, J.; Bonander, C.; Jood, K.; Svensson, M. Cost-effectiveness analysis of left atrial appendage occlusion in patients with atrial fibrillation and contraindication to oral anticoagulation. Eur. Heart J. 2021, 43, 1348–1356. [Google Scholar] [CrossRef]
- Hewage, S.A.; Noviyani, R.; Brain, D.; Sharma, P.; Parsonage, W.; McPhail, S.M.; Barnett, A.; Kularatna, S. Cost-effectiveness of left atrial appendage closure for stroke prevention in atrial fibrillation: A systematic review appraising the methodological quality. Cost Eff. Resour. Alloc. 2023, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. Europace 2020, 22, 184. [Google Scholar] [CrossRef]
- Mesnier, J.; Cruz-González, I.; Arzamendi, D.; Freixa, X.; Nombela-Franco, L.; Peral, V.; Caneiro-Queija, B.; Mangieri, A.; Trejo-Velasco, B.; Asmarats, L.; et al. Incidence and Predictors of Early Death in Patients Undergoing Percutaneous Left Atrial Appendage Closure. JACC Clin. Electrophysiol. 2022, 8, 1093–1102. [Google Scholar] [CrossRef]
- Sharma, S.P.; Cheng, J.; Turagam, M.K.; Gopinathannair, R.; Horton, R.; Lam, Y.-Y.; Tarantini, G.; D’Amico, G.; Rofastes, X.F.; Lange, M.; et al. Feasibility of Left Atrial Appendage Occlusion in Left Atrial Appendage Thrombus. JACC Clin. Electrophysiol. 2020, 6, 414–424. [Google Scholar] [CrossRef]
- Marroquin, L.; Tirado-Conte, G.; Pracoń, R.; Streb, W.; Gutierrez, H.; Boccuzzi, G.; Arzamendi-Aizpurua, D.; Cruz-González, I.; Ruiz-Nodar, J.M.; Kim, J.-S.; et al. Management and outcomes of patients with left atrial appendage thrombus prior to percutaneous closure. Heart 2021, 108, 1098–1106. [Google Scholar] [CrossRef]
- Saw, J.; Holmes, D.R.; Cavalcante, J.L.; Freeman, J.V.; Goldsweig, A.M.; Kavinsky, C.J.; Moussa, I.D.; Munger, T.M.; Price, M.J.; Reisman, M.; et al. SCAI/HRS Expert Consensus Statement on Transcatheter Left Atrial Appendage Closure. JACC Cardiovasc. Interv. 2023, 16, 1384–1400. [Google Scholar] [CrossRef] [PubMed]
- Alsagheir, A.; Koziarz, A.; Belley-Côté, E.P.; Whitlock, R.P. Left Atrial Appendage Occlusion: A Narrative Review. J. Cardiothorac. Vasc. Anesthesia 2019, 33, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; DI Biase, L.; Horton, R.P.; Nguyen, T.; Morhanty, P.; Natale, A. Left Atrial Appendage Studied by Computed Tomography to Help Planning for Appendage Closure Device Placement. J. Cardiovasc. Electrophysiol. 2010, 21, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Anselmino, M.; Scaglione, M.; Di Biase, L.; Gili, S.; Santangeli, P.; Corsinovi, L.; Pianelli, M.; Cesarani, F.; Faletti, R.; Righi, D.; et al. Left atrial appendage morphology and silent cerebral ischemia in patients with atrial fibrillation. Heart Rhythm. 2014, 11, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Lupercio, F.; Ruiz, J.C.; Briceno, D.F.; Romero, J.; Villablanca, P.A.; Berardi, C.; Faillace, R.; Krumerman, A.; Fisher, J.D.; Ferrick, K.; et al. Left atrial appendage morphology assessment for risk stratification of embolic stroke in patients with atrial fibrillation: A meta-analysis. Heart Rhythm 2016, 13, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Breitenstein, A.; Glanzmann, M.; Falk, V.; Maisano, F.; Stämpfli, S.F.; Holy, E.W.; Finlay, M.; Ling, L.-H.; Schilling, R.J.; Lüscher, T.F.; et al. Increased prothrombotic profile in the left atrial appendage of atrial fibrillation patients. Int. J. Cardiol. 2015, 185, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Majunke, N.; Sandri, M.; Adams, V.; Daehnert, I.; Mangner, N.; Schuler, G.; Moebius-Winkler, S. Atrial and Brain Natriuretic Peptide Secretion After Percutaneous Closure of the Left Atrial Appendage With the Watchman Device. J. Invasive Cardiol. 2015, 27, 448–452. [Google Scholar] [PubMed]
- Cruz-Gonzalez, I.; Molinero, J.P.; Valenzuela, M.; Rada, I.; Perez-Rivera, J.A.; Jimenez, A.A.; Gabella, T.; Prieto, A.B.; Polo, J.M.; Sánchez, P.L. Brain natriuretic peptide levels variation after left atrial appendage occlusion. Catheter. Cardiovasc. Interv. 2015, 87, E39–E43. [Google Scholar] [CrossRef]
- Bartus, K.; Podolec, J.; Lee, R.J.; Kapelak, B.; Sadowski, J.; Bartus, M.; Oles, K.; Ceranowicz, P.; Trabka, R.; Litwinowicz, R. Atrial natriuretic peptide and brain natriuretic peptide changes after epicardial percutaneous left atrial appendage suture ligation using LARIAT device. J. Physiol. Pharmacol. 2017, 68, 117–123. [Google Scholar]
- Korsholm, K.; Berti, S.; Iriart, X.; Saw, J.; Wang, D.D.; Cochet, H.; Chow, D.; Clemente, A.; De Backer, O.; Jensen, J.M.; et al. Expert Recommendations on Cardiac Computed Tomography for Planning Transcatheter Left Atrial Appendage Occlusion. JACC Cardiovasc. Interv. 2019, 13, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, A.; Saenz, L.C.; Rosso, R.; Silvestry, F.E.; Callans, D.; Marchlinski, F.E.; Garcia, F. Use of Intracardiac Echocardiography in Interventional Cardiology: Working with the Anatomy Rather Than Fighting It. Circulation 2018, 137, 2278–2294. [Google Scholar] [CrossRef]
- Marmagkiolis, K.; Ates, I.; Kose, G.; Iliescu, C.; Cilingiroglu, M. Effectiveness and safety of same day discharge after left atrial appendage closure under moderate conscious sedation. Catheter. Cardiovasc. Interv. 2020, 97, 912–916. [Google Scholar] [CrossRef]
- Lip, G.Y.; Dagres, N.; Proclemer, A.; Svendsen, J.H.; Pison, L.; Blomstrom-Lundqvist, C. Left atrial appendage occlusion for stroke prevention in atrial fibrillation in Europe: Results of the European Heart Rhythm Association survey. Europace 2013, 15, 141–143. [Google Scholar] [CrossRef]
- Lakkireddy, D.; Lakkireddy, D.; Thaler, D.; Thaler, D.; Ellis, C.R.; Ellis, C.R.; Swarup, V.; Swarup, V.; Sondergaard, L.; Sondergaard, L.; et al. Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis (Amulet IDE): A Randomized, Controlled Trial. Circulation 2021, 144, 1543–1552. [Google Scholar] [CrossRef]
- Kar, S.; Doshi, S.K.; Sadhu, A.; Horton, R.; Osorio, J.; Ellis, C.; Stone, J., Jr.; Shah, M.; Dukkipati, S.R.; Adler, S.; et al. Primary Outcome Evaluation of a Next-Generation Left Atrial Appendage Closure Device: Results from the PINNACLE FLX Trial. Circulation 2021, 143, 1754–1762. [Google Scholar] [CrossRef]
- Dukkipati, S.R.; Kar, S.; Holmes, D.R.; Doshi, S.K.; Swarup, V.; Gibson, D.N.; Maini, B.; Gordon, N.T.; Main, M.L.; Reddy, V.Y. Device-Related Thrombus After Left Atrial Appendage Closure: Incidence, Predictors, and Outcomes. Circulation 2018, 138, 874–885. [Google Scholar] [CrossRef]
- Aminian, A.; Schmidt, B.; Mazzone, P.; Berti, S.; Fischer, S.; Montorfano, M.; Lam, S.C.C.; Lund, J.; Asch, F.M.; Gage, R.; et al. Incidence, Characterization, and Clinical Impact of Device-Related Thrombus Following Left Atrial Appendage Occlusion in the Prospective Global AMPLATZER Amulet Observational Study. JACC Cardiovasc. Interv. 2019, 12, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Busu, T.; Shah, K.; Osman, M.; Alqahtani, F.; Raybuck, B. Incidence and Clinical Impact of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion. JACC Clin. Electrophysiol. 2018, 4, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, A.; Vij, V.; Al-Kassou, B.; Gloekler, S.; Galea, R.; Fürholz, M.; Meier, B.; Valgimigli, M.; O’hara, G.; Arzamendi, D.; et al. Device-Related Thrombus After Left Atrial Appendage Closure: Data on Thrombus Characteristics, Treatment Strategies, and Clinical Outcomes From the EUROC-DRT-Registry. Circ. Cardiovasc. Interv. 2021, 14, e010195. [Google Scholar] [CrossRef] [PubMed]
- Pracon, R.; Bangalore, S.; Dzielinska, Z.; Konka, M.; Kepka, C.; Kruk, M.; Kaczmarska-Dyrda, E.; Petryka-Mazurkiewicz, J.; Bujak, S.; Solecki, M.; et al. Device Thrombosis After Percutaneous Left Atrial Appendage Occlusion Is Related to Patient and Procedural Characteristics but Not to Duration of Postimplantation Dual Antiplatelet Therapy. Circ. Cardiovasc. Interv. 2018, 11, e005997. [Google Scholar] [CrossRef] [PubMed]
- Simard, T.; Jung, R.G.; Lehenbauer, K.; Piayda, K.; Pracoń, R.; Jackson, G.G.; Flores-Umanzor, E.; Faroux, L.; Korsholm, K.; Chun, J.K.; et al. Predictors of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion. J. Am. Coll. Cardiol. 2021, 78, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Main, M.L.; Fan, D.; Reddy, V.Y.; Holmes, D.R.; Gordon, N.T.; Coggins, T.R.; House, J.A.; Liao, L.; Rabineau, D.; Latus, G.G.; et al. Assessment of Device-Related Thrombus and Associated Clinical Outcomes With the WATCHMAN Left Atrial Appendage Closure Device for Embolic Protection in Patients With Atrial Fibrillation (from the PROTECT-AF Trial). Am. J. Cardiol. 2016, 117, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, M.; Bliden, K.P.; Ilkhanoff, L.; Venkataraman, G.; Strickberger, A.; Yazdani, S.; McSwain, R.; Rashid, H.; Navarese, E.P.; Plummer, T.; et al. Detailed thrombogenicity phenotyping and 1 year outcomes in patients undergoing WATCHMAN implantation: (TARGET-WATCHMAN) a case–control study. J. Thromb. Thrombolysis 2020, 50, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Nielsen-Kudsk, J.E.; Bergmann, M.; Daniels, M.J.; Tzikas, A.; Reisman, M.; Rana, B.S. Antithrombotic Therapy and Device-Related Thrombosis Following Endovascular Left Atrial Appendage Closure. JACC Cardiovasc. Interv. 2019, 12, 1067–1076. [Google Scholar] [CrossRef]
- Su, P.; McCarthy, K.P.; Ho, S.Y. Occluding the left atrial appendage: Anatomical considerations. Heart 2008, 94, 1166–1170. [Google Scholar] [CrossRef]
- Raphael, C.; Friedman, P.; Saw, J.; Pislaru, S.; Munger, T.; Holmes, D. Residual leaks following percutaneous left atrial appendage occlusion: Assessment and management implications. EuroIntervention 2017, 13, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.; Varosy, P.D.; Du, C.; Aleong, R.G.; Tumolo, A.Z.; West, J.J.; Tzou, W.S.; Curtis, J.P.; Freeman, J.V.; Friedman, D.J.; et al. Device-Sizing and Associated Complications With Left Atrial Appendage Occlusion: Findings From the NCDR LAAO Registry. Circ. Cardiovasc. Interv. 2022, 15, e012183. [Google Scholar] [CrossRef] [PubMed]
- Viles-Gonzalez, J.F.; Kar, S.; Douglas, P.; Dukkipati, S.; Feldman, T.; Horton, R.; Holmes, D.; Reddy, V.Y. The Clinical Impact of Incomplete Left Atrial Appendage Closure with the Watchman Device in Patients with Atrial Fibrillation: A PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients with Atrial Fibrillation) Substudy. J. Am. Coll. Cardiol. 2012, 59, 923–929. [Google Scholar] [CrossRef]
- Afzal, M.R.; Gabriels, J.K.; Jackson, G.G.; Chen, L.; Buck, B.; Campbell, S.; Sabin, D.F.; Goldner, B.; Ismail, H.; Liu, C.F.; et al. Temporal Changes and Clinical Implications of Delayed Peridevice Leak Following Left Atrial Appendage Closure. JACC Clin. Electrophysiol. 2021, 8, 15–25. [Google Scholar] [CrossRef]
- Alkhouli, M.; Du, C.; Killu, A.; Simard, T.; Noseworthy, P.A.; Friedman, P.A.; Curtis, J.P.; Freeman, J.V.; Holmes, D.R. Clinical Impact of Residual Leaks Following Left Atrial Appendage Occlusion: Insights from the NCDR LAAO Registry. JACC Clin. Electrophysiol. 2022, 8, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; Ellis, C.R.; Nielsen-Kudsk, J.E.; Thaler, D.; Gupta, N.; Koulogiannis, K.; Anderson, J.A.; Gage, R.; Lakkireddy, D. Peridevice Leak After Transcatheter Left Atrial Appendage Occlusion: An Analysis of the Amulet IDE Trial. JACC Cardiovasc. Interv. 2022, 15, 2127–2138. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Horton, R.P.; Di Biase, L.; Bassiouny, M.; Al-Ahmad, A.; Mohanty, S.; Gasperetti, A.; Natale, V.N.; Trivedi, C.; Gianni, C.; et al. First Experience of Transcatheter Leak Occlusion with Detachable Coils Following Left Atrial Appendage Closure. JACC Cardiovasc. Interv. 2020, 13, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; De Backer, O.; Ellis, C.R.; Nielsen-Kudsk, J.E.; Sievert, H.; Natale, A.; Lakkireddy, D.; Holmes, D.R. Peridevice Leak After Left Atrial Appendage Occlusion: Incidence, Mechanisms, Clinical Impact, and Management. JACC Cardiovasc. Interv. 2023, 16, 627–642. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151, Erratum in N. Engl. J. Med. 2010, 363, 1877. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- López-López, J.A.; Sterne, J.A.C.; Thom, H.H.Z.; Higgins, J.P.T.; Hingorani, A.D.; Okoli, G.N.; Davies, P.A.; Bodalia, P.N.; Bryden, P.A.; Welton, N.J.; et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: Systematic review, network meta-analysis, and cost effectiveness analysis. BMJ 2017, 359, j5058, Erratum in BMJ 2018, 361, k2295. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G.; Papavasileiou, V.; Makaritsis, K.; Vemmos, K.; Michel, P.; Lip, G.Y. Real-World Setting Comparison of Nonvitamin-K Antagonist Oral Anticoagulants Versus Vitamin-K Antagonists for Stroke Prevention in Atrial Fibrillation: A Systematic Review and Meta-Analysis. Stroke 2017, 48, 2494–2503. [Google Scholar] [CrossRef]
- Li, G.; Lip, G.Y.H.; Holbrook, A.; Chang, Y.; Larsen, T.B.; Sun, X.; Tang, J.; Mbuagbaw, L.; Witt, D.M.; Crowther, M.; et al. Direct comparative effectiveness and safety between non-vitamin K antagonist oral anticoagulants for stroke prevention in nonvalvular atrial fibrillation: A systematic review and meta-analysis of observational studies. Eur. J. Epidemiol. 2018, 34, 173–190. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Liu, X.; Larsen, T.B.; Witt, D.M.; Ye, Z.; Thabane, L.; Li, G.; Lip, G.Y.H. Comparative effectiveness and safety of direct acting oral anticoagulants in nonvalvular atrial fibrillation for stroke prevention: A systematic review and meta-analysis. Eur. J. Epidemiol. 2021, 36, 793–812. [Google Scholar] [CrossRef] [PubMed]
- Deitelzweig, S.; Bergrath, E.; di Fusco, M.; Kang, A.; Savone, M.; Cappelleri, J.C.; Russ, C.; Betts, M.; Cichewicz, A.; Schaible, K.; et al. Real-world evidence comparing oral anticoagulants in non-valvular atrial fibrillation: A systematic review and network meta-analysis. Futur. Cardiol. 2022, 18, 393–405. [Google Scholar] [CrossRef]
- Freeman, J.V.; Higgins, A.Y.; Wang, Y.; Du, C.; Friedman, D.J.; Daimee, U.A.; Minges, K.E.; Pereira, L.; Goldsweig, A.M.; Price, M.J.; et al. Antithrombotic Therapy After Left Atrial Appendage Occlusion in Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2022, 79, 1785–1798. [Google Scholar] [CrossRef]
- Osman, M.; Busu, T.; Osman, K.; Khan, S.U.; Daniels, M.; Holmes, D.R.; Alkhouli, M. Short-Term Antiplatelet Versus Anticoagulant Therapy After Left Atrial Appendage Occlusion: A Systematic Review and Meta-Analysis. JACC Clin. Electrophysiol. 2020, 6, 494–506. [Google Scholar] [CrossRef]
- Rodriguez-Gabella, T.; Nombela-Franco, L.; Regueiro, A.; Jiménez-Quevedo, P.; Champagne, J.; O’Hara, G.; Bernier, M.; Macaya, C.; Rodés-Cabau, J. Single Antiplatelet Therapy Following Left Atrial Appendage Closure in Patients with Contraindication to Anticoagulation. J. Am. Coll. Cardiol. 2016, 68, 1920–1921. [Google Scholar] [CrossRef]
- Pouru, J.-P.; Jaakkola, S.; Lund, J.; Biancari, F.; Saraste, A.; Airaksinen, K.J. Effectiveness of Only Aspirin or Clopidogrel Following Percutaneous Left Atrial Appendage Closure. Am. J. Cardiol. 2019, 124, 1894–1899. [Google Scholar] [CrossRef] [PubMed]
- Jalal, Z.; Dinet, M.-L.; Combes, N.; Pillois, X.; Renou, P.; Sibon, I.; Iriart, X.; Thambo, J.-B. Percutaneous left atrial appendage closure followed by single antiplatelet therapy: Short- and mid-term outcomes. Arch. Cardiovasc. Dis. 2017, 110, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Hildick-Smith, D.; Landmesser, U.; Camm, A.J.; Diener, H.-C.; Paul, V.; Schmidt, B.; Settergren, M.; Teiger, E.; Nielsen-Kudsk, J.E.; Tondo, C. Left atrial appendage occlusion with the Amplatzer™ Amulet™ device: Full results of the prospective global observational study. Eur. Heart J. 2020, 41, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Tondo, C.; Camm, J.; Diener, H.-C.; Paul, V.; Schmidt, B.; Settergren, M.; Teiger, E.; Nielsen-Kudsk, J.E.; Hildick-Smith, D. Left atrial appendage occlusion with the AMPLATZER Amulet device: One-year follow-up from the prospective global Amulet observational registry. EuroIntervention 2018, 14, e590–e597. [Google Scholar] [CrossRef]
- Paitazoglou, C.; Bergmann, M.W.; Ince, H.; Kische, S.; Romanov, A.; Schmitz, T.; Schmidt, B.; Gori, T.; Meincke, F.; Protopopov, A.V.; et al. True Efficacy of LAA Closure: Patient Outcomes on Long-term Single-Antiplatelet or No Therapy: Insights from the EWOLUTION Registry. J. Invasive Cardiol. 2022, 34, E348–E355. [Google Scholar]
- Mesnier, J.; Cruz-González, I.; Arzamendi, D.; Freixa, X.; Nombela-Franco, L.; Peral, V.; Caneiro-Queija, B.; Mangieri, A.; Trejo-Velasco, B.; Asmarats, L.; et al. Early Discontinuation of Antithrombotic Treatment Following Left Atrial Appendage Closure. Am. J. Cardiol. 2022, 171, 91–98. [Google Scholar] [CrossRef]


| 2020 ESC and 2019 AHA/ACC/HRS guideline recommendations 1,2 | “LAA occlusion may be considered for stroke prevention in patients with AF and contraindications for long-term anticoagulant treatment” (Class of recommendation IIb, Level of evidence B). |
| Meet definition of clinical AF 1 | Minimum duration of an ECG tracing of AF required to establish the diagnosis of clinical AF is at least 30 s or entire 12-lead ECG. |
| High thromboembolic risk |
|
| Contraindication to long-term OAC 3 |
|
| Life expectancy > 1 year | Assessing comorbidities prohibitive to LAAO. |
| Quality of life benefit | Patient–provider shared decision. |
| PASS for WATCHMAN™ FLX | CLOSE for AMPLATZER™ Amulet™ |
|---|---|
| Position: device is distal to or at the ostium of the LAA Anchor: fixation anchors engaged/device is stable Size compression: device is compressed 8–20% of original size Seal: device spans ostium, all lobes of LAA are covered | Closure: At least 2/3 of the device lobe should be distal to the left Circumflex artery on echocardiography Lobe compression: The device Lobe should be slightly compressed and have good apposition to the left atrial appendage wall Orientation: The Orientation of the device lobe must be in line with the axis of the intended landing zone in the left atrial appendage Separation: The disc must be Separated from the lobe Elliptical: The disc will have a concave (Elliptical) shape |
| Immediately after LAAO | Prehospital Discharge | 45–90 Day Follow Up | |
|---|---|---|---|
| Imaging modality | TTE | TTE | TEE/CT |
| Question | Pericardial effusion | Pericardial effusion Device embolization | DRT PDL |
| Trial Acronym | NCT | N | Strategy |
|---|---|---|---|
| ADALA | NCT05632445 | 160 | Apixaban vs. DAPT after LAAO. |
| ANDES | NCT03568890 | 350 | DAPT vs. DOAC for 8 weeks. |
| ASPIRIN-LAAO | NCT03821883 | 1120 | Continuation vs. discontinuation of aspirin 6 months after LAAO. |
| APPROACH | NCT04550637 | 200 | Apixaban for 12 weeks after LAAO. |
| CLOSURE-AF | NCT03463317 | 1512 | DAPT after LAAO. |
| CLEARANCE | NCT04298723 | 530 | DAPT for 3 months after LAAO, followed by aspirin for 12 months. Alternatively, 3 months of NOAC followed by aspirin for 12 months. |
| CATALYST | NCT04226547 | 2650 | DAPT for 3 months after LAAO. |
| CHAMPION-AF | NCT04394546 | 3000 | DOAC or DAPT for 3 months after LAAO. |
| STROKECLOSE | NCT02830152 | 750 | Aspirin ± Clopidogrel for 45 d after LAAO. |
| LAA-KIDNEY | NCT05204212 | 430 | DAPT for 3 months after LAAO. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotta detto Loria, J.; Desch, S.; Pöss, J.; Kirsch, K.; Thiele, H.; Sandri, M. Percutaneous Left Atrial Appendage Occlusion—Current Evidence and Future Directions. J. Clin. Med. 2023, 12, 7292. https://doi.org/10.3390/jcm12237292
Rotta detto Loria J, Desch S, Pöss J, Kirsch K, Thiele H, Sandri M. Percutaneous Left Atrial Appendage Occlusion—Current Evidence and Future Directions. Journal of Clinical Medicine. 2023; 12(23):7292. https://doi.org/10.3390/jcm12237292
Chicago/Turabian StyleRotta detto Loria, Johannes, Steffen Desch, Janine Pöss, Katharina Kirsch, Holger Thiele, and Marcus Sandri. 2023. "Percutaneous Left Atrial Appendage Occlusion—Current Evidence and Future Directions" Journal of Clinical Medicine 12, no. 23: 7292. https://doi.org/10.3390/jcm12237292
APA StyleRotta detto Loria, J., Desch, S., Pöss, J., Kirsch, K., Thiele, H., & Sandri, M. (2023). Percutaneous Left Atrial Appendage Occlusion—Current Evidence and Future Directions. Journal of Clinical Medicine, 12(23), 7292. https://doi.org/10.3390/jcm12237292






