Oxidative Stress: The Role of Estrogen and Progesterone
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Committee
2.2. Patient Characteristics
2.3. Controlled Ovarian Stimulation Procedure
2.4. Study Design
2.5. Body Temperature Evaluation
2.6. Measurement of Hormones
2.7. Measurement of Oxidative Status
2.8. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Hormone Levels
3.3. Body Temperature Values
3.4. Oxidative Status
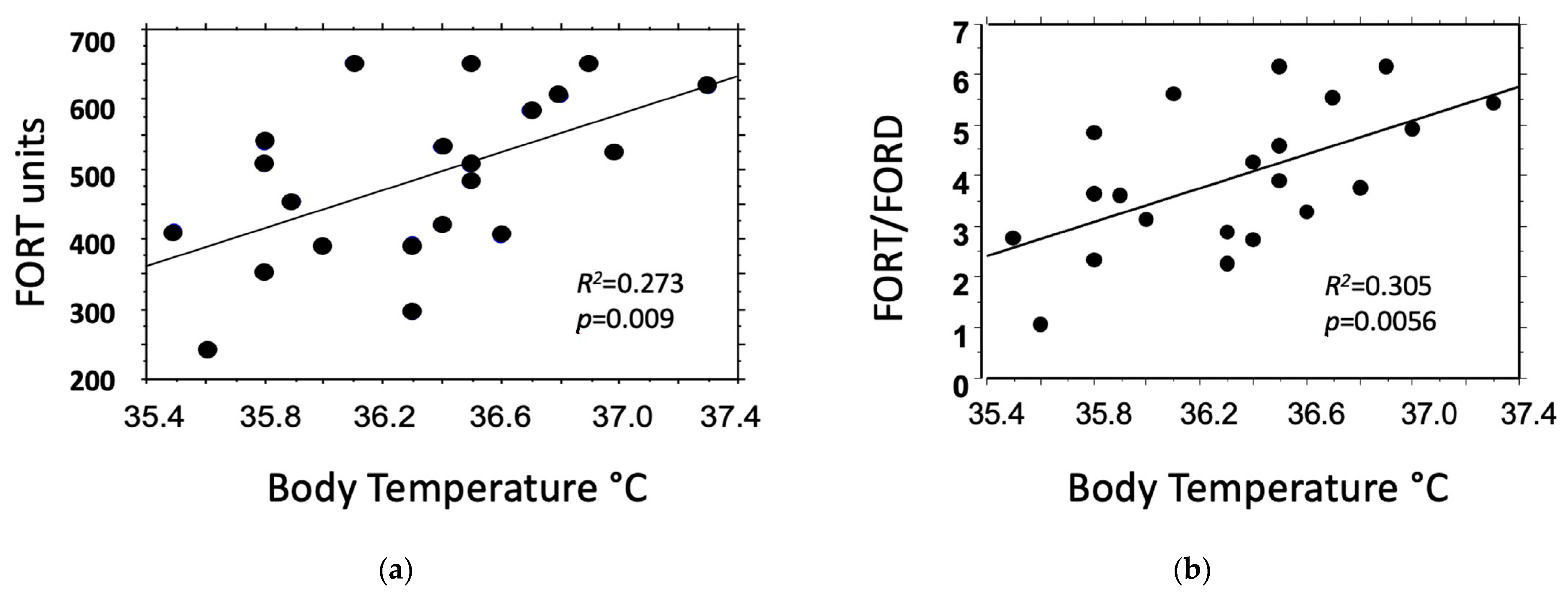
3.5. Relation between Oxidative Status and Body Temperature
4. Discussion
Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Frisard, M.; Ravussin, E. Energy metabolism and oxidative stress: Impact on the metabolic syndrome and the aging process. Endocrine 2006, 29, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Cauci, S.; Buligan, C.; Marangone, M.; Francescato, M.P. Oxidative Stress in Female Athletes Using Combined Oral Contraceptives. Sports Med. Open 2016, 2, 40. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.T.; Kotani, K. Oral contraceptive therapy increases oxidative stress in pre-menopausal women. Int. J. Prev. Med. 2012, 3, 893–896. [Google Scholar] [CrossRef]
- Finco, A.; Belcaro, G.; Cesarone, M.R. Assessment of the activity of an oral contraceptive on the levels of oxidative stress and changes in oxidative stress after co-treatment with two different types of physiological modulators with antioxidant action. Contraception 2011, 84, 418–422. [Google Scholar] [CrossRef]
- Finco, A.; Belcaro, G.; Cesarone, M.R. Evaluation of oxidative stress after treatment with low estrogen contraceptive either alone or associated with specific antioxidant therapy. Contraception 2012, 85, 503–508. [Google Scholar] [CrossRef]
- Morin-Papunen, L.; Martikainen, H.; McCarthy, M.I.; Franks, S.; Sovio, U.; Hartikainen, A.L.; Ruokonen, A.; Leinonen, M.; Laitinen, J.; Järvelin, M.-R.; et al. Comparison of metabolic and inflammatory outcomes in women who used oral contraceptives and the levonorgestrel-releasing intrauterine device in a general population. Am. J. Obstet. Gynecol. 2008, 199, 529.e1–529.e10. [Google Scholar] [CrossRef]
- Pincemail, J.; Vanbelle, S.; Gaspard, U.; Collette, G.; Haleng, J.; Cheramy-Bien, J.; Charlier, C.; Chapelle, J.; Giet, D.; Albert, A.; et al. Effect of different contraceptive methods on the oxidative stress status in women aged 40 48 years from the ELAN study in the province of Liege, Belgium. Hum. Reprod. 2007, 22, 2335–2343. [Google Scholar]
- De Groote, D.; D’Hauterive, S.P.; Pintiaux, A.; Balteau, B.; Gerday, C.; Claesen, J.; Foidart, J.-M. Effects of oral contraception with ethinylestradiol and drospirenone on oxidative stress in women 18–35 years old. Contraception 2009, 80, 187–193. [Google Scholar]
- Biasioli, A.; Xholli, A.; Previtera, F.; Balzano, A.; Capodicasa, V.; Tassi, A.; Londero, A.P.; Cagnacci, A. Systemic Oxidative Stress in Women with Ovarian and Pelvic Endometriosis: Role of Hormonal Therapy. J. Clin. Med. 2022, 11, 7460. [Google Scholar] [CrossRef] [PubMed]
- Cauci, S.; Xodo, S.; Buligan, C.; Colaninno, C.; Barbina, M.; Barbina, G.; Francescato, M.P. Oxidative Stress Is Increased in Combined Oral Contraceptives Users and Is Positively Associated with High-Sensitivity C-Reactive Protein. Molecules 2021, 26, 1070. [Google Scholar] [CrossRef] [PubMed]
- Doshi, S.B.; Agarwal, A. The role of oxidative stress in menopause. J. Midlife Health 2013, 4, 140–146. [Google Scholar] [PubMed]
- Mc Manus, J.; Mc Eneny, J.; Thompson, W.; Young, I.S. The effect of hormone replacement therapy on the oxidation of low density lipoprotein in postmenopausal women. Atherosclerosis 1997, 135, 73–81. [Google Scholar] [CrossRef]
- McGinnis, G.; Kliszczewiscz, B.; Barberio, M.; Ballmann, C.; Peters, B.; Slivka, D.; Dumke, C.; Cuddy, J.; Hailes, W.; Ruby, B.; et al. Acute hypoxia and exercise-induced blood oxidative stress. Int. J. Sport. Nutr. Exerc. Metab. 2014, 24, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Volpe, A.; Paoletti, A.M.; Melis, G.B. Regulation of the 24-hour rhythm of body temperature in menstrual cycles with spontaneous and gonadotropin-induced ovulation. Fertil. Steril. 1997, 68, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Arangino, S.; Tuveri, F.; Paoletti, A.M.; Volpe, A. Regulation of the 24h body temperature rhythm of women in luteal phase: Role of gonadal steroids and prostaglandins. Chronobiol. Int. 2002, 19, 721–730. [Google Scholar] [CrossRef]
- Webb, P. 24-hour energy expenditure and the menstrual cycle. Am. J. Clin. Nutr. 1986, 44, 614–619. [Google Scholar] [CrossRef]
- Baker, F.C.; Waner, J.I.; Vieira, E.F.; Taylor, S.R.; Driver, H.S.; Mitchell, D. Sleep and 24 hour body temperatures: A comparison in young men, naturally cycling women and women taking hormonal contraceptives. J. Physiol. 2001, 530, 565–574. [Google Scholar] [CrossRef]
- Wright, K.P.; Badia, P. Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behav. Brain Res. 1999, 103, 185–194. [Google Scholar] [CrossRef]
- Abe, C.; Miyazawa, T.; Miyazawa, T. Current Use of Fenton Reaction in Drugs and Food. Molecules 2022, 27, 5451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z. Iron and oxidizing species in oxidative stress and Alzheimer’s disease. Aging Med. 2019, 2, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Pavlatou, M.G.; Papastamataki, M.; Apostolakou, F.; Papassotiriou, I.; Tentolouris, N. FORT and FORD: Two simple and rapid assays in the evaluation of oxidative stress in patients with type 2 diabetes mellitus. Metabolism 2009, 58, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.A.; Newell, J.; Burden, R.; Howatson, G.; Pedlar, C.R. Critical Difference and Biological Variation in Biomarkers of Oxidative Stress and Nutritional Status in Athletes. PLoS ONE 2016, 11, e0149927. [Google Scholar] [CrossRef]
- Cagnacci, A.; Cannoletta, M.; Xholli, A.; Piacenti, I.; Palma, F.; Palmieri, B. Folate administration decreases oxidative status and blood pressure in postmenopausal women. Eur. J. Nutr. 2015, 54, 429–435. [Google Scholar] [CrossRef]
- Lorgis, L.; Zeller, M.; Dentan, G.; Sicard, P.; Richard, C.; Buffet, P.; L’huillier, I.; Beer, J.; Cottin, Y.; Rochette, L.; et al. The free oxygen radicals test (FORT) to assess circulating oxidative stress in patients with acute myocardial infarction. Atherosclerosis 2010, 213, 616–621. [Google Scholar] [CrossRef]
- Browne, R.W.; Bloom, M.S.; Schisterman, E.F.; Hovey, K.; Trevisan, M.; Wu, C.; Liu, A.; Wactawski-Wende, J. Analytical and biological variation of biomarkers of oxidative stress during the menstrual cycle. Biomarkers 2008, 13, 160–183. [Google Scholar] [CrossRef][Green Version]
- Quinn, K.M.; Cox, A.J.; Roberts, L.; Pennell, E.N.; McKeating, D.R.; Fisher, J.J.; Perkins, A.V.; Minahan, C. Temporal changes in blood oxidative stress biomarkers across the menstrual cycle and with oral contraceptive use in active women. Eur. J. Appl. Physiol. 2021, 121, 2607–2620. [Google Scholar] [CrossRef]
- Cornelli, U.; Belcaro, G.; Cesarone, M.R.; Finco, A. Analysis of oxidative stress during the menstrual cycle. Reprod. Biol. Endocrinol. 2013, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Massart, A.; Portier, H.; Rosado, F.; Toumi, H.; Filaire, E. Lipid peroxidation in judoists using oral contraceptives. Int. J. Sports Med. 2012, 33, 781–788. [Google Scholar] [CrossRef]
- Borrás, C.; Ferrando, M.; Inglés, M.; Gambini, J.; Lopez-Grueso, R.; Edo, R.; Mas-Bargues, C.; Pellicer, A.; Viña, J. Estrogen Replacement Therapy Induces Antioxidant and Longevity-Related Genes in Women after Medically Induced Menopause. Oxid. Med. Cell Longev. 2021, 2021, 8101615. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxid. Med. Cell Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef]
- Cordoba, P.; Kaaya, A.; Richard, O.; Sutter-Dub, M.T. Inhibition of glucose metabolism by progesterone in adipocytes: Role of protein synthesis. Can. J. Physiol. Pharmacol. 1991, 69, 1861–1867. [Google Scholar] [CrossRef]
- Landau, R.L.; Poulos, J.T. The metabolic influence of progestins. Adv. Metab. Disord. 1971, 5, 119–147. [Google Scholar] [PubMed]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef]
- Grant, L.K.; Gooley, J.J.; St Hilaire, M.A.; Rajaratnam, S.M.W.; Brainard, G.C.; Czeisler, C.A.; Lockley, S.W.; Rahman, S.A. Menstrual phase-dependent differences in neurobehavioral performance: The role of temperature and the progesterone/estradiol ratio. Sleep 2020, 43, zsz227. [Google Scholar] [CrossRef]
- Garelnabi, M.O.; Brown, W.V.; Le, N.A. Evaluation of a novel colorimetric assay for free oxygen radicals as marker of oxidative stress. Clin. Biochem. 2008, 41, 1250–1254. [Google Scholar] [CrossRef]
- Halbrecht, I. Ovarian function and body temperature. Lancet 1945, 2, 668. [Google Scholar] [CrossRef]
- Baker, F.C.; Siboza, F.; Fuller, A. Temperature regulation in women: Effects of the menstrual cycle. Temperature 2020, 7, 226–262. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Arangino, S.; Baldassari, F.; Alessandrini, C.; Landi, S.; Volpe, A. A comparison of the central effects of different progestins used in hormone replacement therapy. Maturitas 2004, 48, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.; Berrino, F.; Clavel-Chapelon, F. Unequal risks for breast cancer associated with different hormone replacement therapies: Results from the E3N cohort study. Breast Cancer Res. Treat. 2008, 107, 103–111. [Google Scholar] [CrossRef] [PubMed]
| Age (yrs) | 37.09 ± 4.06 |
| BMI (kg/m2) | 24.03 ± 4.91 |
| AMH (ng/mL) | 2.52 ± 1.87 |
| Cause of infertility (n) | |
| Idiopathic | 10 |
| Male Factor | 6 |
| Female Factor | 16 |
| Tubaric | 6 |
| Low Reserve | 4 |
| PCOS | 4 |
| Myomas | 2 |
| Smoking (n) | |
| Smokers | 4 |
| Ex-Smokers | 4 |
| Non-Smokers | 24 |
| Physical Activity (h/week) | 1.87 ± 1.89 |
| Use of Alcohol (glass/week) | 0.5 ± 0.3 |
| None | 10 |
| 0 to ≤2 | 18 |
| >2 | 3 |
| A 2nd Day | B Pick-Up | C ET | A vs. B p Value | A vs. C p Value | B vs. C p Value | |
|---|---|---|---|---|---|---|
| Body Temperature * (°C) | 36.3 ± 0.4 | 35.9 ± 0.4 | 36.7 ± 0.3 | 0.001 | 0.001 | 0.001 |
| FORT (units) | 3.92 ± 1.020 | 3.36 ± 0.91 | 4.05 ± 1.01 | 0.023 | 0.584 | 0.006 |
| FORD (units) | 0.93 ± 0.15 | 1.02 ± 0.25 | 0.92 ± 0.14 | 0.085 | 0.783 | 0.052 |
| FORT/FORD | 4.39 ± 1.58 | 3.49 ± 1.24 | 4.53 ± 1.39 | 0.01 | 0.682 | 0.004 |
| Body Temperature (°C) vs. | Coefficient of Regression | 95% Confidence Interval | R2 | p-Value |
|---|---|---|---|---|
| FORT units | 1.031 | 0.291, 1.771 | 0.273 | 0.009 |
| FORD units | −0.225 | −0.417, −0.033 | 0.201 | 0.025 |
| FORT/FORD | 1.685 | 0.556, 2.814 | 0.305 | 0.0056 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cagnacci, A.; Gazzo, I.; Stigliani, S.; Paoletti, A.M.; Anserini, P.; Londero, A.P.; Xholli, A. Oxidative Stress: The Role of Estrogen and Progesterone. J. Clin. Med. 2023, 12, 7304. https://doi.org/10.3390/jcm12237304
Cagnacci A, Gazzo I, Stigliani S, Paoletti AM, Anserini P, Londero AP, Xholli A. Oxidative Stress: The Role of Estrogen and Progesterone. Journal of Clinical Medicine. 2023; 12(23):7304. https://doi.org/10.3390/jcm12237304
Chicago/Turabian StyleCagnacci, Angelo, Irene Gazzo, Sara Stigliani, Anna Maria Paoletti, Paola Anserini, Ambrogio Pietro Londero, and Anjeza Xholli. 2023. "Oxidative Stress: The Role of Estrogen and Progesterone" Journal of Clinical Medicine 12, no. 23: 7304. https://doi.org/10.3390/jcm12237304
APA StyleCagnacci, A., Gazzo, I., Stigliani, S., Paoletti, A. M., Anserini, P., Londero, A. P., & Xholli, A. (2023). Oxidative Stress: The Role of Estrogen and Progesterone. Journal of Clinical Medicine, 12(23), 7304. https://doi.org/10.3390/jcm12237304









