Serous Cystadenoma: A Review on Diagnosis and Management
Abstract
:1. Introduction
2. Presentation
3. Diagnosis
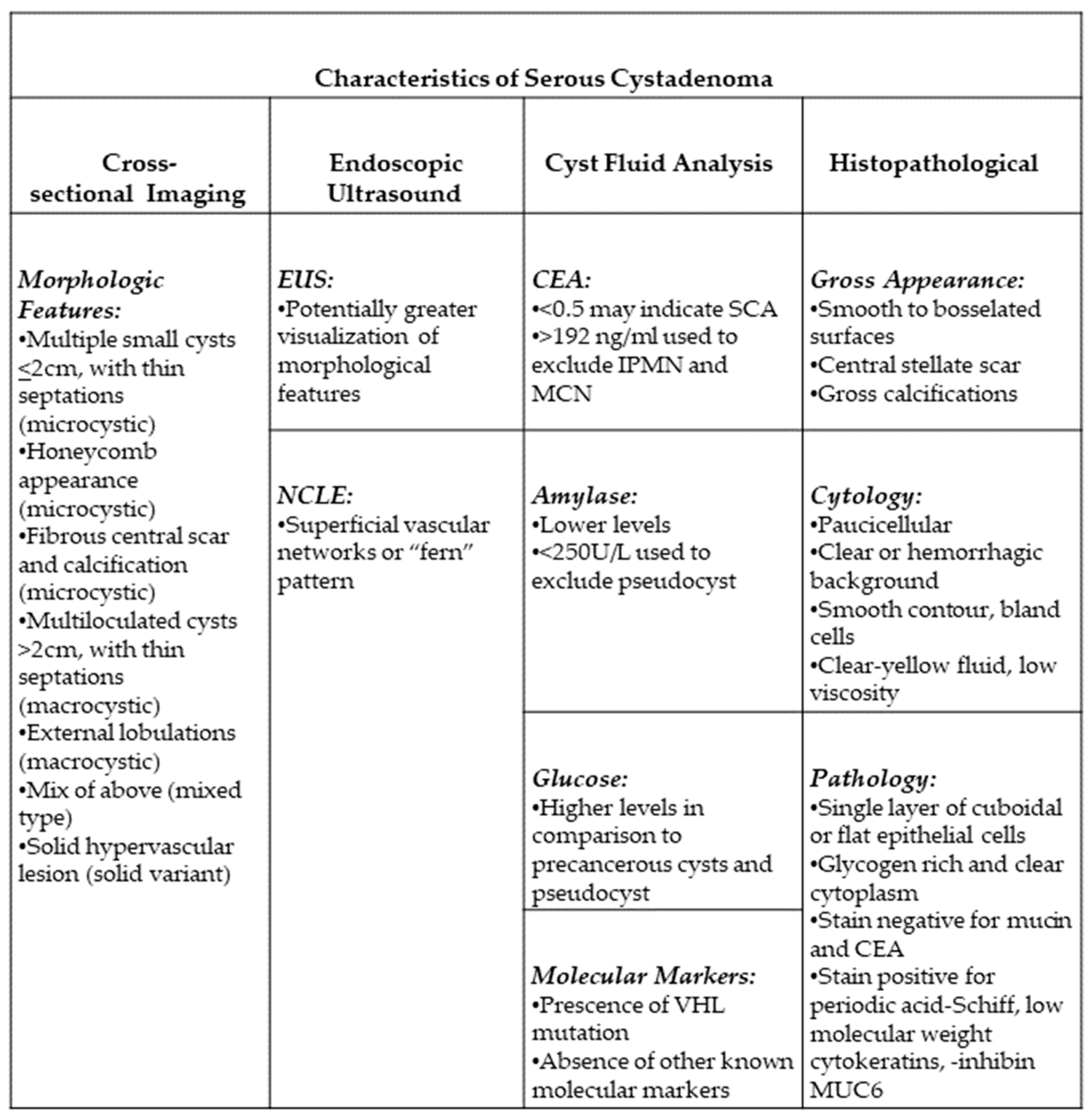
3.1. Morphologic Features of SCA
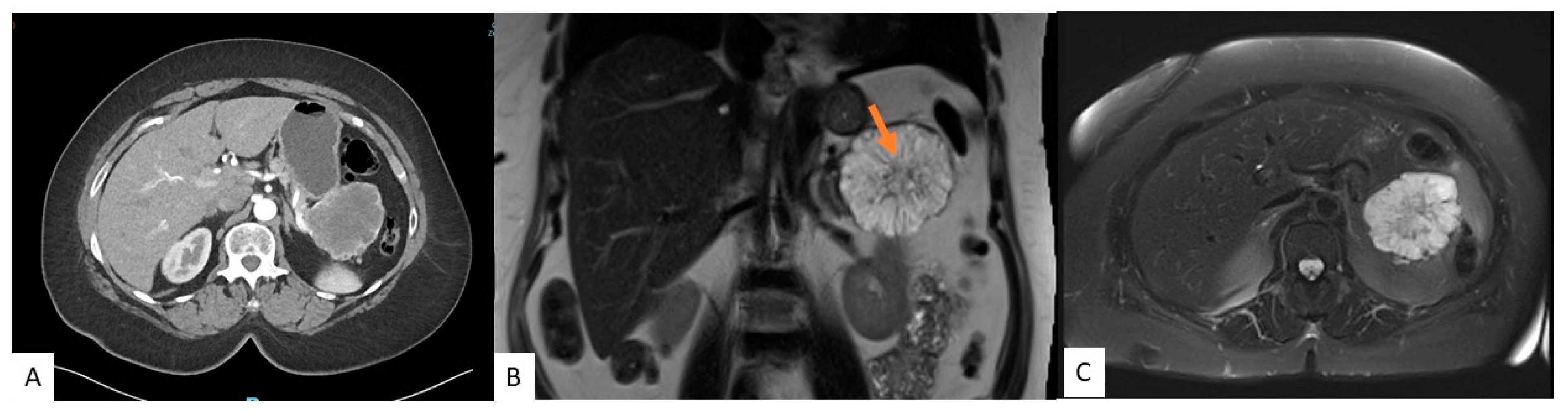
3.2. Cross-Sectional Imaging
3.3. Endoscopic Ultrasound
3.4. Cyst Fluid Analysis
3.5. Confocal Laser Endomicroscopy
4. Histopathological Features
5. Management
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schweber, A.B.; Agarunov, E.; Brooks, C.; Hur, C.; Gonda, T.A. Prevalence, Incidence, and Risk of Progression of Asymptomatic Pancreatic Cysts in Large Sample Real-world Data. Pancreas 2021, 50, 1287–1292. [Google Scholar] [CrossRef]
- Kromrey, M.; Bulow, R.; Hubner, J.; Paperlein, C.; Lerch, M.M.; Ittermann, T.; Volzke, H.; Mayerle, J.; Kuhn, J. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut 2018, 67, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Sekhar, A.; Rofsky, N.M.; Pedrosa, I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am. J. Gastroenterol. 2010, 105, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Jais, B.; Rebours, V.; Malleo, G.; Salvia, R.; Fontana, M.; Maggino, L.; Bassi, C.; Manfredi, R.; Moran, R.; Lennon, A.M.; et al. Serous cystic neoplasm of the pancreas: A multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut 2016, 65, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Compagno, J.; Oertel, J.E. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathologic study of 41 cases. Am. J. Clin. Pathol. 1978, 69, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Thirabanjasak, D.; Basturk, O.; Altinel, D.; Cheng, J.D.; Adsay, N.V. Is serous cystadenoma of the pancreas a model of clear-cell-associated angiogenesis and tumorigenesis? Pancreatology 2009, 9, 182–188. [Google Scholar] [CrossRef]
- Valsangkar, N.P.; Morales-Oyarvide, V.; Thayer, S.P.; Ferrone, C.R.; Wargo, J.A.; Warshaw, A.L.; Fernandez-del Castillo, C. 851 resected cystic tumors of the pancreas: A 33-year experience at the Massachusetts General Hospital. Surgery 2012, 152, 4. [Google Scholar] [CrossRef] [PubMed]
- Galanis, C.; Zamani, A.; Cameron, J.L.; Campbell, K.A.; Lillemoe, K.D.; Caparrelli, D.; Chang, D.; Hruban, R.H.; Yeo, C.J. Resected serous cystic neoplasms of the pancreas: A review of 158 patients with recommendations for treatment. J. Gastrointest. Surg. 2007, 11, 820–826. [Google Scholar] [CrossRef]
- Tseng, J.F.; Warshaw, A.L.; Sahani, D.V.; Lauwers, G.Y.; Rattner, D.W.; Fernandez-del Castillo, C. Serous cystadenoma of the pancreas: Tumor growth rates and recommendations for treatment. Ann. Surg. 2005, 242, 413–421. [Google Scholar] [CrossRef]
- Khashab, M.A.; Shin, E.J.; Amateau, S.; Canto, M.I.; Hruban, R.H.; Fishman, E.K.; Cameron, J.L.; Edil, B.H.; Wolfgang, C.L.; Schulick, R.D.; et al. Tumor size and location correlate with behavior of pancreatic serous cystic neoplasms. Am. J. Gastroenterol. 2011, 106, 1521–1526. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Z.; Chen, H.; Zhang, D.; Wen, L. Discrimination of serous cystadenoma from mucinous cystic neoplasm and branch duct intraductal papillary mucinous neoplasm in the pancreas with CT. Abdom. Radiol. 2020, 45, 2772–2778. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, D.H.; Ko, Y.T.; Lim, J.W.; Kim, H.C.; Kim, K.W. CT of serous cystadenoma of the pancreas and mimicking masses. AJR Am. J. Roentgenol. 2008, 190, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.C.; Singhi, A.D.; Haroun, R.R.; Hruban, R.H.; Fishman, E.K. The many faces of pancreatic serous cystadenoma: Radiologic and pathologic correlation. Diagn. Interv. Imaging 2017, 98, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Maillet, B.; Schroder, S.; Mukai, K.; Kloppel, G. Serous oligocystic and ill-demarcated adenoma of the pancreas: A variant of serous cystic adenoma. Virchows Arch. 1994, 424, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Feller, E.; Schiffman, F.J. Extrahepatic biliary obstruction by lymphoma. Arch. Surg. 1990, 125, 1507–1509. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Kim, S.H.; Kim, M.A.; Lee, J.Y.; Han, J.K.; Choi, B.I. CT imaging spectrum of pancreatic serous tumors: Based on new pathologic classification. Eur. J. Radiol. 2010, 75, 45. [Google Scholar] [CrossRef]
- Fang, X.; Jiang, H.; Cao, K.; Li, J.; Liu, F.; Wang, L.; Lu, J.; Shao, C.; Bian, Y. Distinguishing pancreatic solid serous cystadenomas from nonfunctional pancreatic neuroendocrine tumors by computed tomography: A propensity score analysis. Medicine 2022, 101, e30523. [Google Scholar] [CrossRef]
- Shah, A.A.; Sainani, N.I.; Kambadakone, A.R.; Shah, Z.K.; Deshpande, V.; Hahn, P.F.; Sahani, D.V. Predictive value of multi-detector computed tomography for accurate diagnosis of serous cystadenoma: Radiologic-pathologic correlation. World J. Gastroenterol. 2009, 15, 2739–2747. [Google Scholar] [CrossRef]
- Jones, M.J.; Buchanan, A.S.; Neal, C.P.; Dennison, A.R.; Metcalfe, M.S.; Garcea, G. Imaging of indeterminate pancreatic cystic lesions: A systematic review. Pancreatology 2013, 13, 436–442. [Google Scholar] [CrossRef]
- Bassi, C.; Salvia, R.; Molinari, E.; Biasutti, C.; Falconi, M.; Pederzoli, P. Management of 100 consecutive cases of pancreatic serous cystadenoma: Wait for symptoms and see at imaging or vice versa? World J. Surg. 2003, 27, 319–323. [Google Scholar] [CrossRef]
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B. International Society for Magnetic Resonance in Medicine Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 2017, 16, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Curry, C.A.; Eng, J.; Horton, K.M.; Urban, B.; Siegelman, S.; Kuszyk, B.S.; Fishman, E.K. CT of primary cystic pancreatic neoplasms: Can CT be used for patient triage and treatment? AJR Am. J. Roentgenol. 2000, 175, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Procacci, C.; Biasiutti, C.; Carbognin, G.; Accordini, S.; Bicego, E.; Guarise, A.; Spoto, E.; Andreis, I.A.; De Marco, R.; Megibow, A.J. Characterization of cystic tumors of the pancreas: CT accuracy. J. Comput. Assist. Tomogr. 1999, 23, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Kehagias, D.; Smyrniotis, V.; Kalovidouris, A.; Gouliamos, A.; Kostopanagiotou, E.; Vassiliou, J.; Vlahos, L. Cystic tumors of the pancreas: Preoperative imaging, diagnosis, and treatment. Int. Surg. 2002, 87, 171–174. [Google Scholar] [PubMed]
- Kurita, Y.; Kuwahara, T.; Hara, K.; Mizuno, N.; Okuno, N.; Matsumoto, S.; Obata, M.; Koda, H.; Tajika, M.; Shimizu, Y.; et al. Diagnostic ability of artificial intelligence using deep learning analysis of cyst fluid in differentiating malignant from benign pancreatic cystic lesions. Sci. Rep. 2019, 9, 6893. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, X.; Ou, X.; Zhang, W.; Ma, X. Discrimination of Pancreatic Serous Cystadenomas From Mucinous Cystadenomas With CT Textural Features: Based on Machine Learning. Front. Oncol. 2019, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.C.; Park, S.; Soleimani, S.; Fouladi, D.F.; Shayesteh, S.; He, J.; Javed, A.A.; Wolfgang, C.L.; Vogelstein, B.; Kinzler, K.W.; et al. Classification of pancreatic cystic neoplasms using radiomic feature analysis is equivalent to an experienced academic radiologist: A step toward computer-augmented diagnostics for radiologists. Abdom Radiol. 2022, 47, 4139–4150. [Google Scholar] [CrossRef]
- Sperti, C.; Pasquali, C.; Chierichetti, F.; Liessi, G.; Ferlin, G.; Pedrazzoli, S. Value of 18-fluorodeoxyglucose positron emission tomography in the management of patients with cystic tumors of the pancreas. Ann. Surg. 2001, 234, 675–680. [Google Scholar] [CrossRef]
- Sperti, C.; Pasquali, C.; Decet, G.; Chierichetti, F.; Liessi, G.; Pedrazzoli, S. F-18-fluorodeoxyglucose positron emission tomography in differentiating malignant from benign pancreatic cysts: A prospective study. J. Gastrointest. Surg. 2005, 9, 22–29. [Google Scholar] [CrossRef]
- Sperti, C.; Bissoli, S.; Pasquali, C.; Frison, L.; Liessi, G.; Chierichetti, F.; Pedrazzoli, S. 18-Fluorodeoxyglucose Positron Emission Tomography Enhances Computed Tomography Diagnosis of Malignant Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. 2007, 246, 932–939. [Google Scholar] [CrossRef]
- Kauhanen, S.; Rinta-Kiikka, I.; Kemppainen, J.; Gronroos, J.; Kajander, S.; Seppanen, M.; Alanen, K.; Gullichsen, R.; Nuutila, P.; Ovaska, J. Accuracy of 18F-FDG PET/CT, Multidetector CT, and MR Imaging in the Diagnosis of Pancreatic Cysts: A Prospective Single-Center Study. J. Nucl. Med. 2015, 56, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Mansour, J.C.; Schwartz, L.; Pandit-Taskar, N.; D’Angelica, M.; Fong, Y.; Larson, S.M.; Brennan, M.F.; Allen, P.J. The utility of F-18 fluorodeoxyglucose whole body PET imaging for determining malignancy in cystic lesions of the pancreas. J. Gastrointest. Surg. 2006, 10, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Sedlack, R.; Affi, A.; Vazquez-Sequeiros, E.; Norton, I.D.; Clain, J.E.; Wiersema, M.J. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest. Endosc. 2002, 56, 543–547. [Google Scholar] [CrossRef]
- Kaneto, H.; Endo, T.; Ozeki, I.; Itoh, H.; Sasaki, S.; Mukaiya, M.; Ikeda, K.; Koito, K.; Imai, K. Macrocystic serous cystadenoma of the pancreas: Importance of co-existent tiny cysts depicted by EUS. J. Gastroenterol. 2000, 35, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.A.; Kochman, M.L.; Brensinger, C.; Brugge, W.R.; Faigel, D.O.; Gress, F.G.; Kimmey, M.B.; Nickl, N.J.; Savides, T.J.; Wallace, M.B.; et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest. Endosc. 2003, 58, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Belsley, N.A.; Pitman, M.B.; Lauwers, G.Y.; Brugge, W.R.; Deshpande, V. Serous cystadenoma of the pancreas: Limitations and pitfalls of endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer 2008, 114, 102–110. [Google Scholar] [CrossRef]
- Frossard, J.L.; Amouyal, P.; Amouyal, G.; Palazzo, L.; Amaris, J.; Soldan, M.; Giostra, E.; Spahr, L.; Hadengue, A.; Fabre, M. Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am. J. Gastroenterol. 2003, 98, 1516–1524. [Google Scholar] [CrossRef]
- Abdelkader, A.; Hunt, B.; Hartley, C.P.; Panarelli, N.C.; Giorgadze, T. Cystic Lesions of the Pancreas: Differential Diagnosis and Cytologic-Histologic Correlation. Arch. Pathol. Lab. Med. 2020, 144, 47–61. [Google Scholar] [CrossRef]
- Lilo, M.T.; VandenBussche, C.J.; Allison, D.B.; Lennon, A.M.; Younes, B.K.; Hruban, R.H.; Wolfgang, C.L.; Ali, S.Z. Serous Cystadenoma of the Pancreas: Potentials and Pitfalls of a Preoperative Cytopathologic Diagnosis. Acta Cytol. 2017, 61, 27–33. [Google Scholar] [CrossRef]
- Huang, P.; Staerkel, G.; Sneige, N.; Gong, Y. Fine-needle aspiration of pancreatic serous cystadenoma: Cytologic features and diagnostic pitfalls. Cancer 2006, 108, 239–249. [Google Scholar] [CrossRef]
- Nagula, S.; Kennedy, T.; Schattner, M.A.; Brennan, M.F.; Gerdes, H.; Markowitz, A.J.; Tang, L.; Allen, P.J. Evaluation of cyst fluid CEA analysis in the diagnosis of mucinous cysts of the pancreas. J. Gastrointest. Surg. 2010, 14, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Brugge, W.R.; Lewandrowski, K.; Lee-Lewandrowski, E.; Centeno, B.A.; Szydlo, T.; Regan, S.; del Castillo, C.F.; Warshaw, A.L. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology 2004, 126, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Simons-Linares, C.R.; Yadav, D.; Lopez, R.; Bhatt, A.; Jang, S.; El-Khider, F.; Sanaka, M.; Stevens, T.; Vargo, J.; Chahal, P. The utility of intracystic glucose levels in differentiating mucinous from non-mucinous pancreatic cysts. Pancreatology 2020, 20, 1386–1392. [Google Scholar] [CrossRef]
- Guzman-Calderon, E.; Md, B.M.; Casellas, J.A.; Aparicio, J.R. Intracystic Glucose Levels Appear Useful for Diagnosis of Pancreatic Cystic Lesions: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2022, 67, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.R.; Garg, R.; Rustagi, T. Pancreatic cyst fluid glucose in differentiating mucinous from nonmucinous pancreatic cysts: A systematic review and meta-analysis. Gastrointest. Endosc. 2021, 94, 698–712.e6. [Google Scholar] [CrossRef]
- Park, W.G.; Wu, M.; Bowen, R.; Zheng, M.; Fitch, W.L.; Pai, R.K.; Wodziak, D.; Visser, B.C.; Poultsides, G.A.; Norton, J.A.; et al. Metabolomic-derived novel cyst fluid biomarkers for pancreatic cysts: Glucose and kynurenine. Gastrointest. Endosc. 2013, 78, 295–302.e2. [Google Scholar] [CrossRef]
- Hawes, R.H.; Clancy, J.; Hasan, M.K. Endoscopic ultrasound-guided fine needle aspiration in cystic pancreatic lesions. Clin. Endosc. 2012, 45, 128–131. [Google Scholar] [CrossRef]
- Snozek, C.L.H.; Mascarenhas, R.C.; O’Kane, D.J. Use of cyst fluid CEA, CA19-9, and amylase for evaluation of pancreatic lesions. Clin. Biochem. 2009, 42, 1585–1588. [Google Scholar] [CrossRef]
- Springer, S.; Wang, Y.; Dal Molin, M.; Masica, D.L.; Jiao, Y.; Kinde, I.; Blackford, A.; Raman, S.P.; Wolfgang, C.L.; Tomita, T.; et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015, 149, 1501–1510. [Google Scholar] [CrossRef]
- Paniccia, A.; Polanco, P.M.; Boone, B.A.; Wald, A.I.; McGrath, K.; Brand, R.E.; Khalid, A.; Kubiliun, N.; O’Broin-Lennon, A.M.; Park, W.G.; et al. Prospective, Multi-Institutional, Real-Time Next-Generation Sequencing of Pancreatic Cyst Fluid Reveals Diverse Genomic Alterations That Improve the Clinical Management of Pancreatic Cysts. Gastroenterology 2023, 164, 117–133.e7. [Google Scholar] [CrossRef]
- Reid, M.D.; Choi, H.; Balci, S.; Akkas, G.; Adsay, V. Serous cystic neoplasms of the pancreas: Clinicopathologic and molecular characteristics. Semin. Diagn. Pathol. 2014, 31, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Lonser, R.R.; Glenn, G.M.; Walther, M.; Chew, E.Y.; Libutti, S.K.; Linehan, W.M.; Oldfield, E.H. von Hippel-Lindau disease. Lancet 2003, 361, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Napoleon, B.; Lemaistre, A.; Pujol, B.; Caillol, F.; Lucidarme, D.; Bourdariat, R.; Morellon-Mialhe, B.; Fumex, F.; Lefort, C.; Lepilliez, V.; et al. A novel approach to the diagnosis of pancreatic serous cystadenoma: Needle-based confocal laser endomicroscopy. Endoscopy 2015, 47, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Konjeti, V.R.; McCarty, T.R.; Rustagi, T. Needle-based Confocal Laser Endomicroscopy (nCLE) for Evaluation of Pancreatic Cystic Lesions: A Systematic Review and Meta-analysis. J. Clin. Gastroenterol. 2022, 56, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Colonna, J.; Plaza, J.A.; Frankel, W.L.; Yearsley, M.; Bloomston, M.; Marsh, W.L. Serous cystadenoma of the pancreas: Clinical and pathological features in 33 patients. Pancreatology 2008, 8, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Roldan, J.; Harrison, J.M.; Qadan, M.; Bolm, L.; Baba, T.; Brugge, W.R.; Casey, B.W.; Krishnan, K.; Mino-Kenudson, M.; Pitman, M.B.; et al. Evolving Trends in Pancreatic Cystic Tumors: A 3-Decade Single-Center Experience With 1290 Resections. Ann. Surg. 2023, 277, 491–497. [Google Scholar] [CrossRef]
- Lombardo, C.; Iacopi, S.; Menonna, F.; Napoli, N.; Kauffmann, E.; Bernardini, J.; Cacciato Insilla, A.; Boraschi, P.; Donati, F.; Cappelli, C.; et al. Incidence and reasons of pancreatic resection in patients with asymptomatic serous cystadenoma. Pancreatology 2018, 18, 577–584. [Google Scholar] [CrossRef]
- El-Hayek, K.M.; Brown, N.; O’Rourke, C.; Falk, G.; Morris-Stiff, G.; Walsh, R.M. Rate of growth of pancreatic serous cystadenoma as an indication for resection. Surgery 2013, 154, 794–800, discussion 800–802. [Google Scholar] [CrossRef]
- Malleo, G.; Bassi, C.; Rossini, R.; Manfredi, R.; Butturini, G.; Massignani, M.; Paini, M.; Pederzoli, P.; Salvia, R. Growth pattern of serous cystic neoplasms of the pancreas: Observational study with long-term magnetic resonance surveillance and recommendations for treatment. Gut 2012, 61, 746–751. [Google Scholar] [CrossRef]
- Horvath, K.D.; Chabot, J.A. An aggressive resectional approach to cystic neoplasms of the pancreas. Am. J. Surg. 1999, 178, 269–274. [Google Scholar] [CrossRef]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef] [PubMed]
- European Study Group on Cystic Tumours of the Pancreas European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [CrossRef] [PubMed]
- Megibow, A.J.; Baker, M.E.; Morgan, D.E.; Kamel, I.R.; Sahani, D.V.; Newman, E.; Brugge, W.R.; Berland, L.L.; Pandharipande, P.V. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J. Am. Coll. Radiol. 2017, 14, 911–923. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, K.; Salamone, A.; Manos, L.; Lafaro, K.J.; Afghani, E. Serous Cystadenoma: A Review on Diagnosis and Management. J. Clin. Med. 2023, 12, 7306. https://doi.org/10.3390/jcm12237306
Ning K, Salamone A, Manos L, Lafaro KJ, Afghani E. Serous Cystadenoma: A Review on Diagnosis and Management. Journal of Clinical Medicine. 2023; 12(23):7306. https://doi.org/10.3390/jcm12237306
Chicago/Turabian StyleNing, Kylie, Ashley Salamone, Lindsey Manos, Kelly J. Lafaro, and Elham Afghani. 2023. "Serous Cystadenoma: A Review on Diagnosis and Management" Journal of Clinical Medicine 12, no. 23: 7306. https://doi.org/10.3390/jcm12237306
APA StyleNing, K., Salamone, A., Manos, L., Lafaro, K. J., & Afghani, E. (2023). Serous Cystadenoma: A Review on Diagnosis and Management. Journal of Clinical Medicine, 12(23), 7306. https://doi.org/10.3390/jcm12237306





