Echocardiography and Electrocardiography in Detecting Atrial Cardiomyopathy: A Promising Path to Predicting Cardioembolic Strokes and Atrial Fibrillation
Abstract
:1. Introduction
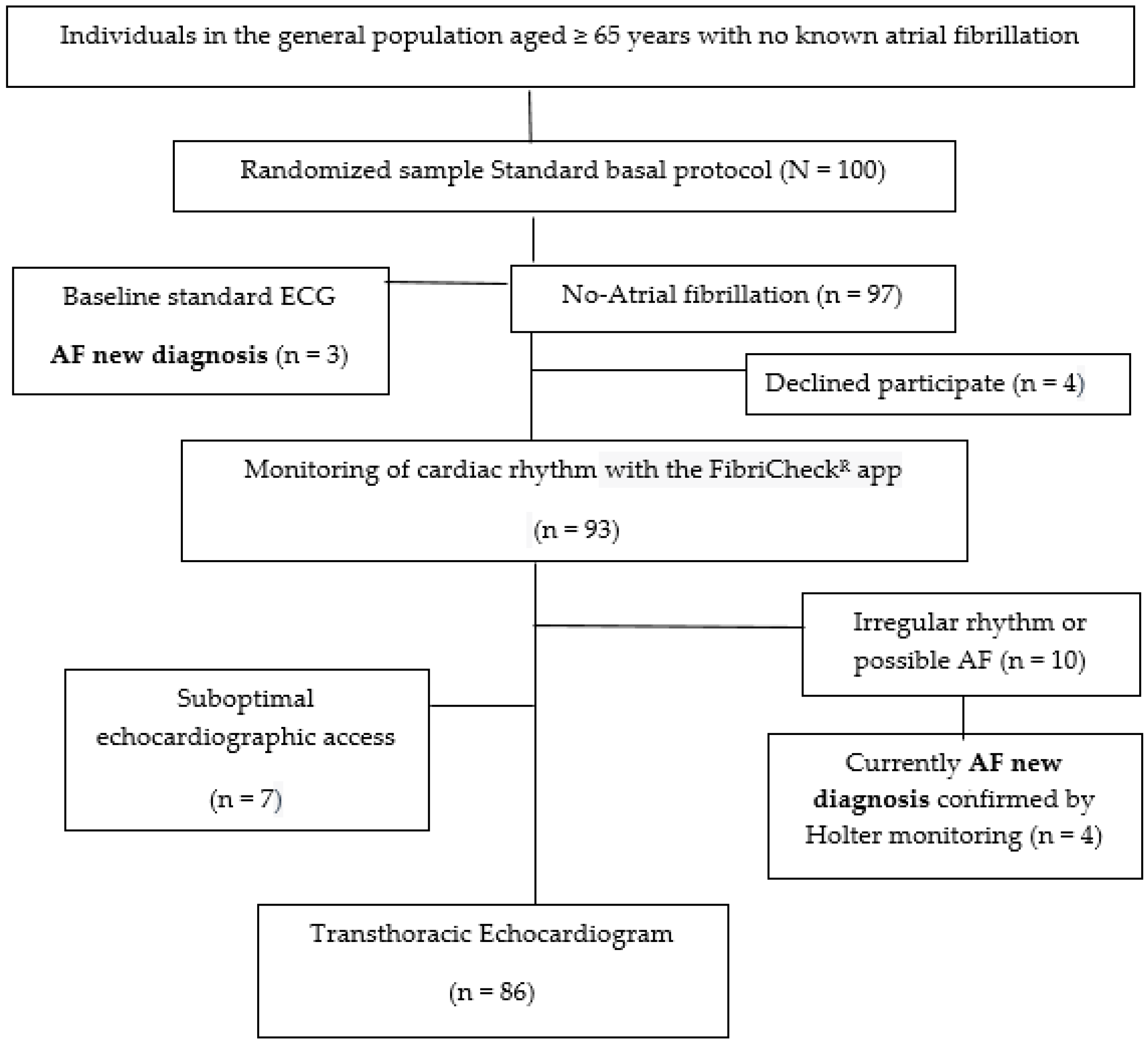
2. Materials and Methods
2.1. Target Population
2.2. Selection Criteria
2.3. Variables
2.4. Intervention
2.4.1. Basal Cardiac Rhythm Monitoring
2.4.2. Electrocardiogram Study
2.4.3. Echocardiogram Study
2.5. Abnormal Range of LA Strain
2.6. Statistical Analysis
3. Results
3.1. Baseline Features
3.2. Echorcardiography Study
3.3. Standard ECG Variables
4. Discussion
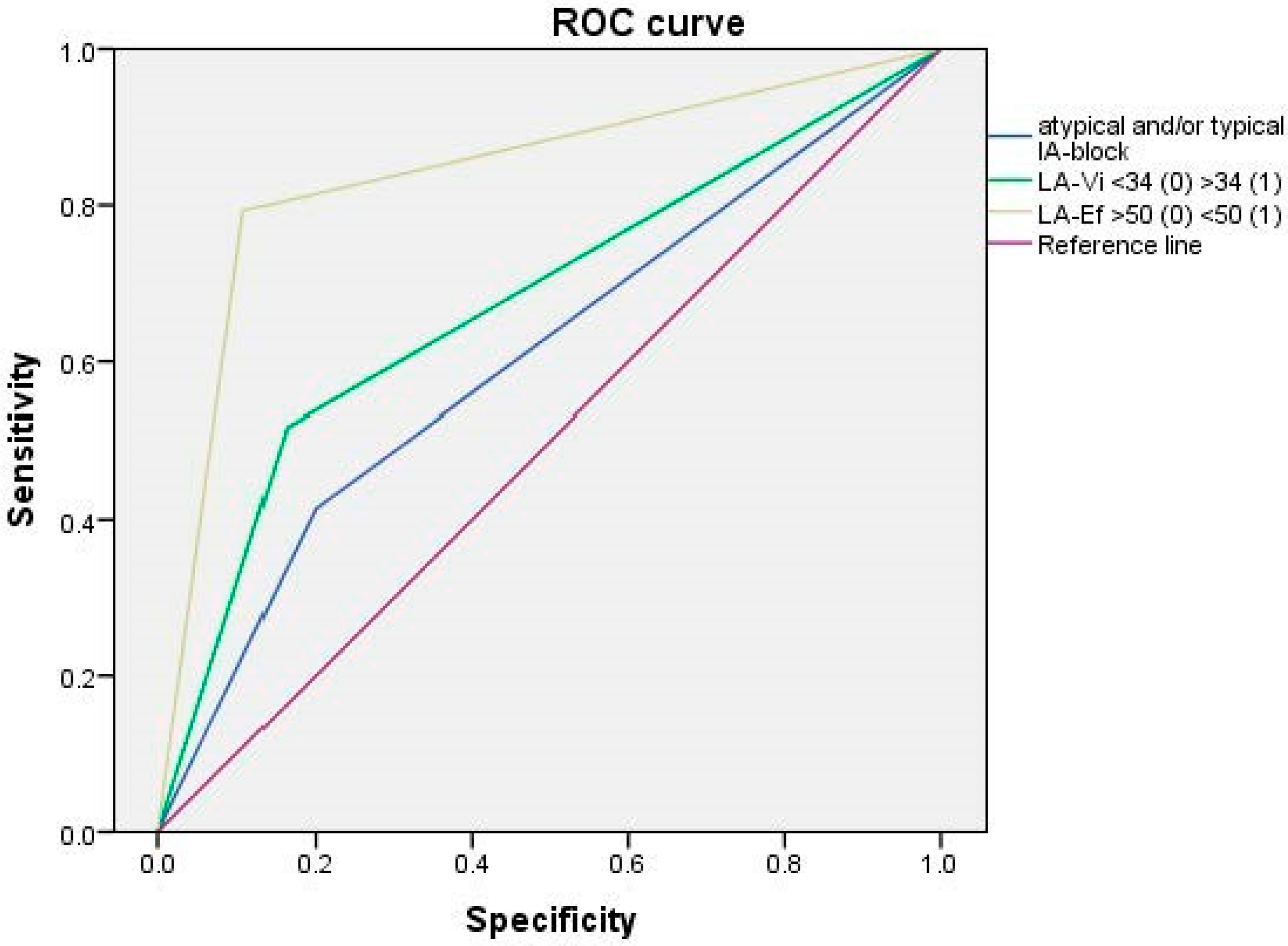
4.1. Relationship between Reduced Strain-R < 26% and the Rest of TTE Parameters
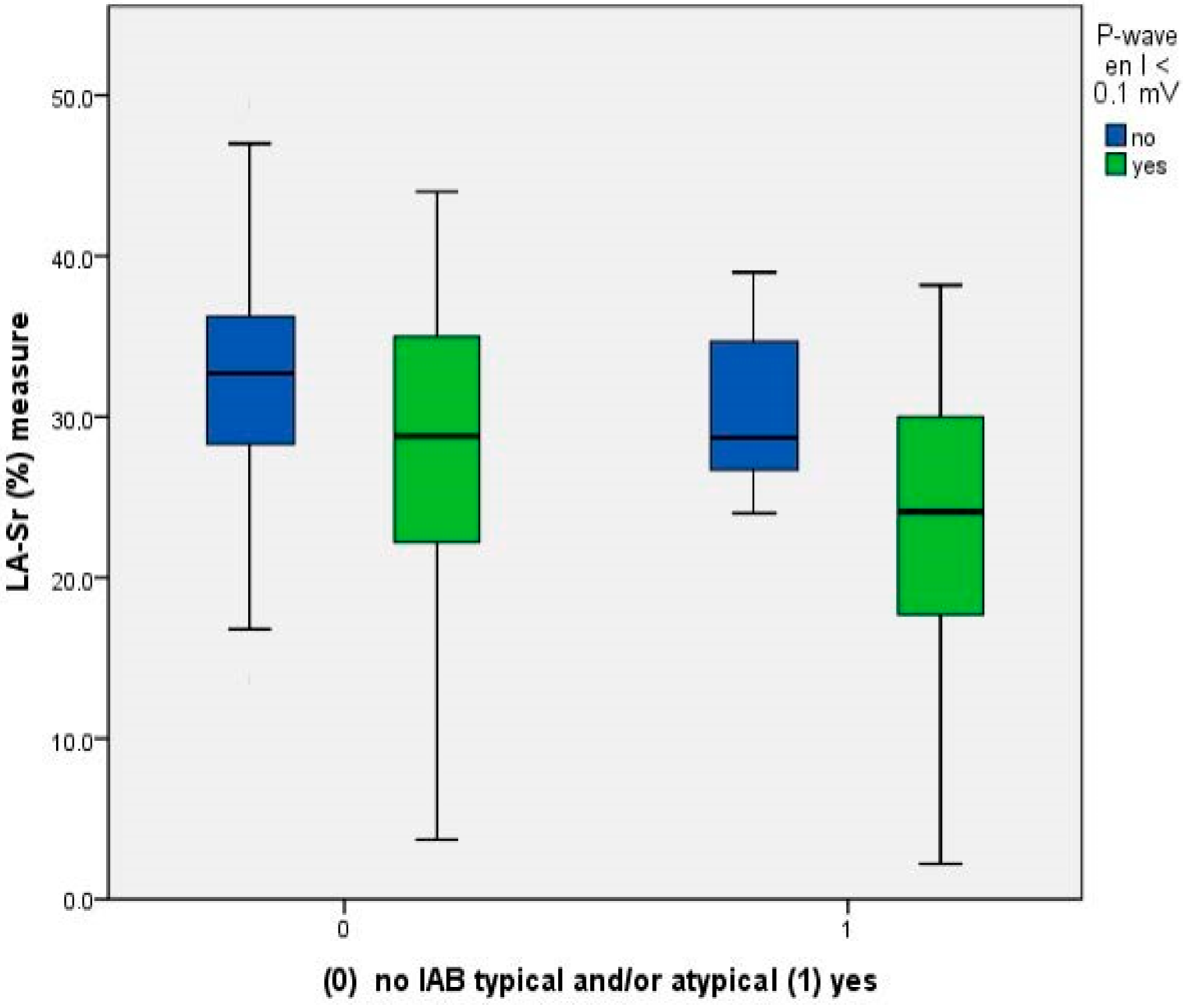
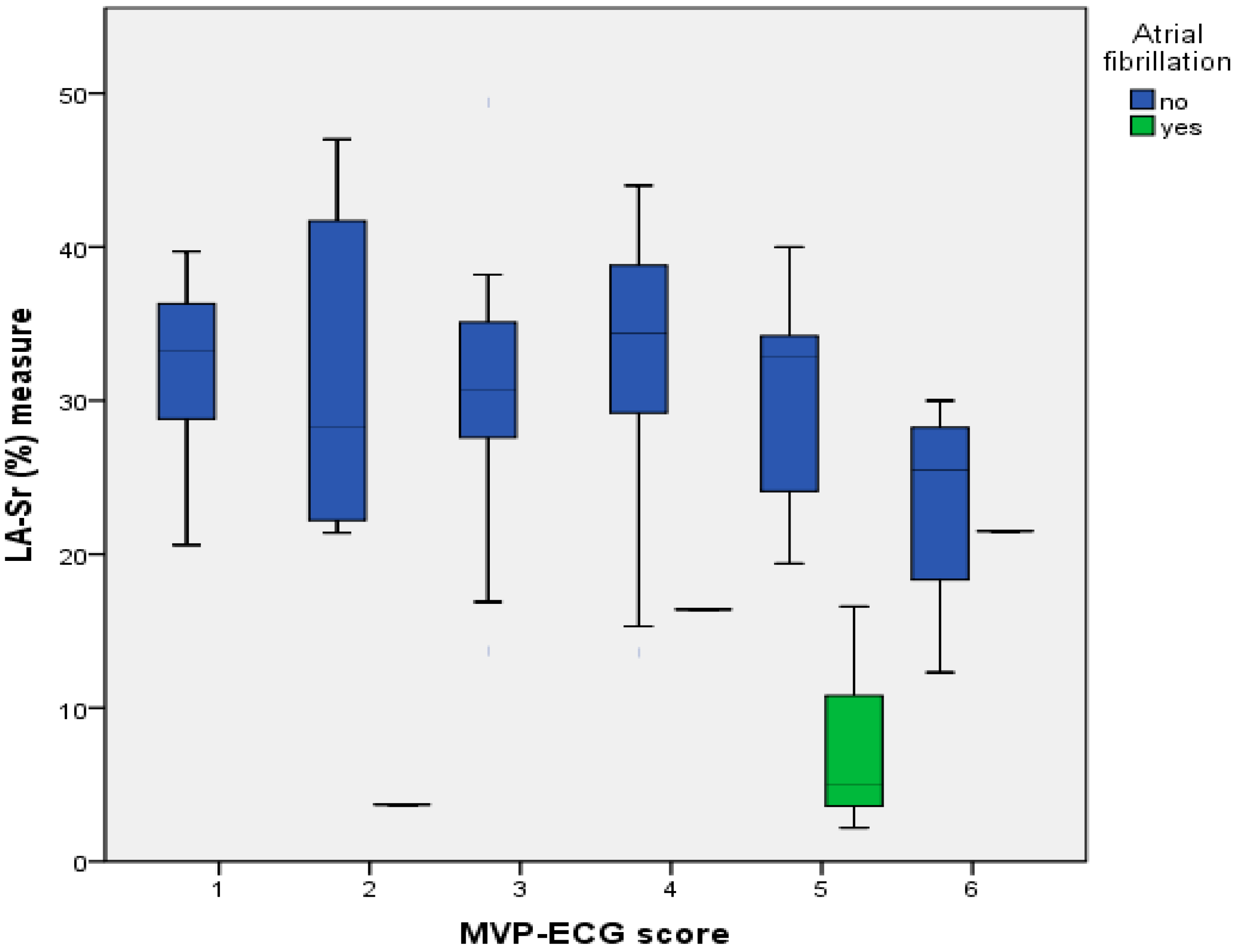
4.2. Relationship between Reduced LA-Sr < 26% and ECG Parameters
4.3. Anticoagulation Therapy versus Aspirin in Patients in Sinus Rhythm
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Stroke Organization. Stroke Alliance for Europe Stroke Action Plan for Europe 2018–2030. Available online: https://www.safestroke.eu/wp-content/uploads/2019/05/SAFE-SAPE-ebook-correct-version_compressed-FINAL-FINAL.pdf (accessed on 10 July 2023).
- Marini, C.; De Santis, F.; Sacco, S.; Russo, T.; Olivieri, L.; Totaro, R.; Carolei, A. Contribution of Atrial Fibrillation to Incidence and Outcome of Ischemic Stroke. Stroke 2005, 36, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Clua-Espuny, J.-L.; Molto-Balado, P.; Lucas-Noll, J.; Panisello-Tafalla, A.; Muria-Subirats, E.; Clua-Queralt, J.; Queralt-Tomas, L.; Reverté-Villarroya, S.; Investigators EBRICTUS Research. Early Diagnosis of Atrial Fibrillation and Stroke Incidence in Primary Care: Translating Measurements into Actions—A Retrospective Cohort Study. Biomedicines 2023, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Hannon, N.; Sheehan, O.; Kelly, L.; Marnane, M.; Merwick, A.; Moore, A.; Kyne, L.; Duggan, J.; Moroney, J.; McCormack, P.M.; et al. Stroke Associated with Atrial Fibrillation—Incidence and Early Outcomes in the North Dublin Population Stroke Study. Cerebrovasc. Dis. 2010, 29, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Palà, E.; Pagola, J.; Juega, J.; Francisco-Pascual, J.; Penalba, A.; Rodriguez, M.; Alfonso, M.D.L.; Arenillas, J.F.; Cabezas, J.A.; Moniche, F.; et al. Proteins and pathways in atrial fibrillation and atrial cardiomyopathy underlying cryptogenic stroke. Int. J. Cardiol. Heart Vasc. 2022, 39, 100977. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Heart Rhythm. 2017, 14, e3–e40. [Google Scholar] [CrossRef] [PubMed]
- Kariki, O.; Vlachos, K.; Dragasis, S.; Tsetika, E.-G.; Perlepe, K.; Saplaouras, A.; Nyktari, E.; Efremidis, M.; Letsas, K.P. Atrial cardiomyopathy: Diagnosis, clinical implications and unresolved issues in anticoagulation therapy. J. Electrocardiol. 2023, 76, 1–10. [Google Scholar] [CrossRef]
- Vera, A.; Cecconi, A.; Ximénez-Carrillo, Á.; Ramos, C.; Martínez-Vives, P.; Lopez-Melgar, B.; Sanz-García, A.; Ortega, G.; Aguirre, C.; Vivancos, J.; et al. Advanced Echocardiography With Left Atrial Strain and Indexed Left Atrial Three-Dimensional Volume for Predicting Underlying Atrial Fibrillation After Cryptogenic Stroke. Am. J. Cardiol. 2022, 185, 87–93. [Google Scholar] [CrossRef]
- Maheshwari, A.; Norby, F.L.; Inciardi, R.M.; Wang, W.; Zhang, M.J.; Soliman, E.Z.; Alonso, A.; Johansen, M.C.; Gottesman, R.F.; Solomon, S.D.; et al. Left Atrial Mechanical Dysfunction and the Risk for Ischemic Stroke in People without Prevalent Atrial Fibrillation or Stroke. Ann. Intern. Med. 2023, 176, 39–48. [Google Scholar] [CrossRef]
- Jain, V.; Ghosh, R.; Gupta, M.; Saijo, Y.; Bansal, A.; Farwati, M.; Marcus, R.; Klein, A.; Xu, B. Contemporary narrative review on left atrial strain mechanics in echocardiography: Cardiomyopathy, valvular heart disease and beyond. Cardiovasc. Diagn. Ther. 2021, 11, 924–938. [Google Scholar] [CrossRef]
- Vera, A.; Cecconi, A.; Ximénez-Carrillo, Á.; Ramos, C.; Martínez-Vives, P.; Lopez-Melgar, B.; Sanz-García, A.; Ortega, G.; Aguirre, C.; Montes, Á.; et al. Left Atrial Strain Predicts Stroke Recurrence and Death in Patients with Cryptogenic Stroke. Am. J. Cardiol. 2023, 210, 51–57. [Google Scholar] [CrossRef]
- Singh, A.; Singulane, C.C.; Miyoshi, T.; Prado, A.D.; Addetia, K.; Bellino, M.; Daimon, M.; Fajardo, P.G.; Kasliwal, R.R.; Kirkpatrick, J.N.; et al. Normal Values of Left Atrial Size and Function and the Impact of Age: Results of the World Alliance Societies of Echocardiography Study. J. Am. Soc. Echocardiogr. 2022, 35, 154–164.e3. [Google Scholar] [CrossRef] [PubMed]
- Conde, D.; Seoane, L.; Gysel, M.; Mitrione, S.; de Luna, A.B.; Baranchuk, A. Bayés’ syndrome: The association between interatrial block and supraventricular arrhythmias. Expert Rev. Cardiovasc. Ther. 2015, 13, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Bayés-De-Luna, A.; Bayés-Genís, A. Clinical implications of advanced interatrial block: Bayés syndrome. Med. Clin. 2021, 156, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.; Bruña, V.; Abizanda, P.; Díez-Villanueva, P.; Formiga, F.; Torres, R.; Carreras, J.; Ayala, R.; Martin-Sánchez, F.J.; Bayés-Genis, A.; et al. Relation of Interatrial Block to Cognitive Impairment in Patients ≥ 70 Years of Age (from the CAMBIAD Case-control Study). Am. J. Cardiol. 2020, 136, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sellés, M.; Martínez-Larrú, M.E.; Ibarrola, M.; Santos, A.; Díez-Villanueva, P.; Bayés-Genis, A.; Baranchuk, A.; Bayés-De-Luna, A.; Elosua, R. Interatrial block and cognitive impairment in the BAYES prospective registry. Int. J. Cardiol. 2020, 321, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sellés, M.; Elosua, R.; Ibarrola, M.; De Andrés, M.; Díez-Villanueva, P.; Bayés-Genis, A.; Baranchuk, A.; Bayés-De-Luna, A.; BAYES Registry Investigators. Advanced interatrial block and P-wave duration are associated with atrial fibrillation and stroke in older adults with heart disease: The BAYES registry. Europace 2020, 22, 1001–1008. [Google Scholar] [CrossRef]
- Berry-Noronha, A.; Bonavia, L.; Wilson, D.; Eranti, A.; Rasmussen, M.U.; Sajadieh, A.; Kreimer, F.; Gotzmann, M.; Sahathevan, R. Predicting risk of AF in ischaemic stroke using sinus rhythm ECG abnormalities: A meta-analysis. Eur. Stroke J. 2023, 8, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Figliozzi, S.; Georgiopoulos, G.; Pateras, K.; Sianis, A.; Previtero, M.; Tondi, L.; Petropoulos, I.; Bragato, R.M.; Papachristidis, A.; Condorelli, G.; et al. Normal ranges of left atrial volumes and ejection fraction by 3D echocardiography in adults: A systematic review and meta-analysis. Int. J. Cardiovasc. Imaging 2022, 38, 1329–1340. [Google Scholar] [CrossRef]
- Tse, G.; Wong, C.W.; Gong, M.; Wong, W.T.; Bazoukis, G.; Wong, S.H.; Li, G.; Wu, W.K.; Tse, L.A.; Lampropoulos, K.; et al. Predictive value of inter-atrial block for new onset or recurrent atrial fibrillation: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 250, 152–156. [Google Scholar] [CrossRef]
- Gentille-Lorente, D.; Salvadó-Usach, T. Clinical, electrical and echocardiographic characteristics of patients with advanced interatrial block. Arch. Cardiol. Mex. 2021, 90, 135–138. [Google Scholar] [CrossRef]
- de Luna, A.B.; Escobar-Robledo, L.A.; Aristizabal, D.; Restrepo, D.W.; Mendieta, G.; van Roessel, A.M.; Elosua, R.; Bayés-Genís, A.; Martínez-Sellés, M.; Baranchuk, A. Atypical advanced interatrial blocks: Definition and electrocardiographic recognition. J. Electrocardiol. 2018, 51, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Gentille-Lorente, D.I.; Scott, L.; Escobar-Robledo, L.A.; Mesa-Maya, M.A.; Carreras-Costa, F.; Baranchuk, A.; Martínez-Sellés, M.; Elosua, R.; Bayés-Genís, A.; Bayés-De-Luna, A. Atypical advanced interatrial block due to giant atrial lipoma. Pacing Clin. Electrophysiol. 2021, 44, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Lacalzada-Almeida, J.; Izquierdo-Gómez, M.M.; Belleyo-Belkasem, C.; Barrio-Martínez, P.; García-Niebla, J.; Elosua, R.; Jiménez-Sosa, A.; Escobar-Robledo, L.A.; de Luna, A.B. Interatrial block and atrial remodeling assessed using speckle tracking echocardiography. BMC Cardiovasc. Disord. 2018, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Montserrat, S.; Gabrielli, L.; Bijnens, B.; Borràs, R.; Berruezo, A.; Poyatos, S.; Brugada, J.; Mont, L.; Sitges, M. Left atrial deformation predicts success of first and second percutaneous atrial fibrillation ablation. Heart Rhythm. 2015, 12, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.; Milden, J.; Hazim, B.; Haseeb, S.; Bayes-Genis, A.; Elosua, R.; Martínez-Sellés, M.; Yeung, C.; Hopman, W.; de Luna, A.B.; et al. New electrocardiographic score for the prediction of atrial fibrillation: The MVP ECG risk score (morphology-voltage-P-wave duration). Ann. Noninvasive Electrocardiol. 2019, 24, e12669. [Google Scholar] [CrossRef]
- Calenda, B.W.; Fuster, V.; Halperin, J.L.; Granger, C.B. Stroke risk assessment in atrial fibrillation: Risk factors and markers of atrial myopathy. Nat. Rev. Cardiol. 2016, 13, 549–559. [Google Scholar] [CrossRef]
- Pathan, F.; D’Elia, N.; Nolan, M.T.; Marwick, T.H.; Negishi, K. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2017, 30, 59–70. [Google Scholar] [CrossRef]
- Park, J.; Hwang, I.; Park, J.J.; Park, J.; Cho, G. Left Atrial Strain to Predict Stroke in Patients With Acute Heart Failure and Sinus Rhythm. J. Am. Heart Assoc. 2021, 10, e020414. [Google Scholar] [CrossRef]
- Sade, L.E.; Keskin, S.; Can, U.; Çolak, A.; Yüce, D.; Çiftçi, O.; Özin, B.; Müderrisoğlu, H. Left atrial mechanics for secondary prevention from embolic stroke of undetermined source. Eur. Heart J. Cardiovasc. Imaging 2020, 23, 381–391. [Google Scholar] [CrossRef]
- Mannina, C.; Ito, K.; Jin, Z.; Yoshida, Y.; Matsumoto, K.; Shames, S.; Russo, C.; Elkind, M.S.V.; Rundek, T.; Yoshita, M.; et al. Association of Left Atrial Strain with Ischemic Stroke Risk in Older Adults. JAMA Cardiol. 2023, 8, 317–325. [Google Scholar] [CrossRef]
- Johansen, M.C.; Wang, W.; Zhang, M.; Knopman, D.S.; Ndumele, C.; Mosley, T.H.; Selvin, E.; Shah, A.M.; Solomon, S.D.; Gottesman, R.F.; et al. Risk of Dementia Associated With Atrial Cardiopathy: The ARIC Study. J. Am. Heart Assoc. 2022, 11, e025646. [Google Scholar] [CrossRef] [PubMed]
- Camara, E.J.N.; Valladares, F.R.D.P.; Key, N.K.; Santana, P.F.; Kawaoka, J.R.; Campos, T.H.; Santana, M.R.d.O.; Cunha, A.C.; Sampaio, D.S.; Santana, G.P.; et al. Indexing of Left Atrial Volume by Body Surface Area and Height in a Brazilian Population without Previous Heart Disease and with a Normal Heart on Echocardiography. Behavior in Obese and Overweight Patients. Cardiol. Cardiovasc. Med. 2023, 7, 25–31. [Google Scholar] [CrossRef]
- Diagnostic Approach of Early Atrial Fibrillation, Silent Stroke and Cognitive Disorder in Patients with High-Risk (PREFA-TE). Grant PERIS 2022 4R22/031 (SLT021/21/000027). ClinicalTrials.gov Identifier (NCT number): NCT05772806. Available online: https://ctv.veeva.com/study/diagnostic-approach-of-early-atrial-fibrillation-silent-stroke-and-cognitive-disorder-in-patients-w (accessed on 1 November 2023).
- Palà, E.; Bustamante, A.; Clua-Espuny, J.L.; Acosta, J.; Gonzalez-Loyola, F.; Santos, S.D.; Ribas-Segui, D.; Ballesta-Ors, J.; Penalba, A.; Giralt, M.; et al. Blood-biomarkers and devices for atrial fibrillation screening: Lessons learned from the AFRICAT (Atrial Fibrillation Research In CATalonia) study. PLoS ONE 2022, 17, e0273571. [Google Scholar] [CrossRef] [PubMed]
- Clua-Espuny, J.-L.; Muria-Subirats, E.; Ballesta-Ors, J.; Lorman-Carbo, B.; Clua-Queralt, J.; Palà, E.; Lechuga-Duran, I.; Gentille-Lorente, D.; Bustamante, A.; Muñoz, M.; et al. Risk of Atrial Fibrillation, Ischemic Stroke and Cognitive Impairment: Study of a Population Cohort ≥ 65 Years of Age. Vasc. Health Risk Manag. 2020, 16, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Proesmans, T.; Mortelmans, C.; Van Haelst, R.; Verbrugge, F.; Vandervoort, P.; Vaes, B. Mobile Phone–Based Use of the Photoplethysmography Technique to Detect Atrial Fibrillation in Primary Care: Diagnostic Accuracy Study of the FibriCheck App. JMIR mHealth uHealth 2019, 7, e12284. [Google Scholar] [CrossRef] [PubMed]
- Mol, D.; Riezebos, R.K.; Marquering, H.A.; Werner, M.E.; Lobban, T.C.; de Jong, J.S.; de Groot, J.R. Performance of an automated photoplethysmography-based artificial intelligence algorithm to detect atrial fibrillation. Cardiovasc. Digit. Health J. 2020, 1, 107–110. [Google Scholar] [CrossRef] [PubMed]
- de Luna, A.B.; Platonov, P.; Cosio, F.G.; Cygankiewicz, I.; Pastore, C.; Baranowski, R.; Bayés-Genis, A.; Guindo, J.; Viñolas, X.; Garcia-Niebla, J.; et al. Interatrial blocks. A separate entity from left atrial enlargement: A consensus report. J. Electrocardiol. 2012, 45, 445–451. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2018, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Cauwenberghs, N.; Haddad, F.; Sabovčik, F.; Kobayashi, Y.; Amsallem, M.; Morris, D.A.; Voigt, J.-U.; Kuznetsova, T. Subclinical left atrial dysfunction profiles for prediction of cardiac outcome in the general population. J. Hypertens. 2020, 38, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Alhakak, A.S.; Biering-Sørensen, S.R.; Møgelvang, R.; Modin, D.; Jensen, G.B.; Schnohr, P.; Iversen, A.Z.; Svendsen, J.H.; Jespersen, T.; Gislason, G.; et al. Usefulness of left atrial strain for predicting incident atrial fibrillation and ischaemic stroke in the general population. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Vyff, F.; Johansen, N.D.; Olsen, F.J.; Duus, L.S.; Lindberg, S.; Fritz-Hansen, T.; Pedersen, S.; Iversen, A.; Galatius, S.; Møgelvang, R.; et al. Left atrial reservoir strain predicts ischaemic stroke after coronary artery bypass grafting independent of postoperative atrial fibrillation. Eur. Heart J. Open 2023, 3, oead045. [Google Scholar] [CrossRef]
- Pagola, J.; González-Alujas, T.; Flores, A.; Muchada, M.; Rodriguez-Luna, D.; Seró, L.; Rubiera, M.; Boned, S.; Ribó, M.; Álvarez-Sabin, J.; et al. Left Atria Strain Is a Surrogate Marker for Detection of Atrial Fibrillation in Cryptogenic Strokes. Stroke 2014, 45, e164–e166. [Google Scholar] [CrossRef]
- Pathan, F.; Sivaraj, E.; Negishi, K.; Rafiudeen, R.; Pathan, S.; D’elia, N.; Galligan, J.; Neilson, S.; Fonseca, R.; Marwick, T.H. Use of Atrial Strain to Predict Atrial Fibrillation After Cerebral Ischemia. JACC Cardiovasc. Imaging 2018, 11, 1557–1565. [Google Scholar] [CrossRef]
- Cameli, M.; Caputo, M.; Mondillo, S.; Ballo, P.; Palmerini, E.; Lisi, M.; Marino, E.; Galderisi, M. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc. Ultrasound 2009, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, T.; Christensen, L.M.; Krieger, D.W.; Mogelvang, R.; Jensen, J.S.; Højberg, S.; Høst, N.; Karlsen, F.M.; Christensen, H. LA Emptying Fraction Improves Diagnosis of Paroxysmal AF after Cryptogenic Ischemic Stroke: Results from the SURPRISE Study. JACC Cardiovasc. Imaging 2014, 7, 962–963. [Google Scholar] [CrossRef]
- Baranchuk, A.; Torner, P.; De Luna, A.B. Bayés Syndrome. What Is It? Circulation 2018, 137, 200–202. [Google Scholar] [CrossRef]
- Bayés-De-Luna, A.; Bacharova, L. New electrocardiographic aspects of the P wave: Its value in clinical cardiology. Ann. Noninvasive Electrocardiol. 2023, 28, e13053. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.U.; Fabricius-Bjerre, A.; Kumarathurai, P.; Larsen, B.S.; Domínguez, H.; Kanters, J.K.; Sajadieh, A. Common source of miscalculation and misclassification of P-wave negativity and P-wave terminal force in lead V1. J. Electrocardiol. 2019, 53, 85–88. [Google Scholar] [CrossRef]
- Sajeev, J.K.; Koshy, A.N.; Dewey, H.; Kalman, J.M.; Bhatia, M.; Roberts, L.; Cooke, J.C.; Frost, T.; Denver, R.; Teh, A.W. Poor reliability of P-wave terminal force V1 in ischemic stroke. J. Electrocardiol. 2019, 52, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, R.K.; Chousou, P.A.; Mukherjee, T.; Pugh, P.J.; Vassiliou, V.S. The predictive value of abnormal P-wave axis for the detection of incident atrial fibrillation: A systematic review with meta-analysis. PLoS ONE 2022, 17, e0278527. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.Y.; Diederichsen, S.Z.; Højberg, S.; Krieger, D.; Graff, C.; Olesen, M.S.; Nielsen, J.B.; Brandes, A.; Køber, L.; Haugan, K.J.; et al. Electrocardiographic Morphology-Voltage-P-Wave-Duration (MVP) Score to Select Patients for Continuous Atrial Fibrillation Screening to Prevent Stroke. Am. J. Cardiol. 2023, 205, 457–464. [Google Scholar] [CrossRef]
- Lorente, D.I.G. Abnormal atrial rhythm in a heterotaxy syndrome. Arch. Cardiovasc. Dis. 2014, 107, 701–703. [Google Scholar] [CrossRef]
- Poli, S.; Meissner, C.; Baezner, H.J.; Kraft, A.; Hillenbrand, F.; Hobohm, C.; Liman, J.; Wachter, R.; Kimmig, H.; Huber, R.; et al. Apixaban for treatment of embolic stroke of undetermined source (ATTICUS) randomized trial—Update of patient characteristics and study timeline after interim analysis. Eur. Heart J. 2021, 42 (Suppl. S1), ehab724.2070. [Google Scholar] [CrossRef]
- Hariharan, N.N.; Patel, K.; Sikder, O.; Perera, K.S.; Diener, H.-C.; Hart, R.G.; Eikelboom, J.W. Oral anticoagulation versus antiplatelet therapy for secondary stroke prevention in patients with embolic stroke of undetermined source: A systematic review and meta-analysis. Eur. Stroke J. 2022, 7, 92–98. [Google Scholar] [CrossRef]
- Healey, J.S.; Lopes, R.D.; Granger, C.B.; Alings, M.; Rivard, L.; McIntyre, W.F.; Atar, D.; Birnie, D.H.; Boriani, G.; Camm, A.J.; et al. Apixaban for Stroke Prevention in Subclinical Atrial Fibrillation. N. Engl. J. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Patrick, S.; Mayer, L.K.; Müller-Edenborn, B.; Eichenlaub, M.; Allgeier, M.; Allgeier, J.; Lehrmann, H.; Ahlgrim, C.; Bohnen, M.; et al. Echocardiographic and Electrocardiographic Determinants of Atrial Cardiomyopathy Identify Patients with Atrial Fibrillation at Risk for Left Atrial Thrombogenesis. J. Clin. Med. 2022, 11, 1332. [Google Scholar] [CrossRef]

| Variables | All | LA-Sr ≥ 26% | LA-Sr < 26% | p |
|---|---|---|---|---|
| N, % | 86 (100.0) | 55 (64.0) | 31 (36.0) | |
| Age, ± SD | 74.9 ± 5.3 | 74.1 ± 5.3 | 76.2 ± 5.6 | 0.083 |
| Women, n (%) | 56 (65.1) | 34 (61.8) | 22 (70.9) | 0.482 |
| Body mass index, ± SD | 32.5 ± 5.7 | 32.5 ± 5.0 | 32.1 ± 7.6 | 0.797 |
| Hypertension, n (%) | 77 (89.5) | 50 (90.9) | 27 (87.1) | 0.717 |
| Dyslipidemia, n (%) | 70 (81.4) | 43 (78.2) | 27(87.1) | 0.394 |
| Diabetes mellitus, n (%) | 50 (58.1) | 34 (61.8) | 16 (51.6) | 0.373 |
| Active smoking, n (%) | 8 (9.30) | 6 (10.9) | 2 (6.4) | 0.705 |
| Chronic renal failure, n (%) | 26 (30.2) | 17 (30.9) | 9 (29.0) | 0.999 |
| Myocardial ischemia, n (%) | 10 (11.6) | 5 (9.1) | 5 (16.1) | 0.485 |
| Peripheral vascular disease, n (%) | 9 (10.5) | 6 (10.9) | 3 (9.7) | 0.999 |
| Heart failure, n (%) | 7 (8.1) | 5 (9.1) | 2 (6.4) | 0.999 |
| CHA2DS2 VASc score, ± SD | 4.1 ± 1.0 | 4.0 ± 1.0 | 4.3 ± 1.2 | 0.251 |
| AF risk [15] 1, ± SD | 8.6 ± 0.5 | 8.6 ± 0.5 | 8.8 ± 0.7 | 0.087 |
| CVD-SCORE 2 ≥ 5%, n (%) | 40 (66.6) | 24 (66.6) | 16 (66.6) | 0.658 |
| REGICOR score 3, ± SD | 6.9 ± 3.9 | 7.3 ± 3.9 | 7.1 ± 3.9 | 0.933 |
| Pfeiffer score, ± SD | 0.8 ± 1.1 | 0.7 ± 0.0 | 1.0 ± 1.2 | 0.158 |
| Epworth score, ± SD | 5.4 ± 6.1 | 5.6 ± 6.2 | 5.2 ± 6.6 | 0.776 |
| Valvular heart disease, n (%) | 4 (4.6) | 3 (5.4) | 1 (3.2) | 0.999 |
| Arterial hypertension, n (%) | 74 (86.0) | 48 (87.3) | 26 (83.9) | 0.749 |
| Statins treatment, n (%) | 50 (58.1) | 35 (63.6) | 15 (48.4) | 0.181 |
| Diabetes treatment, n (%) | 42 (48.8) | 29 (52.7) | 13 (41.9) | 0.375 |
| Antiplatelet drugs, n (%) | 24 (27.9) | 15 (27.3) | 9 (29.0) | 0.999 |
| New atrial fibrillation | 7 | 0 | 7 | <0.001 |
| Variables | All | LA-Sr ≥ 26% | LA-Sr < 26% | p |
|---|---|---|---|---|
| N, % | 86 (100.0) | 55 (64.0) | 31 (36.0) | |
| Age, ± SD | 74.9 ± 5.3 | 74.1 ± 5.3 | 76.2 ± 5.6 | 0.083 |
| Women, n (%) | 56 (65.1) | 34 (61.8) | 22 (70.9) | 0.482 |
| LV 1 Ejection Fraction (%), ± SD | 62.6 ± 6.2 | 63.3 ± 5.6 | 62.1 ± 7.6 | 0.411 |
| LV 1 Ejection Fraction ≤ 50%, n (%) | 2 (2.3) | 0 (0.0) | 2 (6.4) | na 10 |
| LV 1 Ejection Fraction ≤ 40%, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | na 10 |
| LV 1 Diastolic Dysfunction, n (%) | 69 (81.2) | 45 (81.8) | 24 (77.4) | 0.622 |
| LV 1 Diastolic Dysfunction Grade II-IV, n (%) | 11 (12.8) | 5 (9.1) | 6 (19.3) | 0.351 |
| Sigmoid IV 2 Septum, n (%) | 18 (20.9%) | 9 (16.4%) | 9 (29.0%) | 0.179 |
| Sigmoid IV 2 Septum (mm), ± SD | 13.1 ± 1.3 | 12.4 ± 1.0 | 13.7 ± 1.6 | 0.026 |
| LA 3 vol (mL/m2) in 2D-TTE 4, ± SD | 32.0 ± 9.07 | 29.4 ± 6.9 | 36.4 ± 10.4 | <0.001 |
| LA 3 Dilation, n (%) | 24 (28.2) | 9 (16.3) | 15 (48.4) | 0.004 |
| LA 3 Dilation: . mild (34–41.9 mL/m2) . moderate (42–48 mL/m2) . severe (≥48 mL/m2) | 15 (62.5) 3 (12.5) 6 (25.0) | 6 (66.7) 2 (22.2) 1 (11.1) | 9 (60.0) 1 (6.7) 5 (33.3) | 0.306 |
| LA 3 vol (mL/m2) in 3D-TTE 4, ± SD | 38.1 ± 11.0 | 37.1 ± 11.6 | 39.9 ± 10.2 | 0.383 |
| LA 3 Ejection Fraction 2D 8-TTE 4 ± SD | 50.96 ± 10.08 | 55.3 ± 5.38 | 42.4 ± 11.2 | <0.001 |
| LA 3 Ejection Fraction 3D 9-TTE 4, ± SD | 52.7 ± 13.8 | 56.2 ± 13.5 | 44.7 ± 11.4 | 0.001 |
| LA 3 Strain-cd 5, ± SD | 13.03 ± 5.93 | 13.1 ± 4.6 | 11.3 ± 4.4 | 0.069 |
| LA 3 Strain-ct 6, ± SD | 16.3 ± 7.7 | 20.4 ± 5.3 | 8.3 ± 4.5 | <0.001 |
| LA 3 Strain-r 7, ± SD | 28.6 ± 9.5 | 34.2 ± 5.3 | 18.4 ± 6.2 | <0.001 |
| Mitral Regurgitation (grade II–IV), n (%) | 19 (22.1) | 9 (16.4) | 10 (32.2) | 0.119 |
| Mitral Stenosis, n (%) | 6 (7.0) | 2 (3.6) | 4 (12.9) | 0.179 |
| Aortic Regurgitation (grade II–IV), n (%) | 9 (10.5) | 5 (9.1) | 4 (12.9) | 0.823 |
| Aortic Stenosis, n (%) | 4 (4.6) | 1 (1.8) | 3 (9.7) | 0.124 |
| Variables | All | LA-Sr ≥ 26% | LA-Sr < 26% | p |
|---|---|---|---|---|
| N, % | 86 (100.0) | 55 (64) | 31 (36) | |
| Age, ± SD | 74.9 ± 5.3 | 74.1 ± 5.3 | 76.2 ± 5.6 | 0.083 |
| Women, n (%) | 56 (65.1) | 34 (61.8) | 22 (70.9) | 0.482 |
| Heart rate, average ± SD | 69.5 ± 13.0 | 69.5 ± 13.0 | 66.0 ± 13.1 | 0.226 |
| Heart rate < 60 bpm, n (%) | 38 (44.2) | 25 (45.4) | 13 (41.9) | 0.823 |
| P-wave > 120 ms, n (%) | 64 (74.4) | 41 (74.5) | 23 (74.2) | 0.999 |
| Partial IAB 1, n (%) | 39 (45.3) | 30 (55.5) | 9 (29.0) | 0.142 |
| Advanced IAB 2, n (%) | 26 (30.2) | 12 (21.8) | 14 (45.2) | 0.032 |
| Typical advanced IAB, n (%) | 18 (20.9) | 9 (16.3) | 9 (29.0) | 0.272 |
| Atypical advanced IAB, n (%) | 8 (9.3) | 3 (5.4) | 5 (16.1) | 0.008 |
| P-wave < 0.1 mV lead I, n (%) | 43 (50.0) | 21 (38.2) | 22 (71.0) | 0.007 |
| P-wave > 0.04 s in V1, n (%) | 58 (67.4) | 39 (70.9) | 19 (61.3) | 0.339 |
| MVP 3 score (“original”) | 3.5 ± 1.4 | 3.2 ± 1.4 | 3.9 ± 1.37 | 0.038 |
| . low: 0–1, n (%) . intermediate, n (%) . high: 5–6, n (%) | 19 (22.3) 47 (55.3) 19 (22.3) | 14 (25.4) 31(56.4) 10 (18.2) | 5 (16.7) 16 (53.3) 9 (30.0) | |
| MVP 3 score_2 (“modified”) | 3.6 ± 1.5 | 3.2 ± 1.5 | 4.0 ± 1.4 | 0.036 |
| . low: 0–1, n (%) . intermediate, n (%) . high: 5–6, n (%) | 19 (22.3) 41 (48.2) 25 (29.4) | 14 (25.4) 29 (52.7) 12 (21.8) | 5 (16.7) 12 (40.0) 13 (43.3) | |
| QRS > 120 ms, n (%) | 12 (13.9) | 8 (14.5) | 4 (12.9) | 0.999 |
| Sokolow-Lyon and/or Cornell (+) criteria, n (%) | 5 (5.8) | 2 (3.6) | 3 (9.7) | 0.349 |
| Atrioventricular block, n (%) | 14 (16.3) | 9 (16.4) | 5 (16.1) | 0.999 |
| Premature supraventricular beats, n (%) | 5 (5.8) | 2 (3.6) | 3 (9.7) | 0.349 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentille-Lorente, D.; Hernández-Pinilla, A.; Satue-Gracia, E.; Muria-Subirats, E.; Forcadell-Peris, M.J.; Gentille-Lorente, J.; Ballesta-Ors, J.; Martín-Lujan, F.M.; Clua-Espuny, J.L. Echocardiography and Electrocardiography in Detecting Atrial Cardiomyopathy: A Promising Path to Predicting Cardioembolic Strokes and Atrial Fibrillation. J. Clin. Med. 2023, 12, 7315. https://doi.org/10.3390/jcm12237315
Gentille-Lorente D, Hernández-Pinilla A, Satue-Gracia E, Muria-Subirats E, Forcadell-Peris MJ, Gentille-Lorente J, Ballesta-Ors J, Martín-Lujan FM, Clua-Espuny JL. Echocardiography and Electrocardiography in Detecting Atrial Cardiomyopathy: A Promising Path to Predicting Cardioembolic Strokes and Atrial Fibrillation. Journal of Clinical Medicine. 2023; 12(23):7315. https://doi.org/10.3390/jcm12237315
Chicago/Turabian StyleGentille-Lorente, Delicia, Alba Hernández-Pinilla, Eva Satue-Gracia, Eulalia Muria-Subirats, Maria Jose Forcadell-Peris, Jorge Gentille-Lorente, Juan Ballesta-Ors, Francisco Manuel Martín-Lujan, and Josep Lluis Clua-Espuny. 2023. "Echocardiography and Electrocardiography in Detecting Atrial Cardiomyopathy: A Promising Path to Predicting Cardioembolic Strokes and Atrial Fibrillation" Journal of Clinical Medicine 12, no. 23: 7315. https://doi.org/10.3390/jcm12237315
APA StyleGentille-Lorente, D., Hernández-Pinilla, A., Satue-Gracia, E., Muria-Subirats, E., Forcadell-Peris, M. J., Gentille-Lorente, J., Ballesta-Ors, J., Martín-Lujan, F. M., & Clua-Espuny, J. L. (2023). Echocardiography and Electrocardiography in Detecting Atrial Cardiomyopathy: A Promising Path to Predicting Cardioembolic Strokes and Atrial Fibrillation. Journal of Clinical Medicine, 12(23), 7315. https://doi.org/10.3390/jcm12237315






