Small Bowel Capsule Endoscopy and Enteroscopy: A Shoulder-to-Shoulder Race
Abstract
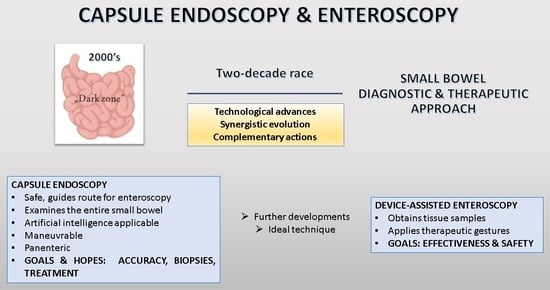
:1. Introduction
2. Small Bowel Exploration—Between Revelations and Unanswered Inquiries
3. The Synergy of Capsule Endoscopy and Enteroscopy in Various Clinical Settings
4. Further Challenges and Goals for the Future
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Triester, S.L.; Leighton, J.A.; Leontiadis, G.I.; Fleischer, D.E.; Hara, A.K.; Heigh, R.I.; Shiff, A.D.; Sharma, V.K. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am. J. Gastroenterol. 2005, 100, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Lukaszewski, K.; Caroline, D.; Parkman, H.; DeSipio, J.; Banson, F.; Bazir, K.; Reddy, L.; Srinivasan, R.; Fisher, R.; et al. A retrospective review of enteroclysis in patients with obscure gastrointestinal bleeding and chronic abdominal pain of undetermined etiology. Dig. Dis. Sci. 2005, 50, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Dionisio, P.M.; Gurudu, S.R.; Leighton, J.A.; Leontiadis, G.I.; Fleischer, D.E.; Hara, A.K.; Heigh, R.I.; Shiff, A.D.; Sharma, V.K. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: A meta-analysis. Am. J. Gastroenterol. 2010, 105, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.Y.; Choi, M.G. Current advance in small bowel tumors. Clin. Endosc. 2011, 44, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Nehme, F.; Goyal, H.; Perisetti, A.; Tharian, B.; Sharma, N.; Tham, T.C.; Chhabra, R. The Evolution of Device-Assisted Enteroscopy: From Sonde Enteroscopy to Motorized Spiral Enteroscopy. Front. Med. 2021, 8, 792668. [Google Scholar] [CrossRef]
- Moeschler, O.; Mueller, M.K. Deep enteroscopy—Indications, diagnostic yield and complications. World J. Gastroenterol. 2015, 21, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Cicero, G.; Mazziotti, S. Crohn’s disease at radiological imaging: Focus on techniques and intestinal tract. Intest. Res. 2021, 19, 365–378. [Google Scholar] [CrossRef]
- Grady, E. Gastrointestinal Bleeding Scintigraphy in the Early 21st Century. J. Nucl. Med. 2016, 57, 252–259. [Google Scholar] [CrossRef]
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless capsule endoscopy. Nature 2000, 405, 417. [Google Scholar] [CrossRef]
- Swain, P. Wireless capsule endoscopy. Gut 2003, 52 (Suppl. 4), 48–50. [Google Scholar] [CrossRef]
- Van de Bruaene, C.; De Looze, D.; Hindryckx, P. Small bowel capsule endoscopy: Where are we after almost 15 years of use? World J. Gastrointest. Endosc. 2015, 7, 13–36. [Google Scholar] [CrossRef]
- Pennazio, M.; Rondonotti, E.; Despott, E.J.; Dray, X.; Keuchel, M.; Moreels, T.; Sanders, D.S.; Spada, C.; Carretero, C.; Cortegoso Valdivia, P.; et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2022. Endoscopy 2023, 55, 58–95. [Google Scholar] [CrossRef]
- Bandorski, D.; Kurniawan, N.; Baltes, P.; Hoeltgen, R.; Hecker, M.; Stunder, D.; Keuchel, M. Contraindications for video capsule endoscopy. World J. Gastroenterol. 2016, 22, 9898–9908. [Google Scholar] [CrossRef]
- Tabet, R.; Nassani, N.; Karam, B.; Shammaa, Y.; Akhrass, P.; Deeb, L. Pooled Analysis of the Efficacy and Safety of Video Capsule Endoscopy in Patients with Implantable Cardiac Devices. Can. J. Gastroenterol. Hepatol. 2019, 2019, 3953807. [Google Scholar] [CrossRef]
- Pitocco, D.; Rizzi, A.; Tortora, A.; Manto, A.; Zaccardi, F.; Ghirlanda, G.; Costamagna, G.; Riccioni, M.E. Possible Radio Interference Between Video Capsule Endoscopy and Second-Generation OmniPod Patch Pump. Diabetes Technol. Ther. 2016, 18, 444–445. [Google Scholar] [CrossRef]
- Koulaouzidis, A.; Iakovidis, D.K.; Karargyris, A.; Rondonotti, E. Wir eless endoscopy in 2020: Will it still be a capsule? World J. Gastroenterol. 2015, 21, 5119–5130. [Google Scholar] [CrossRef]
- Deng, S.; Gu, J.; Jiang, Z.; Cao, Y.; Mao, F.; Xue, Y.; Wang, J.; Dai, K.; Qin, L.; Liu, K.; et al. Application of nanotechnology in the early diagnosis and comprehensive treatment of gastrointestinal cancer. J. Nanobiotechnol. 2022, 20, 415. [Google Scholar] [CrossRef]
- Yamamoto, H.; Despott, E.J.; González-Suárez, B.; Pennazio, M.; Mönkemüller, K. The evolving role of device-assisted enteroscopy: The state of the art as of August 2023. Best Pract. Res. Clin. Gastroenterol. 2023, 64–65, 101858. [Google Scholar] [CrossRef]
- Elena, R.M.; Riccardo, U.; Rossella, C.; Bizzotto, A.; Domenico, G.; Guido, C. Current status of device-assisted enteroscopy: Technical matters, indication, limits and complications. World J. Gastrointest. Endosc. 2012, 4, 4534–4561. [Google Scholar] [CrossRef]
- Tee, H.P.; How, S.H.; Kaffes, A.J. Learning curve for double-balloon enteroscopy: Findings from an analysis of 282 procedures. World J. Gastrointest. Endosc. 2012, 4, 368–372. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Nachbar, L.; Ell, C. Double-balloon enteroscopy (push-and-pull enteroscopy) of the small bowel: Feasibility and diagnostic and therapeutic yield in patients with suspected small bowel disease. Gastrointest. Endosc. 2005, 62, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Liao, Z.; Jiang, Y.P.; Li, Z.S. Indications, detectability, positive findings, total enteroscopy, and complications of diagnostic double-balloon endoscopy: A systematic review of data over the first decade of use. Gastrointest. Endosc. 2011, 74, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Jang, H.J.; Kae, S.H.; Lee, J.G.; Kwon, J.H. Indication, Location of the Lesion, Diagnostic Yield, and Therapeutic Yield of Double-Balloon Enteroscopy: Seventeen Years of Experience. Diagnostics 2022, 12, 2224. [Google Scholar] [CrossRef]
- Hong, S.N.; Kim, E.R.; Ye, B.D.; Jang, H.J.; Jeon, S.R.; Park, S.J.; Im, J.p.; Kim, J.H.; Choi, C.H.; Choi, H.; et al. Indications, diagnostic yield, and complication rate of balloon-assisted enteroscopy (BAE) during the first decade of its use in Korea. Dig. Endosc. 2016, 28, 443–449. [Google Scholar] [CrossRef]
- Mensink, P.B.; Haringsma, J.; Kucharzik, T.; Cellier, C.; Pérez-Cuadrado, E.; Mönkemüller, K.; Gasbarrini, A.; Kaffes, A.J.; Nakamura, K.; Yen, H.H.; et al. Complications of double balloon enteroscopy: A multicenter survey. Endoscopy 2007, 39, 613–615. [Google Scholar] [CrossRef]
- Honda, K.; Itaba, S.; Mizutani, T.; Sumida, Y.; Kanayama, K.; Higuchi, N.; Yoshinaga, S.; Akiho, H.; Kawabe, K.; Arita, Y.; et al. An increase in the serum amylase level in patients after peroral double-balloon enteroscopy: An association with the development of pancreatitis. Endoscopy 2006, 38, 1040–1043. [Google Scholar] [CrossRef]
- Kopácová, M.; Rejchrt, S.; Tachecí, I.; Bures, J. Hyperamylasemia of uncertain significance associated with oral double-balloon enteroscopy. Gastrointest. Endosc. 2007, 66, 1133–1138. [Google Scholar] [CrossRef]
- Aktas, H.; Mensink, P.B.; Haringsma, J.; Kuipers, E.J. Low incidence of hyperamylasemia after proximal double-balloon enteroscopy: Has the insertion technique improved? Endoscopy 2009, 41, 670–673. [Google Scholar] [CrossRef]
- Kawamura, T.; Yasuda, K.; Tanaka, K.; Uno, K.; Ueda, M.; Sanada, K.; Nakajima, M. Clinical evaluation of a newly developed single-balloon enteroscope. Gastrointest. Endosc. 2008, 68, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Takano, N.; Yamada, A.; Watabe, H.; Togo, G.; Yamaji, Y.; Yoshida, H.; Kawabe, T.; Omata, M.; Koike, K. Single-balloon versus double-balloon endoscopy for achieving total enteroscopy: A randomized, controlled trial. Gastrointest. Endosc. 2011, 73, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, B.R.; Sanaka, M.R.; Lopez, A.R.; Vargo, J.J. The clinical utility of single-balloon enteroscopy: A single-center experience of 172 procedures. Gastrointest. Endosc. 2010, 71, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- ASGE Technology Committee; Chauhan, S.; Manfredi, M.; Abu Dayyeh, B.; Enestvedt, B.K.; Fujii-Lau, L.L.; Komanduri, S. Enteroscopy. Gastrointest. Endosc. 2015, 82, 975–990. [Google Scholar] [CrossRef]
- Lenz, P.; Domagk, D. Double- vs. single-balloon vs. spiral enteroscopy. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 303–313. [Google Scholar] [CrossRef]
- Khashab, M.A.; Lennon, A.M.; Dunbar, K.B.; Singh, V.K.; Chandrasekhara, V.; Giday, S.; Canto, M.I.; Buscaglia, J.M.; Kapoor, S.; Shin, E.J.; et al. A comparative evaluation of single-balloon enteroscopy and spiral enteroscopy for patients with mid-gut disorders. Gastrointest. Endosc. 2010, 72, 766–772. [Google Scholar] [CrossRef]
- Marques, M.; Santos-Antunes, J.; Coelho, R.; Cardoso, H.; Vilas Boas, F.; Ribeiro, A.; Macedo, G. Single-balloon enteroscopy efficacy and degree of concordance with noninvasive evaluation of small bowel. Endosc. Int. Open 2017, 5, E96–E102. [Google Scholar] [CrossRef] [PubMed]
- Akerman, P.A.; Agrawal, D.; Chen, W.; Cantero, D.; Avila, J.; Pangtay, J. Spiral enteroscopy: A novel method of enteroscopy by using the Endo-Ease Discovery SB overtube and a pediatric colonoscope. Gastrointest. Endosc. 2009, 69, 327–332. [Google Scholar] [CrossRef]
- Akerman, P.; Agrawal, D.; Cantero, D.; Pangtay, J. Spiral enteroscopy with the new DSB overtube: A novel technique for deep peroral small-bowel intubation. Endoscopy 2008, 40, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Baniya, R.; Upadhaya, S.; Subedi, S.C.; Khan, J.; Sharma, P.; Mohammed, T.S.; Bachuwa, G.; Jamil, L.H. Balloon enteroscopy versus spiral enteroscopy for small-bowel disorders: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 86, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Messer, I.; May, A.; Manner, H.; Ell, C. Prospective, randomized, single-center trial comparing double-balloon enteroscopy and spiral enteroscopy in patients with suspected small-bowel disorders. Gastrointest. Endosc. 2013, 77, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.; Upchurch, B.; Draganov, P.; Binmoeller, K.F.; Haluszka, O.; Jonnalagadda, S.; Okolo, P.; Grimm, I.; Judah, J.; Tokar, J.; et al. Spiral enteroscopy: Prospective U.S. multicenter study in patients with small-bowel disorders. Gastrointest. Endosc. 2010, 72, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Hollerich, J.; Beyna, T. Device-assisted enteroscopy: A review of available techniques and upcoming new technologies. World J. Gastroenterol. 2019, 25, 3538–3545. [Google Scholar] [CrossRef] [PubMed]
- Buscaglia, J.M.; Dunbar, K.B.; Okolo, P.I., 3rd; Judah, J.; Akerman, P.A.; Cantero, D.; Draganov, P.V. The spiral enteroscopy training initiative: Results of a prospective study evaluating the discovery SB overtube device during small bowel enteroscopy (with video). Endoscopy 2009, 41, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, H.; Beyna, T.; Schneider, M.; Devière, J. Novel motorized spiral enteroscopy: First clinical case. VideoGIE 2016, 1, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Mans, L.; Arvanitakis, M.; Neuhaus, H.; Devière, J. Motorized spiral enteroscopy for occult bleeding. Dig. Dis. 2018, 36, 325–327. [Google Scholar] [CrossRef]
- Olympus Medical. Urgent Field Safety Notice QIL FY24-EMEA-10-FY24-OMSC-05 2023 [Urgent Field Safety Notice QIL FY24-EMEA-10-FY24-OMSC-05]. Available online: https://www.igj.nl/binaries/igj/documenten/waarschuwingen/2023/07/11/fsn-qil-fy24-emea-10-fy24-omsc-05-olympus-medical-systems-corporation-powerspiral-intestinal-videoscope-psf-1/IT2081382+FSN-QIL+FY24-EMEA-10-FY24-OMSC-05+Olympus+Medical+Systems+Corporation+PowerSpiral+Intestinal+Videoscope+PSF-1.pdf (accessed on 1 October 2023).
- Adler, S.N.; Bjarnason, I.; Metzger, Y.C. New balloon-guided technique for deep small-intestine endoscopy using standard endoscopes. Endoscopy 2008, 40, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Wild, D.; Shieh, F.; Diehl, D.L.; Fischer, M.; Tamura, W.; Rubin, D.T.; Kumbhaari, V.; Okolo, P.; Storm, A.; et al. Deep enteroscopy with a conventional colonoscope: Initial multicenter study by using a through-the-scope balloon catheter system. Gastrointest. Endosc. 2015, 82, 855–860. [Google Scholar] [CrossRef]
- Kumbhari, V.; Storm, A.C.; Khashab, M.A.; Canto, M.I.; Saxena, P.; Akshintala, V.S.; Messallam, A.A.; Sinbh, V.K.; Lennon, A.M.; Shin, E.J.; et al. Deep enteroscopy with standard endoscopes using a novel through-the- scope balloon. Endoscopy 2014, 46, 685–689. [Google Scholar] [CrossRef]
- Kumbhari, V.; Saxena, P.; Khashab, M.A. A new through-the-scope balloon-assisted deep enteroscopy platform. Gastrointest. Endosc. 2014, 79, 694. [Google Scholar] [CrossRef]
- Shim, K.N.; Jeon, S.R.; Jang, H.J.; Kim, J.; Lim, Y.J.; Kim, K.O.; Song, H.J.; Lee, H.S.; Park, J.J.; Kim, J.H.; et al. Korean Gut Image Study Group. Quality Indicators for Small Bowel Capsule Endoscopy. Clin. Endosc. 2017, 50, 148–160. [Google Scholar] [CrossRef]
- Rizk, M.K.; Sawhney, M.S.; Cohen, J.; Pike, I.M.; Adler, D.G.; Dominitz, J.A.; Lieb, J.G., 2nd; Lieberman, D.A.; Park, W.G.; Shaheen, N.J.; et al. Quality indicators common to all GI endoscopic procedures. Gastrointest. Endosc. 2015, 81, 3–16. [Google Scholar] [CrossRef]
- Leighton, J.A.; Brock, A.S.; Semrad, C.E.; Hass, D.J.; Guda, N.M.; Barkin, J.A.; Eisen, G.M. Quality Indicators for Capsule Endoscopy and Deep Enteroscopy. Am. J. Gastroenterol. 2022, 117, 1780–1796. [Google Scholar] [CrossRef] [PubMed]
- Gerson, L.B.; Fidler, J.L.; Cave, D.R.; Leighton, J.A. ACG Clinical Guideline: Diagnosis and Management of Small Bowel Bleeding. Am. J. Gastroenterol. 2015, 110, 1265–1287; quiz 1288. [Google Scholar] [CrossRef] [PubMed]
- Teshima, C.W.; Kuipers, E.J.; van Zanten, S.V.; Mensink, P.B. Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: An updated meta-analysis. J. Gastroenterol. Hepatol. 2011, 26, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Nakamura, M.; Wu, S.; Uchida, G.; Yamamura, T.; Gao, Y.J.; Goto, H.; Fujishiro, M.; Ge, Z.Z. The role of early video capsule endoscopy in the diagnosis and prognosis of obscure gastrointestinal bleeding: A multi-center propensity score matching study. J. Gastroenterol. Hepatol. 2021, 36, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.C.; Mo, L.R.; Hu, S.C. The Optimal Timing for Using Capsule Endoscopy for Patients with Gastrointestinal Bleeding. Biomed. Res. Int. 2021, 27, 7605324. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, M.M.; Pinho, R.; Fernandes, C.; Rodrigues, A.; Ponte, A.; Gomes, A.C.; Afecto, E.; Correia, J.; Carvalho, J. Diagnostic and therapeutic yields of early capsule endoscopy and device-assisted enteroscopy in the setting of overt GI bleeding: A systematic review with meta-analysis. Gastrointest. Endosc. 2022, 95, 610–625.e9. [Google Scholar] [CrossRef] [PubMed]
- CortegosoValdivia, P.; Skonieczna-Żydecka, K.; Pennazio, M.; Rondonotti, E.; Marlicz, W.; Toth, E.; Koulaouzidis, A. Capsule endoscopy transit-related indicators in choosing the insertion route for double-balloon enteroscopy: A systematic review. Endosc. Int. Open 2021, 9, E163–E170. [Google Scholar] [CrossRef]
- Enns, R.A.; Hookey, L.; Armstrong, D.; Bernstein, C.N.; Heitman, S.J.; Teshima, C.; Leontiadis, G.I.; Tse, F.; Sadowski, D. Clinical Practice Guidelines for the Use of Video Capsule Endoscopy. Gastroenterology 2017, 152, 497–514. [Google Scholar] [CrossRef]
- Koulaouzidis, A.; Rondonotti, E.; Giannakou, A.; Plevris, J.N. Diagnostic yield of small-bowel capsule endoscopy in patients with iron-deficiency anemia: A systematic review. Gastrointest. Endosc. 2012, 76, 983–992. [Google Scholar] [CrossRef]
- Stone, J.; Grover, K.; Bernstein, C.N. The Use of Capsule Endoscopy for Diagnosis of Iron Deficiency Anemia: A Retrospective Analysis. J. Clin. Gastroenterol. 2020, 54, 452–458. [Google Scholar] [CrossRef]
- Olano, C.; Pazos, X.; Avendaño, K.; Calleri, A.; Ketzoian, C. Diagnostic yield and predictive factors of findings in small-bowel capsule endoscopy in the setting of iron-deficiency anemia. Endosc. Int. Open 2018, 6, E688–E693. [Google Scholar] [CrossRef] [PubMed]
- Sealock, R.J.; Thrift, A.P.; El-Serag, H.B.; Sellin, J. Long-term follow up of patients with obscure gastrointestinal bleeding examined with video capsule endoscopy. Medicine 2018, 97, e11429. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, U.; Yung, D.E.; Engel, T.; Vijayan, S.; Har-Noy, O.; Katz, L.; Oliva, S.; Avni, T.; Battat, R.; Eliakim, R.; et al. Diagnostic yield of capsule endoscopy versus magnetic resonance enterography and small bowel contrast ultrasound in the evaluation of small bowel Crohn’s disease: Systematic review and meta-analysis. Dig. Liver Dis. 2017, 49, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Lim, S.; Choi, M.G.; Shim, K.N.; Lee, S.H. Effectiveness of Capsule Endoscopy Compared with Other Diagnostic Modalities in Patients with Small Bowel Crohn’s Disease: A Meta-Analysis. Gut Liver 2017, 11, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Diegoli, M.; Dussias, N.; Salice, M.; Rizzello, F.; Cappelli, A.; Ricci, C.; Gionchetti, P. Performance of Capsule Endoscopy and Cross-Sectional Techniques in Detecting Small Bowel Lesions in Patients with Crohn’s Disease. Crohns Colitis 360 2020, 2, otaa046. [Google Scholar] [CrossRef] [PubMed]
- Prichard, D.O.; Hamilton, Z.; Savage, T.; Smyth, M.; Penner, C.; Lakhani, A.; Carroll, M.W.; Al Sarkhy, A.; Lemberg, D.A.; Enns, R.; et al. Capsule Endoscopy Complements Magnetic Resonance Enterography and Endoscopy in Evaluating Small Bowel Crohn’s Disease. J. Can. Assoc. Gastroenterol. 2019, 3, 279–287. [Google Scholar] [CrossRef]
- Mow, W.S.; Lo, S.K.; Targan, S.R.; Dubinsky, M.C.; Treyzon, L.; Abreu-Martin, M.T.; Papadakis, K.A.; Vasiliauskas, E.A. Initial experience with wireless capsule enteroscopy in the diagnosis and management of inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2004, 2, 31–40. [Google Scholar] [CrossRef]
- Pasha, S.F.; Pennazio, M.; Rondonotti, E.; Wolf, D.; Buras, M.R.; Albert, J.G.; Cohen, S.A.; Cotter, J.; D’Haens, G.; Eliakim, R.; et al. Capsule Retention in Crohn’s Disease: A Meta-analysis. Inflamm. Bowel Dis. 2020, 26, 33–42. [Google Scholar] [CrossRef]
- Gao, Y.; Xin, L.; Wang, Y.X.; Dong, Y.H.; Liao, Z.; Li, Z.S.; Du, Y.Q. Double-balloon enteroscopy for retrieving retained small-bowel video capsule endoscopes: A systematic review. Scand. J. Gastroenterol. 2020, 55, 105–113. [Google Scholar] [CrossRef]
- Han, Z.M.; Qiao, W.G.; Ai, X.Y.; Li, A.M.; Chen, Z.Y.; Feng, X.C.; Zhang, J.; Wan, T.M.; Xu, Z.M.; Bai, Y.; et al. Impact of capsule endoscopy on prevention of postoperative recurrence of Crohn’s disease. Gastrointest. Endosc. 2018, 87, 1489–1498. [Google Scholar] [CrossRef]
- Nishikawa, T.; Nakamura, M.; Yamamura, T.; Maeda, K.; Sawada, T.; Mizutani, Y.; Ishikawa, E.; Ishikawa, T.; Kakushima, N.; Furukawa, K.; et al. Lewis score on capsule endoscopy can predict the prognosis in patients with small bowel lesions of Crohn’s disease. J. Gastroenterol. Hepatol. 2021, 36, 1851–1858. [Google Scholar] [CrossRef]
- Niv, Y. Small-bowel mucosal healing assessment by capsule endoscopy as a predictor of long-term clinical remission in patients with Crohn’s disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 844–848. [Google Scholar] [CrossRef]
- Yablecovitch, D.; Lahat, A.; Neuman, S.; Levhar, N.; Avidan, B.; Ben-Horin, S.; Eliakim, R.; Kopylov, U. The Lewis score or the capsule endoscopy Crohn’s disease activity index: Which one is better for the assessment of small bowel inflammation in established Crohn’s disease? Ther. Adv. Gastroenterol. 2018, 11, 1756283X17747780. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Lahat, A.; Amitai, M.M.; Klang, E.; Yablecovitch, D.; Neuman, S.; Levhar, N.; Selinger, L.; Rozendorn, N.; Turner, D.; et al. Assessment of small bowel mucosal healing by video capsule endoscopy for the prediction of short-term and long-term risk of Crohn’s disease flare: A prospective cohort study. Lancet Gastroenterol. Hepatol. 2019, 4, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Eliakim, R.; Yablecovitch, D.; Lahat, A.; Ungar, B.; Shachar, E.; Carter, D.; Selinger, L.; Neuman, S.; Ben-Horin, S.; Kopylov, U. A novel PillCam Crohn’s capsule score (Eliakim score) for quantification of mucosal inflammation in Crohn’s disease. United Eur. Gastroenterol. J. 2020, 8, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.W.D.; Ellul, P.; Elosua, A.; Fernandez-Urien, I.; Tontini, G.E.; Elli, L.; Eliakim, R.; Kopylov, U.; Koo, S.; Parker, C.; et al. Panenteric capsule endoscopy identifies proximal small bowel disease guiding upstaging and treatment intensification in Crohn’s disease: A European multicentre observational cohort study. United Eur. Gastroenterol. J. 2021, 9, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, M.; Amadi, C.; Gerson, L.B. Retention associated with video capsule endoscopy: Systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 1157–1168.e2. [Google Scholar] [CrossRef] [PubMed]
- Yoo, A.Y.; Lee, B.J.; Kim, W.S.; Kim, S.M.; Kim, S.H.; Joo, M.K.; Kim, H.J.; Park, J.J. Clinicopathological Features of Small Bowel Tumors Diagnosed by Video Capsule Endoscopy and Balloon-Assisted Enteroscopy: A Single Center Experience. Clin. Endosc. 2021, 54, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.J.; Wang, Y.; Xu, X.M.; Su, J.W.; Jiang, W.Y.; Jiang, J.X.; Lin, L.; Zhang, D.Q.; Ding, J.; Chen, L.; et al. Capsule endoscopy and single-balloon enteroscopy in small bowel diseases: Competing or complementary? World J. Gastroenterol. 2016, 22, 10625–10630. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, W.; Chen, G.; Li, Y. Diagnostic Value and Safety of Emergency Single-Balloon Enteroscopy for Obscure Gastrointestinal Bleeding. Gastroenterol. Res. Pract. 2019, 2019, 9026278. [Google Scholar] [CrossRef]
- Kakiya, Y.; Shiba, M.; Okamoto, J.; Kato, K.; Minamino, H.; Ominami, M.; Fukunaga, S.; Nagami, Y.; Sugimori, S.; Tanigawa, T.; et al. A comparison between capsule endoscopy and double balloon enteroscopy using propensity score-matching analysis in patients with previous obscure gastrointestinal bleeding. Scand. J. Gastroenterol. 2017, 52, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.M.; Jung, S.H.; Baek, D.H. Diagnostic Yields and Clinical Impacts of Capsule Endoscopy. Diagnostics 2021, 11, 1842. [Google Scholar] [CrossRef]
- Manguso, N.; Gangi, A.; Johnson, J.; Harit, A.; Nissen, N.; Jamil, L.; Lo, S.; Wachsman, A.; Hendifar, A.; Amersi, F. The role of pre-operative imaging and double balloon enteroscopy in the surgical management of small bowel neuroendocrine tumors: Is it necessary? J. Surg. Oncol. 2018, 117, 207–212. [Google Scholar] [CrossRef]
- Pennazio, M.; Spada, C.; Eliakim, R.; Keuchel, M.; May, A.; Mulder, C.J.; Rondonotti, E.; Adler, S.N.; Albert, J.; Baltes, P.; et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015, 47, 352–376. [Google Scholar] [CrossRef] [PubMed]
- Goverde, A.; Korsse, S.E.; Wagner, A.; van Leerdam, M.E.; Krak, N.C.; Stoker, J.; van Buuren, H.R.; Hofstra, R.M.; Bruno, M.J.; Dewint, P.; et al. Small-bowel Surveillance in Patients with Peutz-Jeghers Syndrome: Comparing Magnetic Resonance Enteroclysis and Double Balloon Enteroscopy. J. Clin. Gastroenterol. 2017, 51, e27–e33. [Google Scholar] [CrossRef] [PubMed]
- Pelizzaro, F.; Marsilio, I.; Fassan, M.; Piazza, F.; Barberio, B.; D’Odorico, A.; Savarino, E.V.; Farinati, F.; Zingone, F. The Risk of Malignancies in Celiac Disease-A Literature Review. Cancers 2021, 13, 5288. [Google Scholar] [CrossRef]
- Ferretti, F.; Branchi, F.; Orlando, S.; Roncoroni, L.; Barigelletti, G.; Fabiano, S.; Vecchi, M.; Penagini, R.; Doneda, L.; Elli, L. Effectiveness of Capsule Endoscopy and Double-Balloon Enteroscopy in Suspected Complicated Celiac Disease. Clin. Gastroenterol. Hepatol. 2022, 20, 941–949.e3. [Google Scholar] [CrossRef]
- Vasilakakis, M.; Koulaouzidis, A.; Yung, D.E.; Plevris, J.N.; Toth, E.; Iakovidis, D.K. Follow-up on: Optimizing lesion detection in small bowel capsule endoscopy and beyond: From present problems to future solutions. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 129–141. [Google Scholar] [CrossRef]
- Xiao, Y.F.; Wu, Z.X.; He, S.; Zhou, Y.Y.; Zhao, Y.B.; He, J.L.; Peng, X.; Yang, Z.X.; Lv, Q.J.; Yang, H.; et al. Fully automated magnetically controlled capsule endoscopy for examination of the stomach and small bowel: A prospective, feasibility, two-centre study. Lancet Gastroenterol. Hepatol. 2021, 6, 914–921. [Google Scholar] [CrossRef]
- Xing, X.; Jia, X.; Meng, M.Q. Bleeding Detection in Wireless Capsule Endoscopy Image Video Using Superpixel-Color Histogram and a Subspace KNN Classifier. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018. [Google Scholar] [CrossRef]
- Aoki, T.; Yamada, A.; Kato, Y.; Saito, H.; Tsuboi, A.; Nakada, A.; Niikura, R.; Fujishiro, M.; Oka, S.; Ishihara, S.; et al. Automatic detection of blood content in capsule endoscopy images based on a deep convolutional neural network. J. Gastroenterol. Hepatol. 2020, 35, 1196–1200. [Google Scholar] [CrossRef]
- Tsuboi, A.; Oka, S.; Aoyama, K.; Saito, H.; Aoki, T.; Yamada, A.; Matsuda, T.; Fujishiro, M.; Ishihara, S.; Nakahori, M.; et al. Artificial intelligence using a convolutional neural network for automatic detection of small-bowel angioectasia in capsule endoscopy images. Dig. Endosc. 2020, 32, 382–390. [Google Scholar] [CrossRef]
- Aoki, T.; Yamada, A.; Aoyama, K.; Saito, H.; Fujisawa, G.; Odawara, N.; Kondo, R.; Tsuboi, A.; Ishibashi, R.; Nakada, A.; et al. Clinical usefulness of a deep learning-based system as the first screening on small-bowel capsule endoscopy reading. Dig. Endosc. 2020, 32, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Klang, E.; Barash, Y.; Margalit, R.Y.; Soffer, S.; Shimon, O.; Albshesh, A.; Ben-Horin, S.; Amitai, M.M.; Eliakim, R.; Kopylov, U. Deep learning algorithms for automated detection of Crohn’s disease ulcers by video capsule endoscopy. Gastrointest. Endosc. 2020, 91, 606–613.e2. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Aoki, T.; Aoyama, K.; Kato, Y.; Tsuboi, A.; Yamada, A.; Fujishiro, M.; Oka, S.; Ishihara, S.; Matsuda, T.; et al. Automatic detection and classification of protruding lesions in wireless capsule endoscopy images based on a deep convolutional neural network. Gastrointest. Endosc. 2020, 92, 144–151.e1. [Google Scholar] [CrossRef]
- Leenhardt, R.; Souchaud, M.; Houist, G.; Le Mouel, J.P.; Saurin, J.C.; Cholet, F.; Rahmi, G.; Leandri, C.; Histace, A.; Dray, X. A neural network-based algorithm for assessing the cleanliness of small bowel during capsule endoscopy. Endoscopy 2021, 53, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Maurice, J.B.; Rochford, A.; Marshall, S.; Sebastian, S.; Dhar, A.; Hayee, B.; Green Endoscopy group. Green endoscopy: Using quality improvement to develop sustainable practice. Front. Gastroenterol. 2021, 13, 342–345. [Google Scholar] [CrossRef]
- Kaan, H.L.; Ho, K.Y. Clinical adoption of robotics in endoscopy: Challenges and solutions. JGH Open 2020, 4, 790–794. [Google Scholar] [CrossRef]
| Criteria | Small Bowel Capsule Endoscopy | Enteroscopy |
|---|---|---|
| Procedure type | Non-invasive | Invasive |
| Patient comfort | Well-tolerated and patient-friendly | May require sedation, potentially causing discomfort |
| Visualization | Enables panenteric mucosal visualization; however, limited by the battery life The application of artificial intelligence has shown potential for enhanced image analysis and interpretation | Facilitates direct, real-time visualization of the small bowel mucosa; however, visualization length is limited |
| Maneuverability | Limited control over the capsule’s movement | Provides control and maneuverability, enables targeted examinations |
| Diagnostic yield | Demonstrates superior diagnostic efficacy for mucosal lesions, particularly in suspected small bowel bleeding No definitive diagnosis, may necessitate biopsy | Exhibits enhanced diagnostic sensitivity for lesions located in the proximal segments of the small bowel, allows tissue sampling |
| Radiation exposure | No radiation exposure | May involve exposure to radiation during fluoroscopy |
| Mutual support | Guides the insertion route for enteroscopy | Targets lesions seen in capsule endoscopy |
| Procedure time | Shorter procedure duration; time needed for capsule ingestion and subsequent image reading | Lengthier procedural timeframe |
| Therapeutic interventions | Primarily a diagnostic modality, without the ability for therapeutic interventions | Facilitates therapeutic maneuvers, including biopsies, polypectomies, and hemostasis, contributing to both diagnosis and treatment |
| Main contraindications and precautions | Gastrointestinal tract obstruction, swallowing disorders | May pose challenges in patients with strictures or significant comorbidities |
| Accessibility | Easier to perform, dedicated training required | Requires specialized training and expertise |
| Complications | Rare complications, as the capsule is naturally excreted | Risk of complications such as perforation and bleeding |
| Cost | Involves costs associated with the capsule and related equipment | Entails increased overall expenses, attributed to specialized equipment, personnel, and facility requirements |
| Objectives and aspirations | Accuracy Biopsies Treatment | Effectiveness Safety |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singeap, A.-M.; Sfarti, C.; Minea, H.; Chiriac, S.; Cuciureanu, T.; Nastasa, R.; Stanciu, C.; Trifan, A. Small Bowel Capsule Endoscopy and Enteroscopy: A Shoulder-to-Shoulder Race. J. Clin. Med. 2023, 12, 7328. https://doi.org/10.3390/jcm12237328
Singeap A-M, Sfarti C, Minea H, Chiriac S, Cuciureanu T, Nastasa R, Stanciu C, Trifan A. Small Bowel Capsule Endoscopy and Enteroscopy: A Shoulder-to-Shoulder Race. Journal of Clinical Medicine. 2023; 12(23):7328. https://doi.org/10.3390/jcm12237328
Chicago/Turabian StyleSingeap, Ana-Maria, Catalin Sfarti, Horia Minea, Stefan Chiriac, Tudor Cuciureanu, Robert Nastasa, Carol Stanciu, and Anca Trifan. 2023. "Small Bowel Capsule Endoscopy and Enteroscopy: A Shoulder-to-Shoulder Race" Journal of Clinical Medicine 12, no. 23: 7328. https://doi.org/10.3390/jcm12237328
APA StyleSingeap, A.-M., Sfarti, C., Minea, H., Chiriac, S., Cuciureanu, T., Nastasa, R., Stanciu, C., & Trifan, A. (2023). Small Bowel Capsule Endoscopy and Enteroscopy: A Shoulder-to-Shoulder Race. Journal of Clinical Medicine, 12(23), 7328. https://doi.org/10.3390/jcm12237328