Biophysical Behavior of Very High-Power Short-Duration Radiofrequency Ablation in Pulmonary Vein Isolation: Fast but Gently—Implications for a Successful Procedure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Procedural Protocol
2.4. The vHP-SD Group
2.5. Control Group
2.6. Statistical Methods
3. Results
3.1. Baseline Data
3.2. Procedural Characteristics
3.3. Biophysical Behavior of Ablation Parameters in the vHP-SD Group vs. Controls
4. Discussion
5. Conclusions
- Procedural characteristics:
- -
- vHP-SD ablation demonstrated reduced RF time, ablation phase time, LA dwelling time, and irrigation volume compared with the standard technique. These findings have the potential to lower the risk associated with transmural lesions and fluid overload. This suggests the feasibility of achieving fewer complications while maintaining acute success, though further studies with larger populations are essential for confirmation.
- Physical behavior:
- -
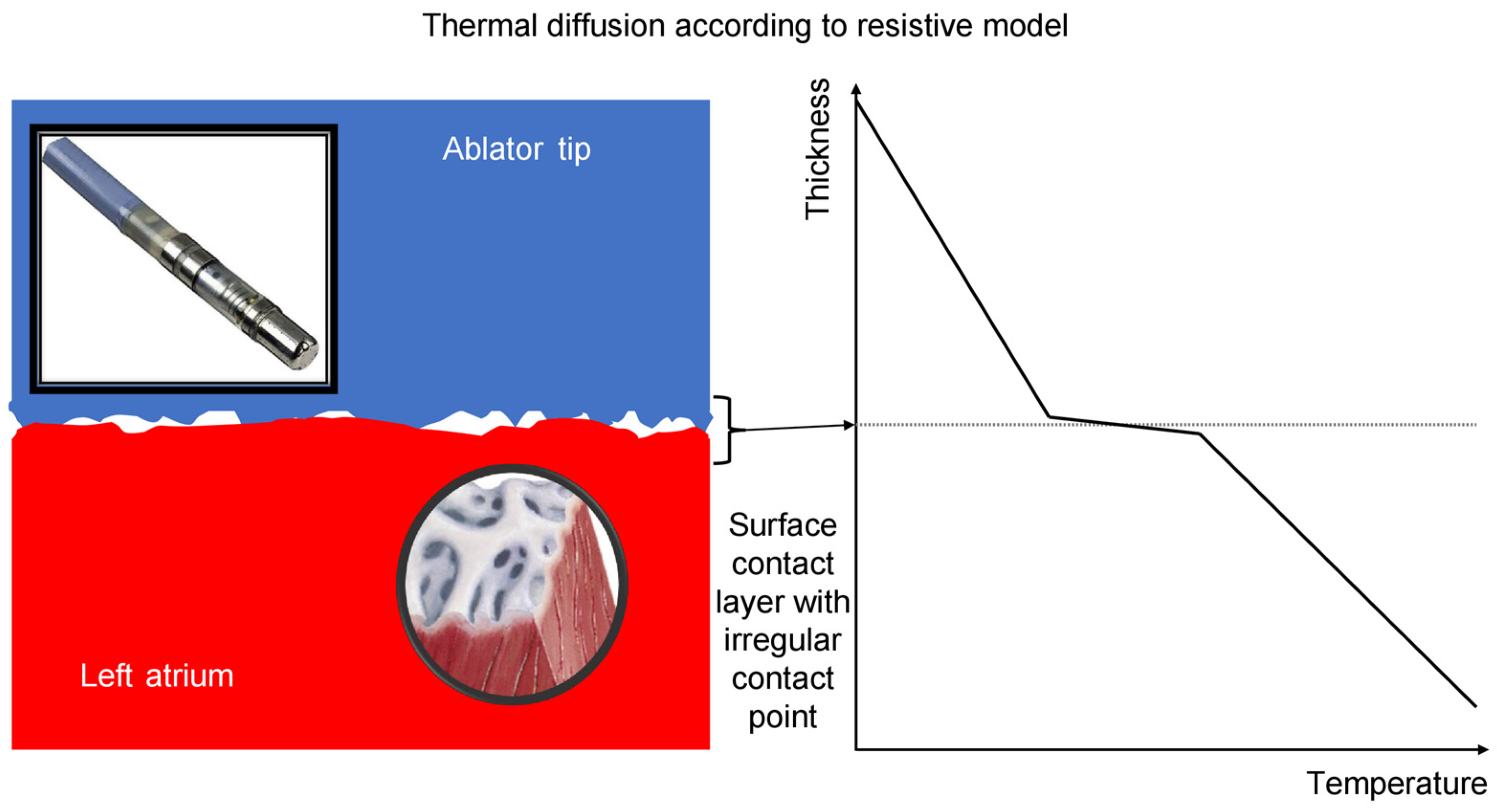
- In both arms, temperature increase proved to be predictive of an impedance drop of >10 Ohm and, consequently, tissue lesion.
- -
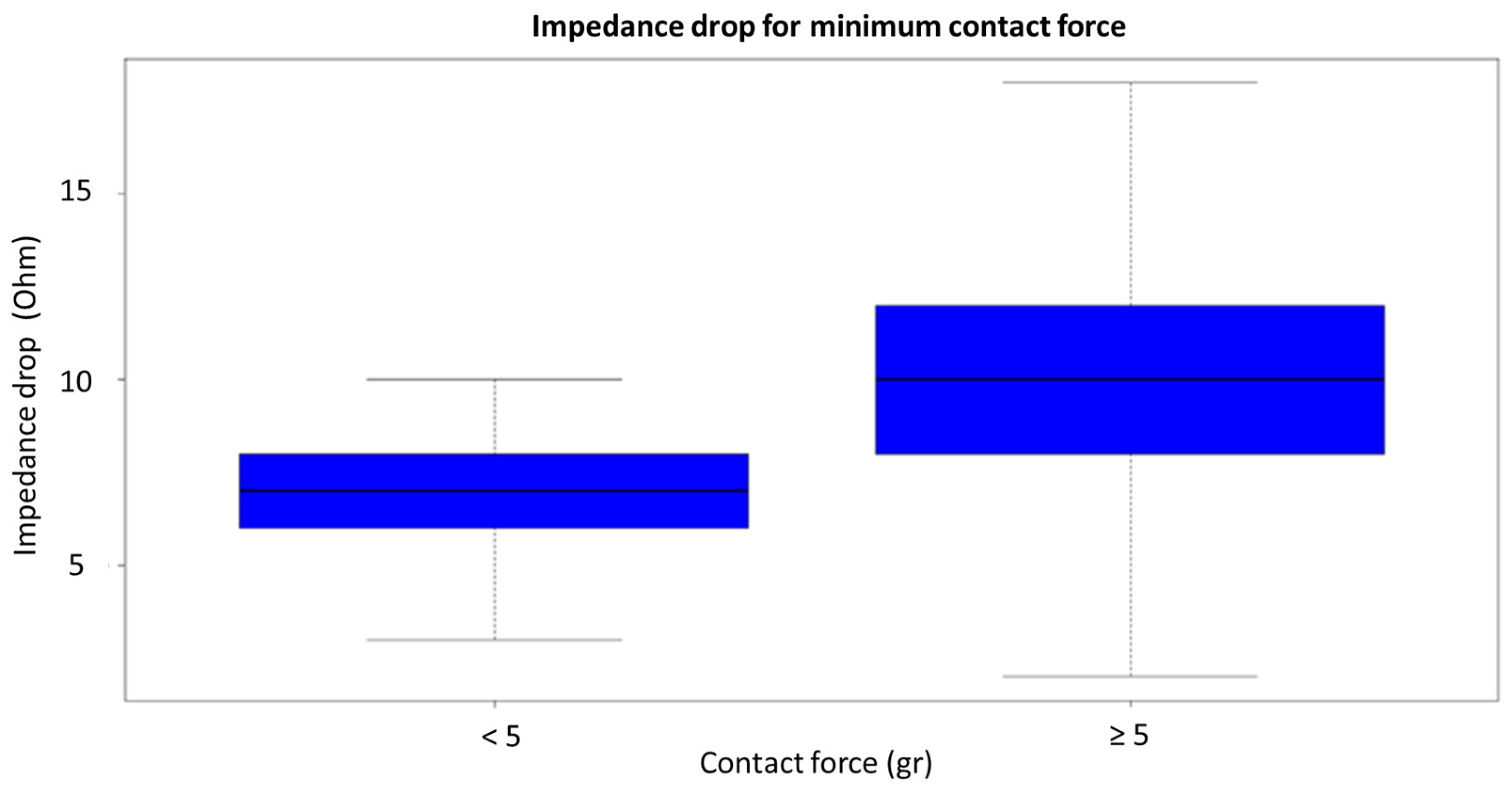
- In vHP-SD ablation, a minimum contact force of >5 g (rather than the mean contact force throughout the application) predicted an impedance drop of >10 Ohm and tissue lesion in our population. This differs from the standard technique, where the mean contact force (not the minimum value) is predictive of an impedance drop.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lévy, S.; Steinbeck, G.; Santini, L.; Nabauer, M.; Maceda, D.P.; Kantharia, B.; Saksena, S.; Cappato, R. Management of atrial fibrillation: Two decades of progress—A scientific statement from the European Cardiac Arrhythmia Society. J. Interv. Card. Electrophysiol. 2022, 65, 287–326. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. Corrigendum to: 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Hear. J. 2021, 42, 373–498. [Google Scholar]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS focused update of the 2014 aha/acc/hrs guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar] [PubMed]
- Cappato, R.; Ali, H. Surveys and registries on catheter ablation of atrial fibrillation: Fifteen years of history. Circ. Arrhythm. Electrophysiol. 2021, 14, e008073. [Google Scholar] [CrossRef] [PubMed]
- Tilz, R.R.; Heeger, C.H.; Wick, A.; Saguner, A.M.; Metzner, A.; Rillig, A.; Wohlmut, P.; Reissmann, B.; Lemes, C.; Maurer, T.; et al. Ten-year clinical outcome after circumferential pulmonary vein isolation utilizing the hamburg approach in patients with symptomatic drug-refractory paroxysmal atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2018, 11, e005250. [Google Scholar] [CrossRef]
- Haines, D.E. Determinants of Lesion Size During Radiofrequency Catheter Ablation: The Role of Electrode-Tissue Contact Pressure and Duration of Energy Delivery. J. Cardiovasc. Electrophysiol. 1991, 2, 509–515. [Google Scholar] [CrossRef]
- Hussein, A.; Das, M.; Riva, S.; Morgan, M.; Ronayne, C.; Sahni, A.; Shaw, M.; Todd, D.; Hall, M.; Modi, S.; et al. Use of ablation index-guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients: The PRAISE study results. Circ. Arrhythm. Electrophysiol. 2018, 11, e006576. [Google Scholar] [CrossRef]
- Hussein, A.; Das, M.; Chaturvedi, V.; Asfour, I.K.; Daryanani, N.; Morgan, M.; Ronayne, C.; Shaw, M.; Snowdon, R.; Gupta, D. Prospective use of Ablation Index targets improves clinical outcomes following ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2017, 28, 1037–1047. [Google Scholar] [CrossRef]
- Kottmaier, M.; Popa, M.; Bourier, F.; Reents, T.; Cifuentes, J.; Semmler, V.; Telishevska, M.; Otgonbayar, U.; Koch-Buttner, K.; Lennerz, C.; et al. Safety and outcome of very high-power short-duration ablation using 70 W for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Europace 2020, 22, 388–393. [Google Scholar] [CrossRef]
- Barkagan, M.; Contreras-Valdes, F.M.; Leshem, E.; Buxton, A.E.; Nakagawa, H.; Anter, E. High-power and short-duration ablation for pulmonary vein isolation: Safety, efficacy, and long-term durability. J. Cardiovasc. Electrophysiol. 2018, 29, 1287–1296. [Google Scholar] [CrossRef]
- Osorio, J.; Hussein, A.A.; Delaughter, M.C.; Monir, G.; Natale, A.; Dukkipati, S.; Oza, S.; Daoud, E.; Di Biase, L.; Mansour, M.; et al. Very high-power short-duration, temperature-controlled radiofrequency ablation in paroxysmal atrial fibrillation: The prospective multicenter Q-FFICIENCY trial. JACC Clin. Electrophysiol. 2023, 9, 468–480. [Google Scholar] [CrossRef]
- Leshem, E.; Zilberman, I.; Tschabrunn, C.M.; Barkagan, M.; Contreras-Valdes, F.M.; Govari, A.; Anter, E. High-power and short-duration ablation for pulmonary vein isolation: Biophysical characterization. JACC Clin. Electrophysiol. 2018, 4, 467–479. [Google Scholar] [CrossRef]
- Chinitz, J.; Michaud, G.; Stephenson, K. Impedance-guided Radiofrequency Ablation: Using Impedance to Improve Ablation Outcomes. J. Innov. Card. Rhythm. Manag. 2017, 8, 2868–2873. [Google Scholar] [CrossRef]
- Stabile, G.; Schillaci, V.; Strisciuglio, T.; Arestia, A.; Agresta, A.; Shopova, G.; De Simone, A.; Solimene, F. In vivo biophysical characterization of very high power, short duration, temperature-controlled lesions. Pacing Clin. Electrophysiol. 2021, 44, 1717–1723. [Google Scholar] [CrossRef]
- Sciacca, V.; Fink, T.; Körperich, H.; Bergau, L.; Guckel, D.; Nischik, F.; Eckstein, J.; Braun, M.; El Hamriti, M.; Imnadze, G.; et al. Magnetic resonance assessment of left atrial scar formation following a novel very high-power short-duration workflow for atrial fibrillation ablation. Europace 2023, 25, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Thiagalingam, A.; D’avila, A.; Foley, L.; Guerrero, J.L.; Lambert, H.; Leo, G.; Ruskin, J.N.; Reddy, V.Y. Importance of Catheter Contact Force During Irrigated Radiofrequency Ablation: Evaluation in a Porcine Ex Vivo Model Using a Force-Sensing Catheter. J. Cardiovasc. Electrophysiol. 2010, 21, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Hartung, W.M.; Burton, M.E.; Deam, A.G.; Walter, P.F.; McTEAGUE, K.; Langberg, J.J. Estimation of Temperature During Radiofrequency Catheter Ablation Using Impedance Measurements. Pacing Clin. Electrophysiol. 1995, 18, 2017–2021. [Google Scholar] [CrossRef] [PubMed]
- De Bortoli, A.; Sun, L.; Solheim, E.; Hoff, P.I.; Schuster, P.; Ohm, O.; Chen, J. Ablation Effect Indicated by Impedance Fall is Correlated with Contact Force Level During Ablation for Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2013, 24, 1210–1215. [Google Scholar] [CrossRef]
- Nakagawa, H.; Ikeda, A.; Sharma, T.; Govari, A.; Ashton, J.; Maffre, J.; Lifshitz, A.; Fuimaono, K.; Yokoyama, K.; Wittkampf, F.H.; et al. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with high powershort duration and moderate power-moderate duration: Effects of thermal latency and contact force on lesion formation. Circ. Arrhythm. Electrophysiol. 2021, 14, e009899. [Google Scholar] [CrossRef]
- Özışık, M.N. Heat Conduction, 2nd ed.; Wiley: New Work, NY, USA, 1993. [Google Scholar]
- Yamashita, S.; Mizukami, A.; Ono, M.; Hiroki, J.; Miyakuni, S.; Ueshima, D.; Matsumura, A.; Miyazaki, S.; Sasano, T. Higher power achieves greater local impedance drop, shorter ablation time, and more transmural lesion formation in comparison to lower power in local impedance guided radiofrequency ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2023, 34, 1869–1877. [Google Scholar] [CrossRef]
- de Groot, N.M.; Houben, R.P.; Smeets, J.L.; Boersma, E.; Schotten, U.; Schalij, M.J.; Crijns, H.; Allessie, M.A. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: Epicardial breakthrough. Circulation 2010, 122, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- de Groot, N.; van der Does, L.; Yaksh, A.; Lanters, E.; Teuwen, C.; Knops, P.; van de Woestijne, P.; Bekkers, J.; Kik, C.; Bogers, A.; et al. Direct Proof of Endo-Epicardial Asynchrony of the Atrial Wall During Atrial Fibrillation in Humans. Circ. Arrhythm. Electrophysiol. 2016, 9, e003648. [Google Scholar] [CrossRef] [PubMed]

| Clinical Parameters | Control (20) | vHP-SD (20) | p-Value | |
|---|---|---|---|---|
| Male, n (%) | 21 (52.5) | 13 (65.5) | 8 (40) | 0.123 |
| Age (years) | 63.2 ± 8.5 | 64.4 ± 7.7 | 61.5 ± 9.7 | 0.383 |
| BMI (kg/m2) | 26.3 ± 2.9 | 26.9 ± 3.0 | 25.5 ± 2.7 | 0.223 |
| Hypertension, n (%) | 7 (17.5) | 3 (15) | 4 (20) | 0.677 |
| CAD, n (%) | 0 (0) | 0 (0) | 0 (0) | 0.999 |
| Diabetes, n (%) | 6 (15) | 4 (20) | 2 (10) | 0.376 |
| Smokers, n (%) | 7 (17.5) | 3 (15) | 4 (20) | 0.677 |
| Previous ablation, n (%) | 0 (0) | 0 (0) | 0 (0) | 0.999 |
| CHA2DS2VASC score | 1.2 | 1.1 ± 0.7 | 1.3 ± 0.6 | 0.338 |
| Time from first episode to ablation (mo) | 37 ± 19 | 40 ± 20 | 35 ± 17 | 0.399 |
| Medications | ||||
| Ic AAD, n (%) | 9 (22.5) | 4 (20) | 5 (25) | 0.705 |
| Amiodarone, n (%) | 4 (10) | 2 (10) | 2 (10) | 0.999 |
| Anticoagulant, n (%) | 12 (30) | 5 (25) | 7 (35) | 0.490 |
| Betablocker, n (%) | 15 (37.5) | 6 (30) | 9 (45) | 0.327 |
| RAAS, n (%) | 6 (15) | 3 (15) | 3 (15) | 0.999 |
| Other, n (%) | 3 (7.5) | 2 (10) | 1 (5) | 0.520 |
| Echocardiography | ||||
| LV-EF (%) | 59.9 ± 5.0 | 60.5 ± 4.5 | 59.3 ± 5.7 | 0.626 |
| LA diameter, mm | 37 ± 8 | 36.1 ± 10 | 38.3 ± 6 | 0.404 |
| LA volume (mL/mq) | 30 ± 6 | 29 ± 5 | 31 ± 7 | 0.305 |
| TAPSE, mm | 20 ± 5 | 20 ± 6 | 21 ± 4 | 0.539 |
| PASP, mmHg | 28 ± 6 | 27 ± 7 | 29 ± 5 | 0.305 |
| vHP-SD | Control | p-Value | |
|---|---|---|---|
| Total RF time (s) | 229 [202; 273] | 1270 [1106; 1492] | <0.001 |
| RF applications (n) | 58 [51; 64] | 46 [41; 55] | 0.002 |
| Irrigation fluid (mL) | 165 [139; 185] | 404 [336; 472] | <0.001 |
| Mapping time (min) | 14 ± 3.5 | 12.5 ± 3.3 | 0.278 |
| Ablation phase (min) | 25 ± 5 | 39 ± 9 | <0.001 |
| LA dwelling time (min) | 47 ± 10 | 56 ± 12 | 0.023 |
| Model 1 | p-Value | Model 2 | p-Value | Model 3 | p-Value | Model 4 | p-Value | Log Model | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| T increase for ST | 0.36 [0.28; 0.44] | <0.001 | 0.55 [0.45; 0.65] | <0.001 | 0.55 [0.47; 0.63] | <0.001 | 0.74 [0.64; 0.84] | <0.001 | 1.34 [1.25; 1.43] | <0.001 |
| T increase for QDot | 0.40 [0.28; 0.52] | <0.001 | 0.52 [0.36; 0.68] | <0.001 | 0.52 [0.38; 0.66] | <0.001 | 0.36 [0.28; 0.44] | <0.001 | 1.24 [1.19; 1.31] | <0.001 |
| Starting impedance | 0.39 [0.35; 0.43] | <0.001 | 0.13 [0.11; 0.15] | <0.001 | 0.14 [0.12; 0.16] | <0.001 | 0.39 [0.35; 0.43] | <0.001 | 1.12 [1.02; 1.04] | <0.001 |
| F min for ST | - | - | - | - | 0.75 [0.41; 1.09] | <0.001 | - | - | - | - |
| F med for ST | - | - | 2.41 [1.45; 3.37] | <0.001 | - | - | - | - | - | - |
| F max for ST | 2.75 [2.09; 3.41] | <0.001 | - | - | - | - | - | - | - | - |
| F min for QDot | - | - | - | - | −0.26 [−0.62; 0.10] | 0.171 | - | - | - | - |
| F med for QDot | - | - | −0.31 [−1.11; 0.49] | 0.440 | - | - | - | - | - | - |
| F max for QDot | 0.04 [−0.58; 0.66] | 0.910 | - | - | - | - | - | - | - | - |
| F min > 5 g for Qdot | - | - | - | - | - | - | 2.83 [1.17; 4.49] | 0.005 | 2.63 [1.37; 5.07] | 0.003 |
| F min > 5 g for ST | - | - | - | - | - | - | 1.43 [−1.51; 4.37] | 0.092 | 0.69 [0.28; 1.72] | 0.419 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celentano, E.; Cristiano, E.; Ignatiuk, B.; Bia, E.; Girotto, L.; Tarantino, N.; De Groot, N.M.S. Biophysical Behavior of Very High-Power Short-Duration Radiofrequency Ablation in Pulmonary Vein Isolation: Fast but Gently—Implications for a Successful Procedure. J. Clin. Med. 2023, 12, 7332. https://doi.org/10.3390/jcm12237332
Celentano E, Cristiano E, Ignatiuk B, Bia E, Girotto L, Tarantino N, De Groot NMS. Biophysical Behavior of Very High-Power Short-Duration Radiofrequency Ablation in Pulmonary Vein Isolation: Fast but Gently—Implications for a Successful Procedure. Journal of Clinical Medicine. 2023; 12(23):7332. https://doi.org/10.3390/jcm12237332
Chicago/Turabian StyleCelentano, Eduardo, Ernesto Cristiano, Barbara Ignatiuk, Elena Bia, Lorenzo Girotto, Nicola Tarantino, and Natasja M. S. De Groot. 2023. "Biophysical Behavior of Very High-Power Short-Duration Radiofrequency Ablation in Pulmonary Vein Isolation: Fast but Gently—Implications for a Successful Procedure" Journal of Clinical Medicine 12, no. 23: 7332. https://doi.org/10.3390/jcm12237332
APA StyleCelentano, E., Cristiano, E., Ignatiuk, B., Bia, E., Girotto, L., Tarantino, N., & De Groot, N. M. S. (2023). Biophysical Behavior of Very High-Power Short-Duration Radiofrequency Ablation in Pulmonary Vein Isolation: Fast but Gently—Implications for a Successful Procedure. Journal of Clinical Medicine, 12(23), 7332. https://doi.org/10.3390/jcm12237332







