A Meta-Analysis of Short-Term Outcomes of TAVR versus SAVR in Bicuspid Aortic Valve Stenosis and TAVR Results in Different Bicuspid Valve Anatomies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria, Databases, and Search Strategy
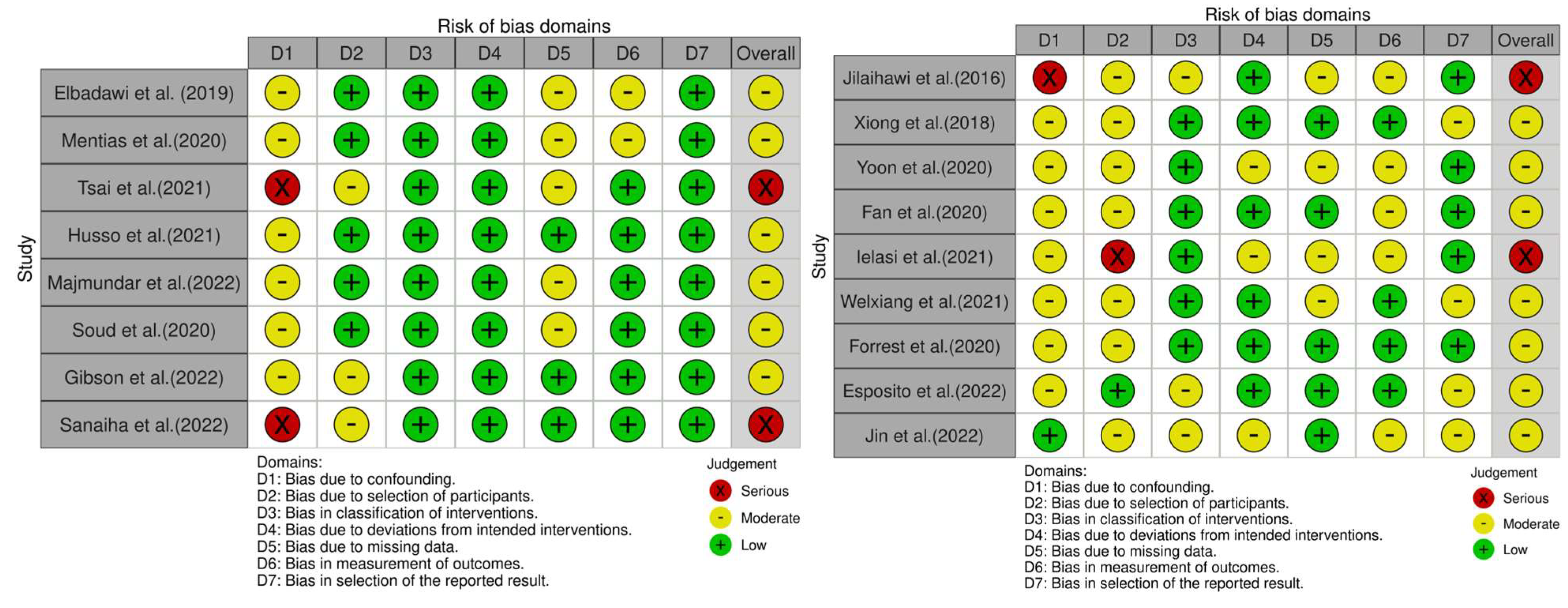
2.2. Assessment of Risk of Bias
2.3. Statistical Analysis
3. Results
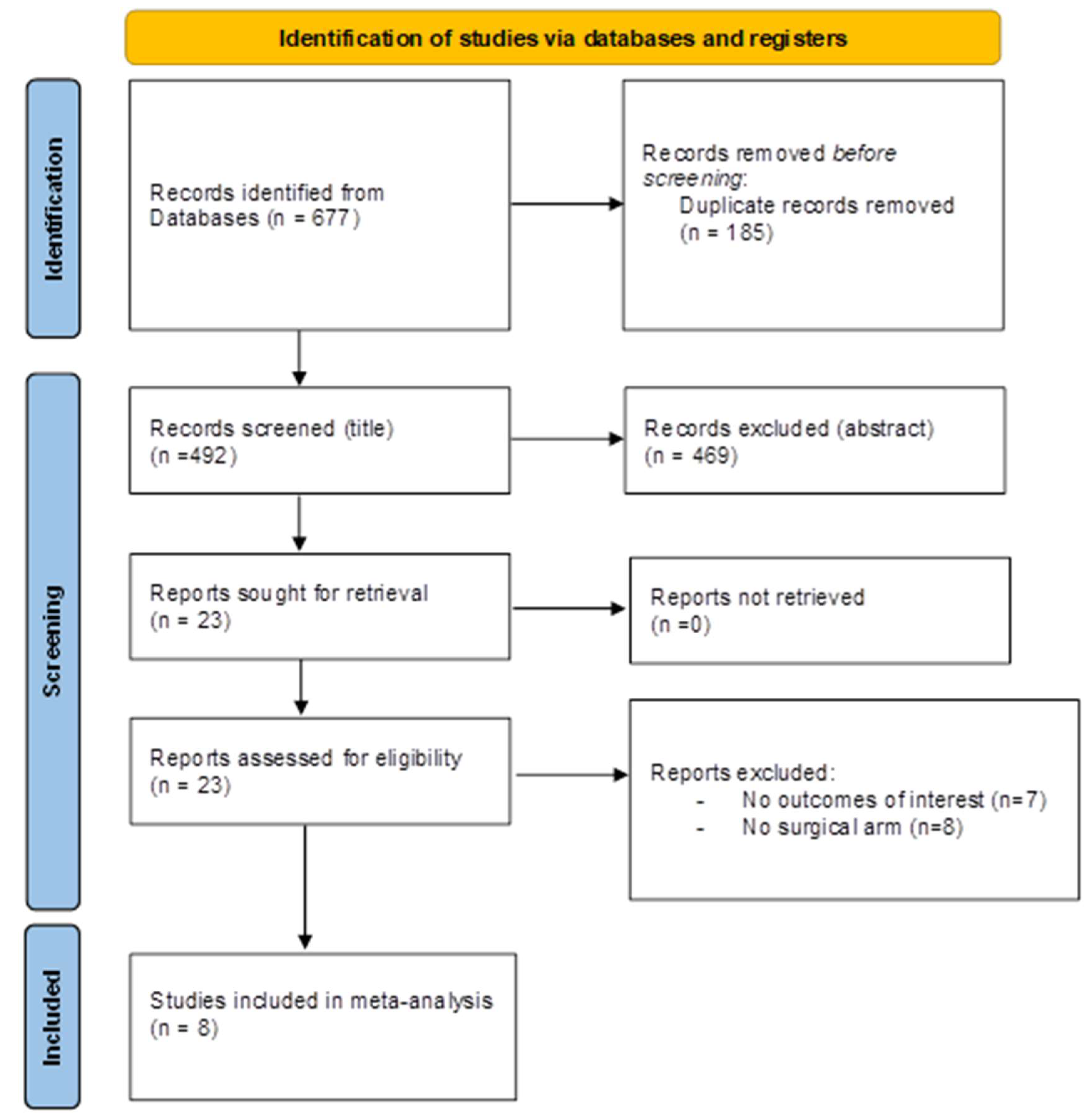
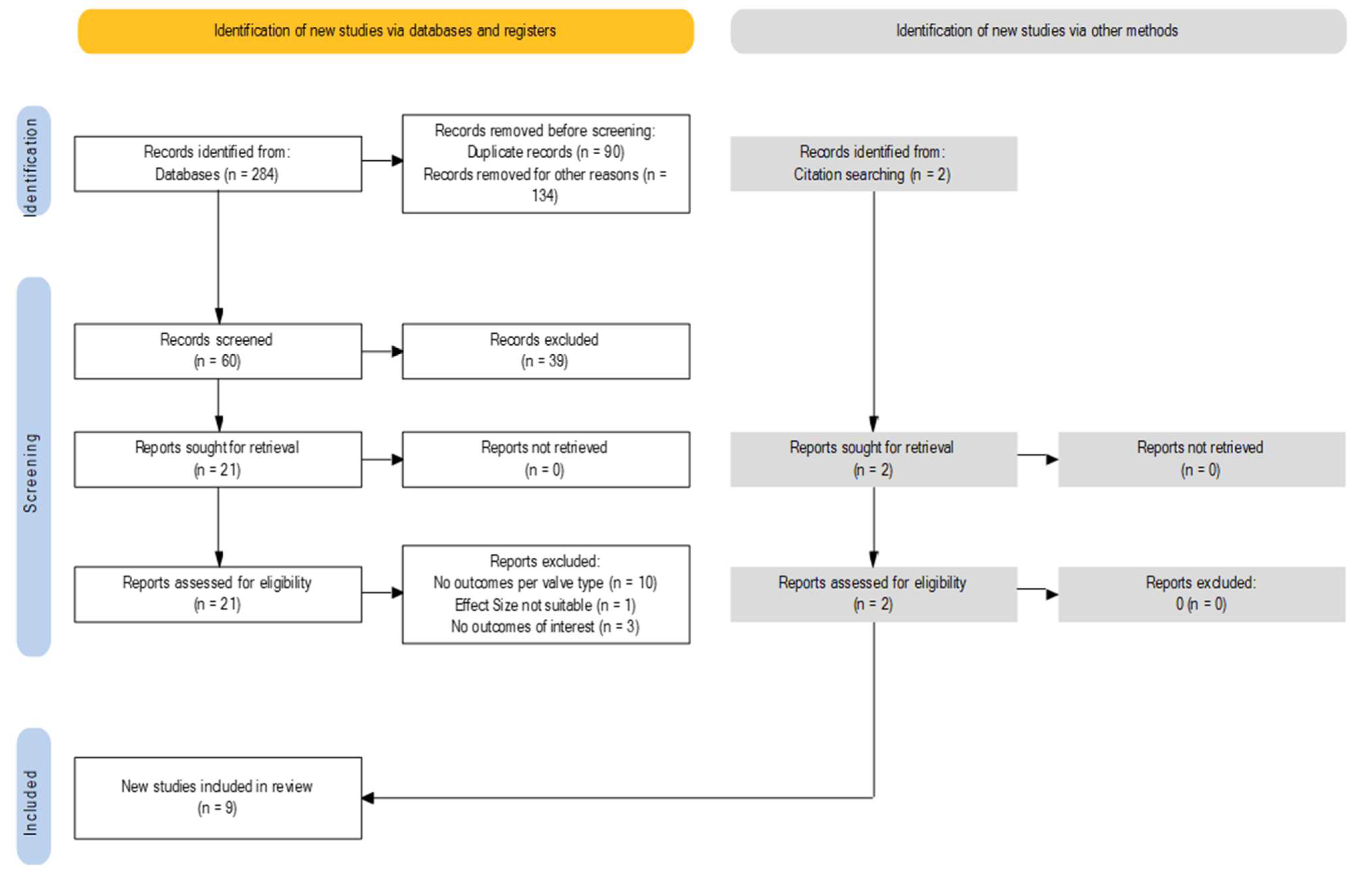
3.1. Study Selection and Characteristics
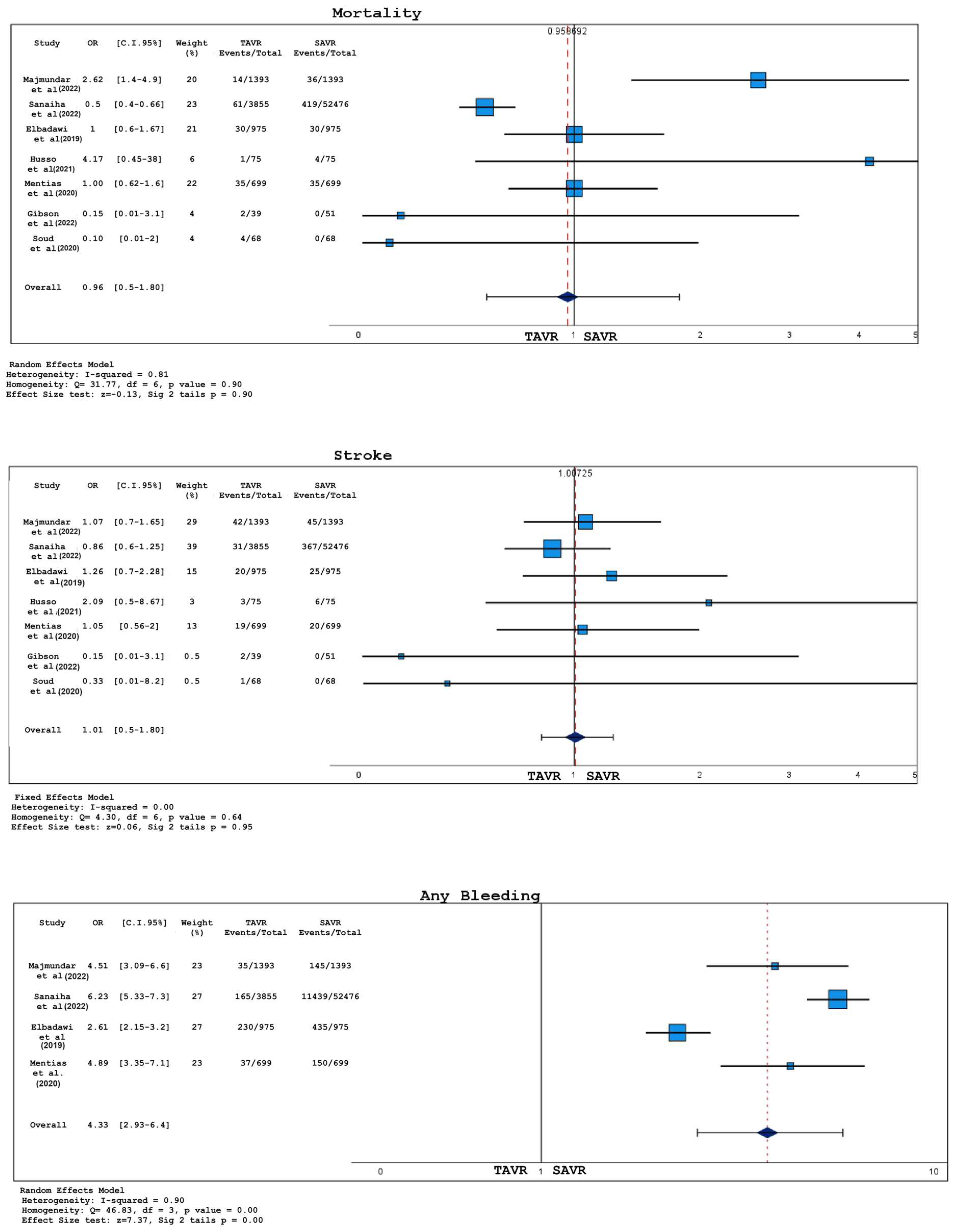
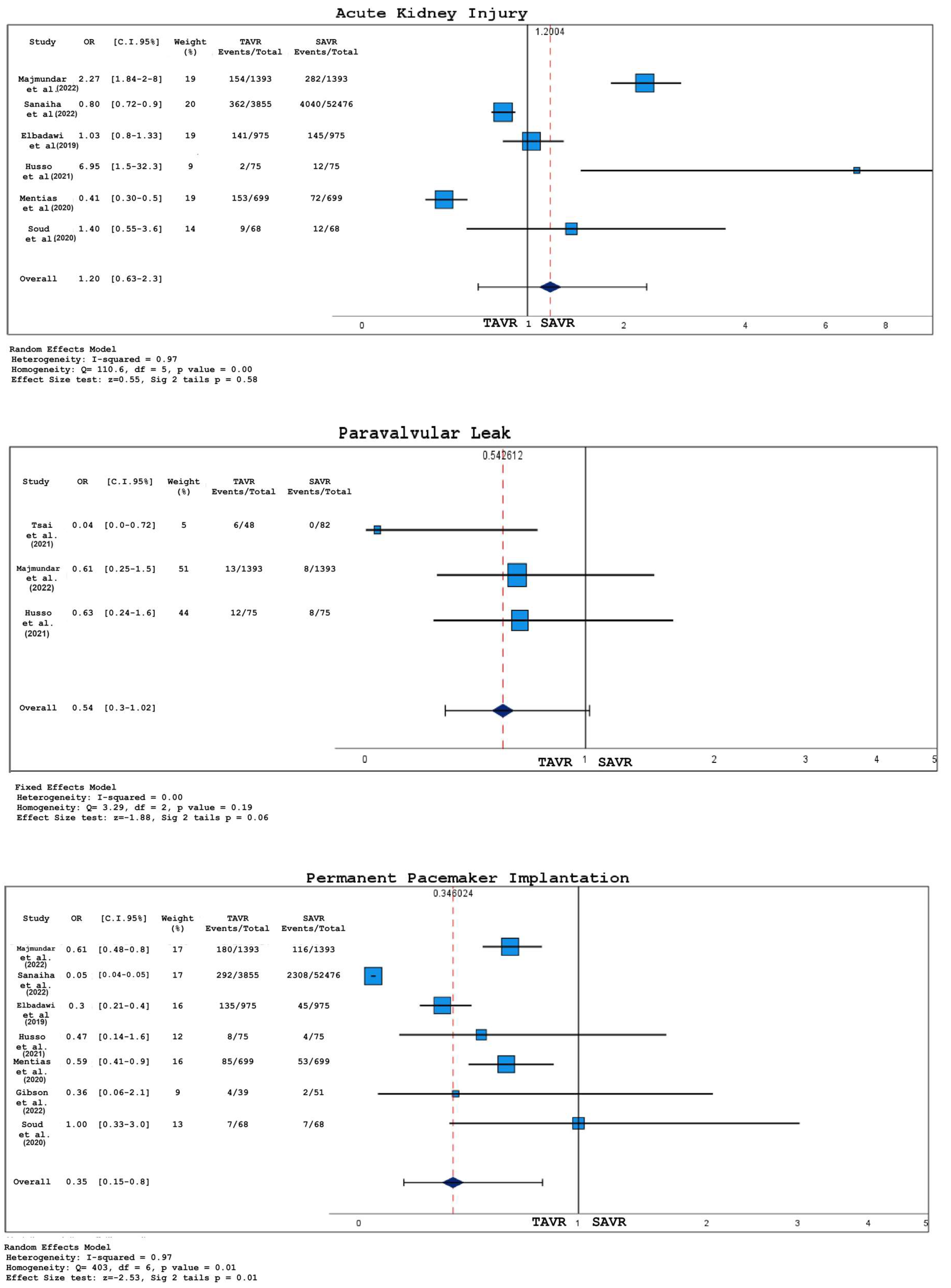
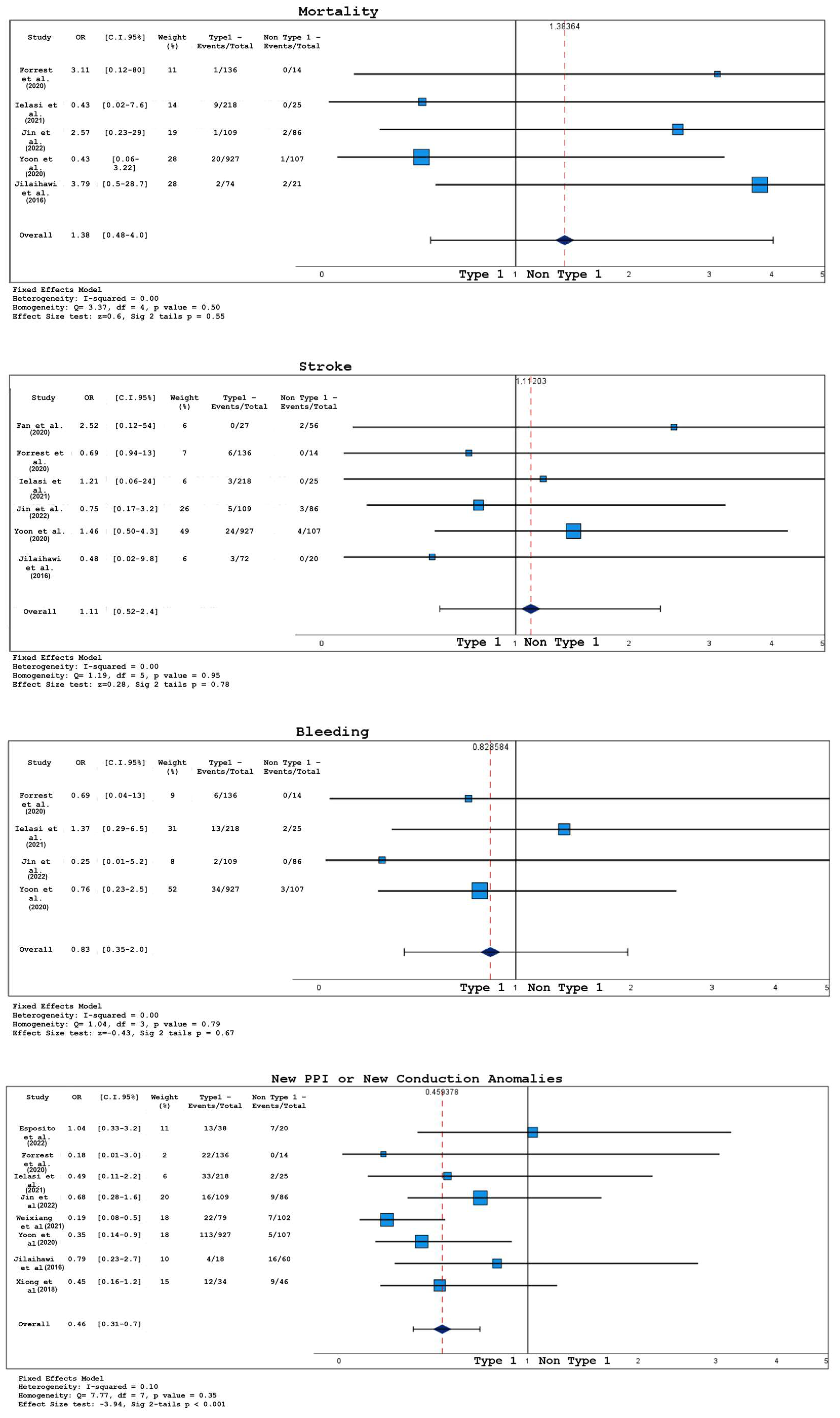
3.2. Analysis of Short-Term Outcomes
4. Discussion
- There was no significant difference in 30-day deaths, stroke, or acute kidney injury (AKI) between TAVR and SAVR;
- Bleedings were more common in the SAVR group;
- Patients with BAS who underwent TAVR had a higher probability of permanent pacemaker implantation (PPI) compared to those treated with SAVR;
- Even when not reaching statistically significant thresholds, TAVR was associated with a major incidence of paravalvular leakage and a shorter hospital stay, as opposed to SAVR;
- According to the Sievers classification, TAVR patients with type 1 BAV had lower post-operative transvalvular gradients but an increased risk of PPI and new conduction abnormalities compared to those with type 0 or type 2.
5. Limitations
6. Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Ward, C. Clinical significance of the bicuspid aortic valve. Heart 2000, 83, 81–85. [Google Scholar] [CrossRef]
- Kusner, J.J.; Brown, J.Y.; Gleason, T.G.; Edelman, E.R. The Natural History of Bicuspid Aortic Valve Disease. Struct. Heart 2022, 7, 100119. [Google Scholar] [CrossRef]
- Sievers, H.-H.; Schmidtke, C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J. Thorac. Cardiovasc. Surg. 2007, 133, 1226–1233. [Google Scholar] [CrossRef]
- I Michelena, H.; Della Corte, A.; Evangelista, A.; Maleszewski, J.J.; Edwards, W.D.; Roman, M.J.; Devereux, R.B.; Fernández, B.; Asch, F.M.; Barker, A.J.; et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. Eur. J. Cardio-Thoracic Surg. 2021, 60, 448–476. [Google Scholar] [CrossRef]
- Mordi, I.; Tzemos, N. Bicuspid Aortic Valve Disease: A Comprehensive Review. Cardiol. Res. Pract. 2012, 2012, 196037. [Google Scholar] [CrossRef]
- Perrin, N.; Ibrahim, R.; Dürrleman, N.; Basmadjian, A.; Leroux, L.; Demers, P.; Modine, T.; Ben Ali, W. Bicuspid Aortic Valve Stenosis: From Pathophysiological Mechanism, Imaging Diagnosis, to Clinical Treatment Methods. Front. Cardiovasc. Med. 2021, 8, 798949. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Borger, M.A.; David, T.E. Management of the Valve and Ascending Aorta in Adults with Bicuspid Aortic Valve Disease. Semin. Thorac. Cardiovasc. Surg. 2005, 17, 143–147. [Google Scholar] [CrossRef]
- Goland, S.; Czer, L.S.; De Robertis, M.A.; Mirocha, J.; Kass, R.M.; Fontana, G.P.; Chang, W.; Trento, A. Risk Factors Associated With Reoperation and Mortality in 252 Patients After Aortic Valve Replacement for Congenitally Bicuspid Aortic Valve Disease. Ann. Thorac. Surg. 2007, 83, 931–937. [Google Scholar] [CrossRef]
- Girdauskas, E.; Disha, K.; Borger, M.A.; Kuntze, T. Long-term prognosis of ascending aortic aneurysm after aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J. Thorac. Cardiovasc. Surg. 2014, 147, 276–282. [Google Scholar] [CrossRef]
- McKellar, S.H.; Michelena, H.I.; Li, Z.; Schaff, H.V.; Sundt, T.M., 3rd. Long-term risk of aortic events following aortic valve replacement in patients with bicuspid aortic valves. Am. J. Cardiol. 2010, 106, 1626–1633. [Google Scholar] [CrossRef]
- Russo, C.F.; Mazzetti, S.; Garatti, A.; Ribera, E.; Milazzo, A.; Bruschi, G.; Lanfranconi, M.; Colombo, T.; Vitali, E. Aortic complications after bicuspid aortic valve replacement: Long-term results. Ann. Thorac. Surg. 2002, 74, S1773–S1776; discussion S1792–S1799. [Google Scholar] [CrossRef]
- Adams, D.H.; Popma, J.J.; Reardon, M.J. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N. Engl. J. Med. 2014, 371, 967–968. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Kapadia, S.; Webb, J.G.; Yoon, S.-H.; Trento, A.; Svensson, L.G.; Herrmann, H.C.; et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 799–809. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Forrest, J.K.; Ramlawi, B.; Deeb, G.M.; Zahr, F.; Song, H.K.; Kleiman, N.S.; Chetcuti, S.J.; Michelena, H.I.; Mangi, A.A.; Skiles, J.A.; et al. Transcatheter Aortic Valve Replacement in Low-risk Patients With Bicuspid Aortic Valve Stenosis. JAMA Cardiol. 2021, 6, 50–57. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Gibson, B.; Tanner, R.; Traynor, B.; Casserly, I. 45 Short term outcomes following transcatheter versus surgical aortic valve replacement for the treatment of bicuspid aortic valve stenosis. Heart 2022, 108 (Suppl. S3), A37–A38. [Google Scholar] [CrossRef]
- Majmundar, M.; Kumar, A.; Doshi, R.; Shariff, M.; Krishnaswamy, A.; Reed, G.W.; Brockett, J.; Lahorra, J.L.; Svensson, L.S.; Puri, R.; et al. Early outcomes of transcatheter versus surgical aortic valve implantation in patients with bicuspid aortic valve stenosis. EuroIntervention 2022, 18, 23–32. [Google Scholar] [CrossRef]
- Sanaiha, Y.; Hadaya, J.E.; Tran, Z.; Shemin, R.J.; Benharash, P. Transcatheter and Surgical Aortic Valve Replacement in Patients With Bicuspid Aortic Valve Stenosis. Ann. Thorac. Surg. 2023, 115, 611–618. [Google Scholar] [CrossRef]
- Soud, M.; Al-Khadra, Y.; Darmoch, F.; Pacha, H.M.; Fanari, Z.; Alraies, M.C. Transcatheter aortic valve replacement in patients with bicuspid aortic valve stenosis: National trends and in-hospital outcomes. Avicenna J. Med. 2020, 10, 22–28. [Google Scholar] [CrossRef]
- Tsai, H.; Lin, Y.; Wu, I.; Kuo, L.; Chen, B.; Shen, S.; Hsu, W.; Huang, H. Major adverse cardiac events and functional capacity in patients at intermediate risk undergoing transcatheter versus surgical aortic valve replacement for aortic stenosis with bicuspid valves. J. Card. Surg. 2021, 36, 828–833. [Google Scholar] [CrossRef]
- Elbadawi, A.; Saad, M.; Elgendy, I.Y.; Barssoum, K.; Omer, M.A.; Soliman, A.; Almahmoud, M.F.; Ogunbayo, G.O.; Mentias, A.; Gilani, S.; et al. Temporal Trends and Outcomes of Transcatheter Versus Surgical Aortic Valve Replacement for Bicuspid Aortic Valve Stenosis. JACC Cardiovasc. Interv. 2019, 12, 1811–1822. [Google Scholar] [CrossRef]
- Mentias, A.; Sarrazin, M.V.; Desai, M.Y.; Saad, M.; Horwitz, P.A.; Kapadia, S.; Girotra, S. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Bicuspid Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2020, 75, 2518–2519. [Google Scholar] [CrossRef]
- Husso, A.; Airaksinen, J.; Juvonen, T.; Laine, M.; Dahlbacka, S.; Virtanen, M.; Niemelä, M.; Mäkikallio, T.; Savontaus, M.; Eskola, M.; et al. Transcatheter and surgical aortic valve replacement in patients with bicuspid aortic valve. Clin. Res. Cardiol. 2021, 110, 429–439. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Kim, W.-K.; Dhoble, A.; Pio, S.M.; Babaliaros, V.; Jilaihawi, H.; Pilgrim, T.; De Backer, O.; Bleiziffer, S.; Vincent, F.; et al. Bicuspid Aortic Valve Morphology and Outcomes After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 1018–1030. [Google Scholar] [CrossRef]
- Jilaihawi, H.; Chen, M.; Webb, J.; Himbert, D.; Ruiz, C.E.; Rodés-Cabau, J.; Pache, G.; Colombo, A.; Nickenig, G.; Lee, M.; et al. A Bicuspid Aortic Valve Imaging Classification for the TAVR Era. JACC Cardiovasc. Imaging 2016, 9, 1145–1158. [Google Scholar] [CrossRef]
- Xiong, T.; Liao, Y.; Li, Y.; Zhao, Z.; Wei, X.; Tsauo, J.; Xu, Y.; Feng, Y.; Chen, M. Permanent pacemaker implantation after transcatheter aortic valve replacement in bicuspid aortic valve patients. J. Interv. Cardiol. 2018, 31, 878–884. [Google Scholar] [CrossRef]
- Ou, Y.-W.; He, J.-J.; Zhou, X.; Li, G.-Y.; Liao, Y.-B.; Wei, X.; Peng, Y.; Feng, Y.; Chen, M. The incidence and predictors of high-degree atrioventricular block in patients with bicuspid aortic valve receiving self-expandable transcatheter aortic valve implantation. J. Geriatr. Cardiol. 2021, 18, 825–835. [Google Scholar] [CrossRef]
- Esposito, G.; Kumar, N.; Pugliese, F.; Sayers, M.; Chow, A.W.; Kennon, S.; Ozkor, M.; Mathur, A.; Baumbach, A.; Lloyd, G.; et al. Predictors of post-TAVI conduction abnormalities in patients with bicuspid aortic valves. Open Heart 2022, 9, e001995. [Google Scholar] [CrossRef]
- Jin, Q.; Chen, S.; Yang, X.; Li, M.; Li, W.; Zhang, X.; Zhou, D.; Lam, Y.-Y.; Ge, J. Clinical outcomes of bicuspid versus tricuspid aortic valve stenosis after transcatheter aortic valve replacement with self-expandable valves. BMC Cardiovasc. Disord. 2022, 22, 540. [Google Scholar] [CrossRef]
- Ielasi, A.; Moscarella, E.; Mangieri, A.; Giannini, F.; Tchetchè, D.; Kim, W.-K.; Sinning, J.-M.; Landes, U.; Kornowski, R.; De Backer, O.; et al. Procedural and clinical outcomes of type 0 versus type 1 bicuspid aortic valve stenosis undergoing trans-catheter valve replacement with new generation devices: Insight from the BEAT international collaborative registry. Int. J. Cardiol. 2021, 325, 109–114. [Google Scholar] [CrossRef]
- Fan, J.; Fang, X.; Liu, C.; Zhu, G.; Hou, C.R.; Jiang, J.; Lin, X.; Wang, L.; He, Y.; Zhu, Q.; et al. Brain Injury After Transcatheter Replacement of Bicuspid Versus Tricuspid Aortic Valves. J. Am. Coll. Cardiol. 2020, 76, 2579–2590. [Google Scholar] [CrossRef]
- Tarantini, G.; Fabris, T. Transcatheter Aortic Valve Replacement for Bicuspid Aortic Valve Stenosis: A Practical Operative Overview. Circ. Cardiovasc. Interv. 2021, 14, e009827. [Google Scholar] [CrossRef]
- Das, R.; Puri, R. Transcatheter Treatment of Bicuspid Aortic Valve Disease: Imaging and Interventional Considerations. Front. Cardiovasc. Med. 2018, 5, 91. [Google Scholar] [CrossRef]
- Pineda, A.M.; Rymer, J.; Wang, A.; Banks, A.Z.; Koweek, L.H.; Plichta, R.; Williams, A.; Vavalle, J.P.; Halim, S.; Kiefer, T.; et al. Transcatheter aortic valve replacement for patients with severe bicuspid aortic stenosis. Am. Heart J. 2020, 224, 105–112. [Google Scholar] [CrossRef]
- Fu, B.; Chen, Q.; Zhao, F.; Guo, Z.; Jiang, N.; Wang, X.; Wang, W.; Han, J.; Yang, L.; Zhu, Y.; et al. Efficacy and safety of transcatheter aortic valve implantation in patients with severe bicuspid aortic stenosis. Ann. Transl. Med. 2020, 8, 873. [Google Scholar] [CrossRef]
- Waksman, R.; Craig, P.E.; Torguson, R.; Asch, F.M.; Weissman, G.; Ruiz, D.; Gordon, P.; Ehsan, A.; Parikh, P.; Bilfinger, T.; et al. Transcatheter Aortic Valve Replacement in Low-Risk Patients With Symptomatic Severe Bicuspid Aortic Valve Stenosis. JACC Cardiovasc. Interv. 2020, 13, 1019–1027. [Google Scholar] [CrossRef]
- Kochman, J.; Zbroński, K.; Kołtowski, Ł.; Parma, R.; Ochała, A.; Huczek, Z.; Rymuza, B.; Wilimski, R.; Dąbrowski, M.; Witkowski, A.; et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve stenosis utilizing the next-generation fully retrievable and repositionable valve system: Mid-term results from a prospective multicentre registry. Clin. Res. Cardiol. 2020, 109, 570–580. [Google Scholar] [CrossRef]
- Williams, M.R.; Jilaihawi, H.; Makkar, R.; O’neill, W.W.; Guyton, R.; Malaisrie, S.C.; Brown, D.L.; Blanke, P.; Leipsic, J.A.; Pibarot, P.; et al. The PARTNER 3 Bicuspid Registry for Transcatheter Aortic Valve Replacement in Low-Surgical-Risk Patients. JACC Cardiovasc. Interv. 2022, 15, 523–532. [Google Scholar] [CrossRef]
- Forrest, J.K.; Kaple, R.K.; Ramlawi, B.; Gleason, T.G.; Meduri, C.U.; Yakubov, S.J.; Jilaihawi, H.; Liu, F.; Reardon, M.J. Transcatheter Aortic Valve Replacement in Bicuspid Versus Tricuspid Aortic Valves From the STS/ACC TVT Registry. JACC Cardiovasc. Interv. 2020, 13, 1749–1759. [Google Scholar] [CrossRef]
- Nuyens, P.; De Backer, O.; Sathananthan, J.; Jørgensen, T.H.; Treede, H.; Leipsic, J.A.; Bax, J.J.; Webb, J.G.; Mehran, R.; Chen, M.; et al. TAVR in Bicuspid Aortic Stenosis: Current Evidence and Proposal for a Randomized Controlled Trial Design. JACC Cardiovasc. Interv. 2023, 16, 1682–1687. [Google Scholar] [CrossRef]
- Mylotte, D.; Lefevre, T.; Søndergaard, L.; Watanabe, Y.; Modine, T.; Dvir, D.; Bosmans, J.; Tchetche, D.; Kornowski, R.; Sinning, J.-M.; et al. Transcatheter Aortic Valve Replacement in Bicuspid Aortic Valve Disease. J. Am. Coll. Cardiol. 2014, 64, 2330–2339. [Google Scholar] [CrossRef]
- Perlman, G.Y.; Blanke, P.; Dvir, D.; Pache, G.; Modine, T.; Barbanti, M.; Holy, E.W.; Treede, H.; Ruile, P.; Neumann, F.-J.; et al. Bicuspid aortic valve stenosis: Favorable early outcomes with a next-generation transcatheter heart valve in a multicenter study. JACC Cardiovasc. Interv. 2016, 9, 817–824. [Google Scholar] [CrossRef]
- Kang, J.J.; Fialka, N.M.; El-Andari, R.; Watkins, A.; Hong, Y.; Mathew, A.; Bozso, S.J.; Nagendran, J. Surgical vs transcatheter aortic valve replacement in bicuspid aortic valve stenosis: A systematic review and meta-analysis. Trends Cardiovasc. Med. 2023. ahead of print. [Google Scholar] [CrossRef]
- Sakurai, Y.; Yokoyama, Y.; Kuno, T.; Takagi, H.; Mentias, A.; Thourani, V.H.; Latib, A.; Kaneko, T. Transcatheter versus surgical aortic valve replacement for stenotic bicuspid aortic valve: Systematic review and meta-analysis. JTCVS Open 2023, 13, 75–94. [Google Scholar] [CrossRef]
- Montalto, C.; Sticchi, A.; Crimi, G.; Laricchia, A.; Khokhar, A.A.; Giannini, F.; Reimers, B.; Colombo, A.; Latib, A.; Waksman, R.; et al. Outcomes After Transcatheter Aortic Valve Replacement in Bicuspid Versus Tricuspid Anatomy: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Interv. 2021, 14, 2144–2155. [Google Scholar] [CrossRef]
- Takagi, H.; Hari, Y.; Kawai, N.; Kuno, T.; Ando, T. Meta-analysis of transcatheter aortic valve implantation for bicuspid versus tricuspid aortic valves. J. Cardiol. 2019, 74, 40–48. [Google Scholar] [CrossRef]
- Zhou, D.; Yidilisi, A.; Fan, J.; Zhang, Y.; Dai, H.; Zhu, G.; Guo, Y.; He, Y.; Zhu, Q.; Lin, X.; et al. Three-year outcomes of transcatheter aortic valve implantation for bicuspid versus tricuspid aortic stenosis. EuroIntervention 2022, 18, 193–202. [Google Scholar] [CrossRef]
- Vincent, F.; Ternacle, J.; Denimal, T.; Shen, M.; Redfors, B.; Delhaye, C.; Simonato, M.; Debry, N.; Verdier, B.; Shahim, B.; et al. Transcatheter Aortic Valve Replacement in Bicuspid Aortic Valve Stenosis. Circulation 2021, 143, 1043–1061. [Google Scholar] [CrossRef]
- Gaudino, M.; Benesch, C.; Bakaeen, F.; DeAnda, A.; Fremes, S.E.; Glance, L.; Messé, S.R.; Pandey, A.; Rong, L.Q.; On behalf of the American Heart Association Council on Cardiovascular Surgery; et al. Considerations for Reduction of Risk of Perioperative Stroke in Adult Patients Undergoing Cardiac and Thoracic Aortic Operations: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e193–e209. [Google Scholar] [CrossRef]
- Généreux, P.; Cohen, D.J.; Williams, M.R.; Mack, M.; Kodali, S.K.; Svensson, L.G.; Kirtane, A.J.; Xu, K.; McAndrew, T.C.; Makkar, R.; et al. Bleeding complications after surgical aortic valve replacement compared with transcatheter aortic valve replacement: Insights from the PARTNER I Trial (Placement of Aortic Transcatheter Valve). J. Am. Coll. Cardiol. 2014, 63, 1100–1109. [Google Scholar] [CrossRef]
- Faroux, L.; Chen, S.; Muntané-Carol, G.; Regueiro, A.; Philippon, F.; Sondergaard, L.; Jørgensen, T.H.; Lopez-Aguilera, J.; Kodali, S.; Leon, M.; et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: A systematic review and meta-analysis. Eur. Heart J. 2020, 41, 2771–2781. [Google Scholar] [CrossRef]
- Sá, M.P.; Jacquemyn, X.; Eynde, J.V.D.; Tasoudis, P.; Erten, O.; Sicouri, S.; Macedo, F.Y.; Pasala, T.; Kaple, R.; Weymann, A.; et al. Impact of Paravalvular Leak on Outcomes After Transcatheter Aortic Valve Implantation: Meta-Analysis of Kaplan-Meier-derived Individual Patient Data. Struct. Heart 2023, 7, 100118. [Google Scholar] [CrossRef]
- Hamdan, A.; Nassar, M.; Schwammenthal, E.; Perlman, G.; Arow, Z.; Lessick, J.; Kerner, A.; Barsheshet, A.; Assa, H.V.; Assali, A.; et al. Short membranous septum length in bicuspid aortic valve stenosis increases the risk of conduction disturbances. J. Cardiovasc. Comput. Tomogr. 2021, 15, 339–347. [Google Scholar] [CrossRef]
- Kim, W.-K.; Bhumimuang, K.; Renker, M.; Fischer-Rasokat, U.; Möllmann, H.; Walther, T.; Choi, Y.-H.; Nef, H.; Hamm, C.W. Determinants of paravalvular leakage following transcatheter aortic valve replacement in patients with bicuspid and tricuspid aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1387–1396. [Google Scholar] [CrossRef]
- Popma, J.J.; Gleason, T.G.; Yakubov, S.J.; Harrison, J.K.; Forrest, J.K.; Maini, B.; Ruiz, C.E.; Pinto, D.S.; Costa, M.; Resar, J.; et al. Relationship of Annular Sizing Using Multidetector Computed Tomographic Imaging and Clinical Outcomes After Self-Expanding CoreValve Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2016, 9, e003282. [Google Scholar] [CrossRef]
- Arora, S.; Strassle, P.D.; Kolte, D.; Ramm, C.J.; Falk, K.; Jack, G.; Caranasos, T.G.; Cavender, M.A.; Rossi, J.S.; Vavalle, J.P. Length of Stay and Discharge Disposition After Transcatheter Versus Surgical Aortic Valve Replacement in the United States. Circ. Cardiovasc. Interv. 2018, 11, e006929. [Google Scholar] [CrossRef]
- Siu, S.C.; Silversides, C.K. Bicuspid Aortic Valve Disease. J. Am. Coll. Cardiol. 2010, 55, 2789–2800. [Google Scholar] [CrossRef]
- Mauri, V.; Reimann, A.; Stern, D.; Scherner, M.; Kuhn, E.; Rudolph, V.; Rosenkranz, S.; Eghbalzadeh, K.; Friedrichs, K.; Wahlers, T.; et al. Predictors of Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement With the SAPIEN 3. JACC Cardiovasc. Interv. 2016, 9, 2200–2209. [Google Scholar] [CrossRef]
- Fujita, B.; Kütting, M.; Seiffert, M.; Scholtz, S.; Egron, S.; Prashovikj, E.; Börgermann, J.; Schäfer, T.; Scholtz, W.; Preuss, R.; et al. Calcium distribution patterns of the aortic valve as a risk factor for the need of permanent pacemaker implantation after transcatheter aortic valve implantation. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1385–1393. [Google Scholar] [CrossRef]
- Frangieh, A.H.; Michel, J.; Deutsch, O.; Joner, M.; Pellegrini, C.; Rheude, T.; Bleiziffer, S.; Kasel, A.M. Aortic annulus sizing in stenotic bicommissural non-raphe-type bicuspid aortic valves: Reconstructing a three-dimensional structure using only two hinge points. Clin. Res. Cardiol. 2019, 108, 6–15. [Google Scholar] [CrossRef]
- Bugani, G.; Pagnesi, M.; Tchetchè, D.; Kim, W.K.; Khokhar, A.; Sinning, J.M.; Landes, U.; Kornowski, R.; Codner, P.; De Backer, O.; et al. Predictors of high residual gradient after transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Clin. Res. Cardiol. 2021, 110, 667–675. [Google Scholar] [CrossRef]
- Du, Y.; Wang, Z.; Liu, W.; Guo, Y.; Han, W.; Shen, H.; Jia, S.; Yu, Y.; Han, K.; Shi, D.; et al. Transcatheter Aortic Valve Implantation in Sievers Type 0 vs. Type 1 Bicuspid Aortic Valve Morphology: Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 771789. [Google Scholar] [CrossRef]
- Al-Asad, K.S.; Salazar, A.M.; Radwan, Y.; Wang, E.; Salam, M.F.; Sabanci, R.; Saeed, M.; Halboni, A.; Al-Abcha, A.; Abela, G. Transcatheter Aortic Valve Replacement in Bicuspid Versus Tricuspid Aortic Valve Stenosis: Meta-Analysis and Systemic Review. Am. J. Cardiol. 2023, 203, 105–112. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Tian, R.; Zong, M.; Gu, X.; Xu, F.; Chen, Y.; Li, C. Outcomes of Cerebral Embolic Protection for Bicuspid Aortic Valve Stenosis Undergoing Transcatheter Aortic Valve Replacement. J. Am. Heart Assoc. 2023, 12, e028890. [Google Scholar] [CrossRef]
- Forrest, J.K.; Deeb, G.M.; Yakubov, S.J.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Teirstein, P.S.; Tchétché, D.; Huang, J.; et al. 4-Year Outcomes of Patients With Aortic Stenosis in the Evolut Low Risk Trial. J. Am. Coll. Cardiol. 2023, 82, 2163–2165. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Pibarot, P.; Hahn, R.T.; Genereux, P.; Kodali, S.K.; Kapadia, S.R.; Cohen, D.J.; Pocock, S.J.; et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. New Engl. J. Med. 2023, 389, 1949–1960. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Ihlemann, N.; Jørgensen, T.H.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Five-Year Clinical and Echocardiographic Outcomes From the NOTION Randomized Clinical Trial in Patients at Lower Surgical Risk. Circulation 2019, 139, 2714–2723. [Google Scholar] [CrossRef]

| Study | Publication Year | Country | Adjustment | Patients (N) | ||
|---|---|---|---|---|---|---|
| Total | TAVR | SAVR | ||||
| 1-Gibson et al. [21] | 2022 | Ireland | NA | 100 | 39 | 51 |
| 2-Majmundar et al. [22] | 2021 | US | PSM | 2786 | 1393 | 1393 |
| 3-Sanaiha et al. [23] | 2022 | US | UM | 56,331 | 3855 | 52,476 |
| 4-Soud et al. [24] | 2020 | US | PSM | 136 | 68 | 68 |
| 5-Tsai et al. [25] | 2020 | Taiwan | Matched for age and sex | 130 | 48 | 82 |
| 6-Elbadawi et al. [26] | 2019 | US | PSM | 1950 | 975 | 975 |
| 7-Mentias et al. [27] | 2020 | US | PSM | 1398 | 699 | 699 |
| 8-Husso et al. [28] | 2021 | Finland | PSM | 150 | 75 | 75 |
| Study | Publication year | Country | Adjustment | Patients (N) | ||
| Total | Type 1 | Non Type 1 | ||||
| 1-Yoon et al. [29] | 2020 | America | UM | 1034 | 927 | 107 |
| 2-Jilaihawi et al. [30] | 2016 | Mixed | UM | 95 | 74 | 21 |
| 3-Xiong et al. [31] | 2018 | Asia | UM | 80 | 34 | 46 |
| 4-Forrest et al. [20] | 2020 | America | UM | 150 | 136 | 14 |
| 5-Welixiang et al. [32] | 2021 | Asia | UM | 181 | 79 | 102 |
| 6-Esposito et al. [33] | 2022 | Europe | UM | 38 | 25 | 13 |
| 7-Jin et al. [34] | 2022 | Asia | UM | 195 | 109 | 86 |
| 8-Ielasi et al. [35] | 2020 | Europe | UM | 243 | 218 | 25 |
| 9-Fan et al. [36] | 2020 | Asia | UM | 83 | 27 | 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Improta, R.; Di Pietro, G.; Kola, N.; Birtolo, L.I.; Colantonio, R.; Bruno, E.; Tocci, M.; Giansante, A.; Sannino, M.; Zullino, V.; et al. A Meta-Analysis of Short-Term Outcomes of TAVR versus SAVR in Bicuspid Aortic Valve Stenosis and TAVR Results in Different Bicuspid Valve Anatomies. J. Clin. Med. 2023, 12, 7371. https://doi.org/10.3390/jcm12237371
Improta R, Di Pietro G, Kola N, Birtolo LI, Colantonio R, Bruno E, Tocci M, Giansante A, Sannino M, Zullino V, et al. A Meta-Analysis of Short-Term Outcomes of TAVR versus SAVR in Bicuspid Aortic Valve Stenosis and TAVR Results in Different Bicuspid Valve Anatomies. Journal of Clinical Medicine. 2023; 12(23):7371. https://doi.org/10.3390/jcm12237371
Chicago/Turabian StyleImprota, Riccardo, Gianluca Di Pietro, Novis Kola, Lucia Ilaria Birtolo, Riccardo Colantonio, Emanuele Bruno, Marco Tocci, Alessandra Giansante, Michele Sannino, Veronica Zullino, and et al. 2023. "A Meta-Analysis of Short-Term Outcomes of TAVR versus SAVR in Bicuspid Aortic Valve Stenosis and TAVR Results in Different Bicuspid Valve Anatomies" Journal of Clinical Medicine 12, no. 23: 7371. https://doi.org/10.3390/jcm12237371
APA StyleImprota, R., Di Pietro, G., Kola, N., Birtolo, L. I., Colantonio, R., Bruno, E., Tocci, M., Giansante, A., Sannino, M., Zullino, V., Monosilio, S., Cimino, S., Maestrini, V., Severino, P., Badagliacca, R., Lavalle, C., Celli, P., Saade, W., Musto, C., ... Mancone, M. (2023). A Meta-Analysis of Short-Term Outcomes of TAVR versus SAVR in Bicuspid Aortic Valve Stenosis and TAVR Results in Different Bicuspid Valve Anatomies. Journal of Clinical Medicine, 12(23), 7371. https://doi.org/10.3390/jcm12237371









