Pancreatic Ductal Adenocarcinoma: Update of CT-Based Radiomics Applications in the Pre-Surgical Prediction of the Risk of Post-Operative Fistula, Resectability Status and Prognosis
Abstract
:1. Introduction
1.1. Resectability Status
1.2. Post-Operative Pancreatic Fistula
1.3. Prognosis
2. Methods
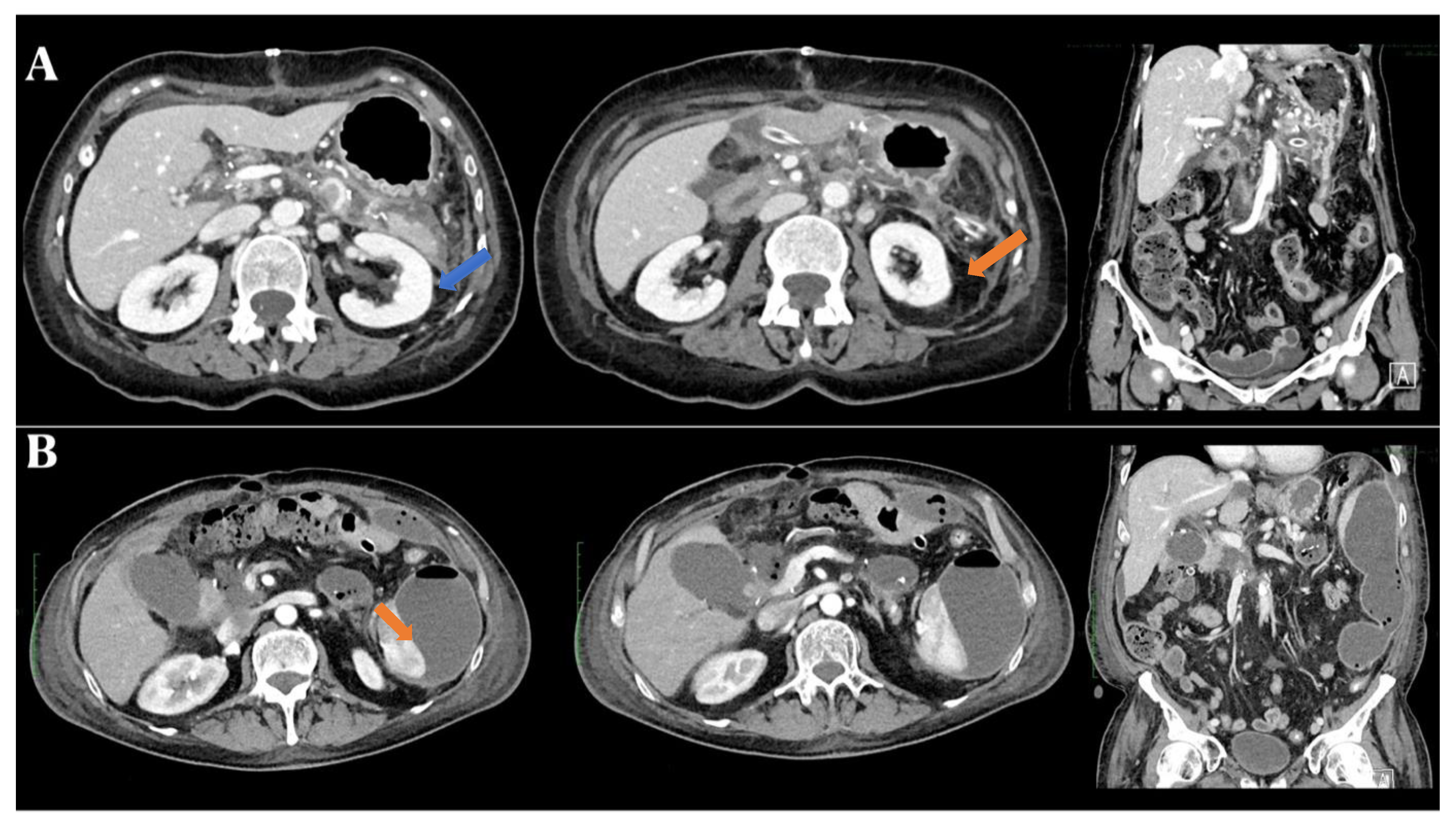
3. Results
4. Discussion
4.1. Resectability Status
4.2. Post-Operative Pancreatic Fistula
4.3. Prognosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koopmann, B.D.; Dunnewind, N.; van Duuren, L.A.; Lansdorp-Vogelaar, I.; Naber, S.K.; Cahen, D.L.; Bruno, M.J.; de Kok, I.M.; Dutch Pancreatic Cancer Group. The Natural Disease Course of Pancreatic Cyst-Associated Neoplasia, Dysplasia, and Ductal Adenocarcinoma: Results of a Microsimulation Model. Gastroenterology 2023, 165, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Casadei, R.; Ricci, C.; Ingaldi, C.; Alberici, L.; De Raffele, E.; Barcia, B.; Mosconi, C.; Diegoli, M.; Di Marco, M.; Brandi, G.; et al. Evolving knowledge in surgical oncology of pancreatic cancer: From theory to clinical practice-a fifteen-year journey at a tertiary referral centre. Updates Surg. 2022, 74, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Alcalá, S.; Mayoral-Varo, V.; Ruiz-Cañas, L.; López-Gil, J.C.; Heeschen, C.; Martín-Pérez, J.; Sainz, B., Jr. Targeting SRC Kinase Signaling in Pancreatic Cancer Stem Cells. Int. J. Mol. Sci. 2020, 21, 7437. [Google Scholar] [CrossRef] [PubMed]
- Avella, P.; Vaschetti, R.; Cappuccio, M.; Gambale, F.; Rafanelli, F.; Brunese, M.C.; Guerra, G.; Scacchi, A.; Rocca, A. The role of liver surgery in simultaneous synchronous colorectal liver metastases and colorectal cancer resections: A literature review of 1730 patients underwent open and minimally invasive surgery. Minerva Surg. 2022, 77, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Scacchi, A.; Cappuccio, M.; Avella, P.; Bugiantella, W.; De Rosa, M.; Costa, G.; Polistena, A.; Codacci-Pisanelli, M.; Amato, B.; et al. Robotic surgery for colorectal liver metastases resection: A systematic review. Int. J. Med. Robot. 2021, 17, e2330. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.M.; Wo, J.Y.; Ferrone, C.R.; Horick, N.K.; Keane, F.K.; Qadan, M.; Lillemoe, K.D.; Hong, T.S.; Clark, J.W.; Blaszkowsky, L.S.; et al. Intraoperative Radiation Therapy (IORT) for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma (BR/LA PDAC) in the Era of Modern Neoadjuvant Treatment: Short-Term and Long-Term Outcomes. Ann. Surg. Oncol. 2020, 27, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; de Gramont, A.; Figueras, J.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; Sobrero, A.; et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat. Rev. 2015, 41, 729–741. [Google Scholar] [CrossRef]
- Wang, G.; Lei, W.; Duan, S.; Cao, A.; Shi, H. Preoperative evaluating early recurrence in resectable pancreatic ductal adenocarcinoma by using CT radiomics. Abdom. Radiol. 2023. ahead of print. [Google Scholar] [CrossRef]
- DiMagno, E.P.; Reber, H.A.; Tempero, M.A. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American Gastroenterological Association. Gastroenterology 1999, 117, 1464–1484. [Google Scholar] [CrossRef]
- Müller, P.C.; Frey, M.C.; Ruzza, C.M.; Nickel, F.; Jost, C.; Gwerder, C.; Hackert, T.; Z’Graggen, K.; Kessler, U. Neoadjuvant Chemotherapy in Pancreatic Cancer: An Appraisal of the Current High-Level Evidence. Pharmacology 2021, 106, 143–153. [Google Scholar] [CrossRef]
- Fogel, E.L.; Shahda, S.; Sandrasegaran, K.; DeWitt, J.; Easler, J.J.; Agarwal, D.M.; Eagleson, M.; Zyromski, N.J.; House, M.G.; Ellsworth, S.; et al. A Multidisciplinary Approach to Pancreas Cancer in 2016: A Review. Am. J. Gastroenterol. 2017, 112, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Casadei, R.; Ricci, C.; Ingaldi, C.; Alberici, L.; Minni, F. Contemporary indications for upfront total pancreatectomy. Updates Surg. 2021, 73, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, T.; Di Gioia, A.; Andrianello, S.; Marchegiani, G.; Bassi, C. Pancreatoduodenectomy associated with colonic resections: Indications, pitfalls, and outcomes. Updates Surg. 2021, 73, 379–390. [Google Scholar] [CrossRef]
- Dbouk, M.; Katona, B.W.; Brand, R.E.; Chak, A.; Syngal, S.; Farrell, J.J.; Kastrinos, F.; Stoffel, E.M.; Blackford, A.L.; Rustgi, A.K.; et al. The Multicenter Cancer of Pancreas Screening Study: Impact on Stage and Survival. J. Clin. Oncol. 2022, 40, 3257–3266. [Google Scholar] [CrossRef] [PubMed]
- Khadka, R.; Tian, W.; Hao, X.; Koirala, R. Risk factor, early diagnosis and overall survival on outcome of association between pancreatic cancer and diabetes mellitus: Changes and advances, a review. Int. J. Surg. 2018, 52, 342–346. [Google Scholar] [CrossRef]
- Hackert, T.; Klaiber, U.; Pausch, T.; Mihaljevic, A.L.; Büchler, M.W. Fifty Years of Surgery for Pancreatic Cancer. Pancreas 2020, 49, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Friess, H.; Bauden, M.; Samnegård, J.; Andersson, R. Pancreatic cancer: Disease dynamics, tumor biology and the role of the microenvironment. Oncotarget 2018, 9, 6644–6651. [Google Scholar] [CrossRef]
- Ettrich, T.J.; Sturm, N.; Güthle, M.; Hüttner, F.J.; Perkhofer, L. Pancreatic Cancer: Current Multimodality Treatment Options and the Future Impact of Molecular Biological Profiling. Visc. Med. 2022, 38, 20–29. [Google Scholar] [CrossRef]
- Evans, D.B. What Makes a Pancreatic Cancer Resectable? Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 300–305. [Google Scholar] [CrossRef]
- Pajewska, M.; Partyka, O.; Czerw, A.; Deptała, A.; Cipora, E.; Gąska, I.; Wojtaszek, M.; Sygit, K.; Sygit, M.; Krzych-Fałta, E.; et al. Management of Metastatic Pancreatic Cancer-Comparison of Global Guidelines over the Last 5 Years. Cancers 2023, 15, 4400. [Google Scholar] [CrossRef]
- Rocca, A.; Calise, F.; Marino, G.; Montagnani, S.; Cinelli, M.; Amato, B.; Guerra, G. Primary giant hepatic neuroendocrine carcinoma: A case report. Int. J. Surg. 2014, 12 (Suppl. S1), S218–S221. [Google Scholar] [CrossRef] [PubMed]
- Al-Hawary, M.M.; Francis, I.R.; Chari, S.T.; Fishman, E.K.; Hough, D.M.; Lu, D.S.; Macari, M.; Megibow, A.J.; Miller, F.H.; Mortele, K.J.; et al. Pancreatic ductal adenocarcinoma radiology reporting template: Consensus statement of the society of abdominal radiology and the american pancreatic association. Gastroenterology 2014, 146, 291–304.e291. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Morana, G.; D’Onofrio, M.; Fusco, R.; Coppola, F.; Grassi, F.; Cappabianca, S.; Reginelli, A.; Maggialetti, N.; Buccicardi, D.; et al. Structured Reporting of Computed Tomography and Magnetic Resonance in the Staging of Pancreatic Adenocarcinoma: A Delphi Consensus Proposal. Diagnostics 2021, 11, 2033. [Google Scholar] [CrossRef] [PubMed]
- Danti, G.; Flammia, F.; Matteuzzi, B.; Cozzi, D.; Berti, V.; Grazzini, G.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Gastrointestinal neuroendocrine neoplasms (GI-NENs): Hot topics in morphological, functional, and prognostic imaging. Radiol. Med. 2021, 126, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Javadi, S.; Karbasian, N.; Bhosale, P.; de Castro Faria, S.; Le, O.; Katz, M.H.; Koay, E.J.; Tamm, E.P. Imaging findings of recurrent pancreatic cancer following resection. Abdom. Radiol. 2018, 43, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Casadei, R.; Ricci, C.; Ingaldi, C.; Cornacchia, A.; Migliori, M.; Di Marco, M.; Pagano, N.; Serra, C.; Alberici, L.; Minni, F. External validation of nomogram for predicting malignant intraductal papillary mucinous neoplasm (IPMN): From the theory to the clinical practice using the decision curve analysis model. Updates Surg. 2021, 73, 429–438. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Costa, G.; De Rosa, M.; Codacci Pisanelli, M.; Frezza, B.; De Prizio, M.; Bravi, I.; Scacchi, A.; Gallo, G.; Amato, B.; et al. Minimally Invasive Approach to Gastric GISTs: Analysis of a Multicenter Robotic and Laparoscopic Experience with Literature Review. Cancers 2021, 13, 4351. [Google Scholar] [CrossRef]
- Jajodia, A.; Wang, A.; Alabousi, M.; Wilks, C.; Kulkarni, A.; van der Pol, C.B. MRI vs. CT for pancreatic adenocarcinoma vascular invasion: Comparative diagnostic test accuracy systematic review and meta-analysis. Eur. Radiol. 2023, 33, 6883–6891. [Google Scholar] [CrossRef]
- Harrington, K.A.; Shukla-Dave, A.; Paudyal, R.; Do, R.K.G. MRI of the Pancreas. J. Magn. Reson. Imaging 2021, 53, 347–359. [Google Scholar] [CrossRef]
- Miller, F.H.; Lopes Vendrami, C.; Hammond, N.A.; Mittal, P.K.; Nikolaidis, P.; Jawahar, A. Pancreatic Cancer and Its Mimics. Radiographics 2023, 43, e230054. [Google Scholar] [CrossRef] [PubMed]
- Gassert, F.G.; Ziegelmayer, S.; Luitjens, J.; Gassert, F.T.; Tollens, F.; Rink, J.; Makowski, M.R.; Rübenthaler, J.; Froelich, M.F. Additional MRI for initial M-staging in pancreatic cancer: A cost-effectiveness analysis. Eur. Radiol. 2022, 32, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Jaén-Torrejimeno, I.; López-Guerra, D.; Rojas-Holguín, A.; De-Armas-Conde, N.; Blanco-Fernández, G. Resection of isolated pancreatic metastases from pulmonary neoplasia: A systematic review. Updates Surg. 2022, 74, 1817–1825. [Google Scholar] [CrossRef]
- Ottens, T.; Barbieri, S.; Orton, M.R.; Klaassen, R.; van Laarhoven, H.W.M.; Crezee, H.; Nederveen, A.J.; Zhen, X.; Gurney-Champion, O.J. Deep learning DCE-MRI parameter estimation: Application in pancreatic cancer. Med. Image Anal. 2022, 80, 102512. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, C.Q.; Chen, T.W.; Tang, M.Y.; Zhang, X.M. Molecular Imaging with MRI: Potential Application in Pancreatic Cancer. BioMed Res. Int. 2015, 2015, 624074. [Google Scholar] [CrossRef]
- Chu, L.C.; Park, S.; Kawamoto, S.; Yuille, A.L.; Hruban, R.H.; Fishman, E.K. Pancreatic Cancer Imaging: A New Look at an Old Problem. Curr. Probl. Diagn. Radiol. 2021, 50, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Stoop, T.F.; van Veldhuisen, E.; van Rijssen, L.B.; Klaassen, R.; Gurney-Champion, O.J.; de Hingh, I.H.; Busch, O.R.; van Laarhoven, H.W.M.; van Lienden, K.P.; Stoker, J.; et al. Added value of 3T MRI and the MRI-halo sign in assessing resectability of locally advanced pancreatic cancer following induction chemotherapy (IMAGE-MRI): Prospective pilot study. Langenbeck’s Arch. Surg. 2022, 407, 3487–3499. [Google Scholar] [CrossRef]
- Chen, F.M.; Ni, J.M.; Zhang, Z.Y.; Zhang, L.; Li, B.; Jiang, C.J. Presurgical Evaluation of Pancreatic Cancer: A Comprehensive Imaging Comparison of CT Versus MRI. Am. J. Roentgenol. 2016, 206, 526–535. [Google Scholar] [CrossRef]
- Caranci, F.; Brunese, L.; Reginelli, A.; Napoli, M.; Fonio, P.; Briganti, F. Neck neoplastic conditions in the emergency setting: Role of multidetector computed tomography. Semin. Ultrasound CT MRI 2012, 33, 443–448. [Google Scholar] [CrossRef]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef]
- Kwan, J.Y.Y.; Su, J.; Huang, S.H.; Ghoraie, L.S.; Xu, W.; Chan, B.; Yip, K.W.; Giuliani, M.; Bayley, A.; Kim, J.; et al. Radiomic Biomarkers to Refine Risk Models for Distant Metastasis in HPV-related Oropharyngeal Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, X.; Wang, Y.; Ding, X. Prognosis prediction of extremity and trunk wall soft-tissue sarcomas treated with surgical resection with radiomic analysis based on random survival forest. Updates Surg. 2022, 74, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Brunese, M.C.; Fantozzi, M.R.; Fusco, R.; De Muzio, F.; Gabelloni, M.; Danti, G.; Borgheresi, A.; Palumbo, P.; Bruno, F.; Gandolfo, N.; et al. Update on the Applications of Radiomics in Diagnosis, Staging, and Recurrence of Intrahepatic Cholangiocarcinoma. Diagnostics 2023, 13, 1488. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Galdiero, R.; Maggialetti, N.; Silvestro, L.; De Bellis, M.; Di Girolamo, E.; Grazzini, G.; Chiti, G.; et al. Risk Assessment and Pancreatic Cancer: Diagnostic Management and Artificial Intelligence. Cancers 2023, 15, 351. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Brunese, M.C.; Santone, A.; Avella, P.; Bianco, P.; Scacchi, A.; Scaglione, M.; Bellifemine, F.; Danzi, R.; Varriano, G.; et al. Early Diagnosis of Liver Metastases from Colorectal Cancer through CT Radiomics and Formal Methods: A Pilot Study. J. Clin. Med. 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Maggialetti, N.; Capasso, R.; Pinto, D.; Carbone, M.; Laporta, A.; Schipani, S.; Piccolo, C.L.; Zappia, M.; Reginelli, A.; D’Innocenzo, M.; et al. Diagnostic value of computed tomography colonography (CTC) after incomplete optical colonoscopy. Int. J. Surg. 2016, 33 (Suppl. S1), S36–S44. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, S.; Lang, H. Neoadjuvant Therapy of Pancreatic Cancer: Definitions and Benefits. Int. J. Mol. Sci. 2017, 18, 1622. [Google Scholar] [CrossRef]
- Gugenheim, J.; Crovetto, A.; Petrucciani, N. Neoadjuvant therapy for pancreatic cancer. Updates Surg. 2022, 74, 35–42. [Google Scholar] [CrossRef]
- Van Dam, J.L.; Janssen, Q.P.; Besselink, M.G.; Homs, M.Y.V.; van Santvoort, H.C.; van Tienhoven, G.; de Wilde, R.F.; Wilmink, J.W.; van Eijck, C.H.J.; Groot Koerkamp, B. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: A meta-analysis of randomised controlled trials. Eur. J. Cancer 2022, 160, 140–149. [Google Scholar] [CrossRef]
- Ferrari, C.; Maggialetti, N.; Masi, T.; Nappi, A.G.; Santo, G.; Niccoli Asabella, A.; Rubini, G. Early Evaluation of Immunotherapy Response in Lymphoma Patients by 18F-FDG PET/CT: A Literature Overview. J. Pers. Med. 2021, 11, 217. [Google Scholar] [CrossRef]
- Danti, G.; Addeo, G.; Cozzi, D.; Maggialetti, N.; Lanzetta, M.M.; Frezzetti, G.; Masserelli, A.; Pradella, S.; Giovagnoni, A.; Miele, V. Relationship between diagnostic imaging features and prognostic outcomes in gastrointestinal stromal tumors (GIST). Acta Biomed. 2019, 90, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Kauffmann, E.; Cacace, C.; Menonna, F.; Caramella, D.; Cappelli, C.; Campani, D.; Cacciato Insilla, A.; Vasile, E.; Vivaldi, C.; et al. Factors predicting survival in patients with locally advanced pancreatic cancer undergoing pancreatectomy with arterial resection. Updates Surg. 2021, 73, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Mungroop, T.H.; van Rijssen, L.B.; van Klaveren, D.; Smits, F.J.; van Woerden, V.; Linnemann, R.J.; de Pastena, M.; Klompmaker, S.; Marchegiani, G.; Ecker, B.L.; et al. Alternative Fistula Risk Score for Pancreatoduodenectomy (a-FRS): Design and International External Validation. Ann. Surg. 2019, 269, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Schlanger, D.; Graur, F.; Popa, C.; Moiș, E.; Al Hajjar, N. The role of artificial intelligence in pancreatic surgery: A systematic review. Updates Surg. 2022, 74, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yu, S.; Zhang, Y.; Zhu, L.; Chen, S.; Liu, Y. CT-Based Radiomics Nomogram Improves Risk Stratification and Prediction of Early Recurrence in Hepatocellular Carcinoma After Partial Hepatectomy. Front. Oncol. 2022, 12, 896002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Zhang, G.; Qiu, X.; Tan, W.; Yin, X.; Liao, L. Deep Learning with Radiomics for Disease Diagnosis and Treatment: Challenges and Potential. Front. Oncol. 2022, 12, 773840. [Google Scholar] [CrossRef]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef]
- Cardenas, C.E.; Yang, J.; Anderson, B.M.; Court, L.E.; Brock, K.B. Advances in Auto-Segmentation. Semin. Radiat. Oncol. 2019, 29, 185–197. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, G.; Pandey, S.; Yeh, C.H.; Wang, J.J.; Lin, C.Y.; Ho, T.Y.; Ko, S.F.; Ng, S.H. Fully automated segmentation and radiomics feature extraction of hypopharyngeal cancer on MRI using deep learning. Eur. Radiol. 2023, 33, 6548–6556. [Google Scholar] [CrossRef]
- Ma, M.; Gan, L.; Liu, Y.; Jiang, Y.; Xin, L.; Qin, N.; Cheng, Y.; Liu, Q.; Xu, L.; Zhang, Y.; et al. Radiomics features based on automatic segmented MRI images: Prognostic biomarkers for triple-negative breast cancer treated with neoadjuvant chemotherapy. Eur. J. Radiol. 2022, 146, 110095. [Google Scholar] [CrossRef]
- Yao, J.; Shi, Y.; Cao, K.; Lu, L.; Lu, J.; Song, Q.; Jin, G.; Xiao, J.; Hou, Y.; Zhang, L. DeepPrognosis: Preoperative prediction of pancreatic cancer survival and surgical margin via comprehensive understanding of dynamic contrast-enhanced CT imaging and tumor-vascular contact parsing. Med. Image Anal. 2021, 73, 102150. [Google Scholar] [CrossRef] [PubMed]
- Avanzo, M.; Wei, L.; Stancanello, J.; Vallières, M.; Rao, A.; Morin, O.; Mattonen, S.A.; El Naqa, I. Machine and deep learning methods for radiomics. Med. Phys. 2020, 47, e185–e202. [Google Scholar] [CrossRef] [PubMed]
- Stanzione, A.; Verde, F.; Romeo, V.; Boccadifuoco, F.; Mainenti, P.P.; Maurea, S. Radiomics and machine learning applications in rectal cancer: Current update and future perspectives. World J. Gastroenterol. 2021, 27, 5306–5321. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, Y.; Jiang, S.; Fan, R.; Zhang, H.; Jiang, W. Application of radiomics and machine learning in head and neck cancers. Int. J. Biol. Sci. 2021, 17, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Currie, G.; Hawk, K.E.; Rohren, E.; Vial, A.; Klein, R. Machine Learning and Deep Learning in Medical Imaging: Intelligent Imaging. J. Med. Imaging Radiat. Sci. 2019, 50, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.; Doken, S.; Zhuang, T.; Wingerter, D.; Gidwani, M.; Mistry, N.; Ladic, L.; Kamen, A.; Abazeed, M.E. An image-based deep learning framework for individualizing radiotherapy dose. Lancet Digit. Health 2019, 1, e136–e147. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Lu, J.; Zhang, H.; Adeli, E.; Wang, J.; Yu, Z.; Liu, L.; Wang, Q.; Wu, J.; Shen, D. Multi-Channel 3D Deep Feature Learning for Survival Time Prediction of Brain Tumor Patients Using Multi-Modal Neuroimages. Sci. Rep. 2019, 9, 1103. [Google Scholar] [CrossRef]
- Nie, D.; Zhang, H.; Adeli, E.; Liu, L.; Shen, D. 3D Deep Learning for Multi-modal Imaging-Guided Survival Time Prediction of Brain Tumor Patients. Med. Image Comput. Comput. Assist. Interv. 2016, 9901, 212–220. [Google Scholar] [CrossRef]
- Manimegalai, P.; Suresh Kumar, R.; Valsalan, P.; Dhanagopal, R.; Vasanth Raj, P.T.; Christhudass, J. 3D Convolutional Neural Network Framework with Deep Learning for Nuclear Medicine. Scanning 2022, 2022, 9640177. [Google Scholar] [CrossRef]
- Yee, E.; Ma, D.; Popuri, K.; Chen, S.; Lee, H.; Chow, V.; Ma, C.; Wang, L.; Beg, M.F. 3D hemisphere-based convolutional neural network for whole-brain MRI segmentation. Comput. Med. Imaging Graph. 2022, 95, 102000. [Google Scholar] [CrossRef]
- Yuan, H.; Fan, Z.; Wu, Y.; Cheng, J. An efficient multi-path 3D convolutional neural network for false-positive reduction of pulmonary nodule detection. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, M.; Uzunoglu, F.G.; Adham, M.; Imrie, C.; Milicevic, M.; Sandberg, A.A.; Asbun, H.J.; Bassi, C.; Büchler, M.; Charnley, R.M.; et al. Borderline resectable pancreatic cancer: A consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014, 155, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Falconi, M. Total pancreatectomy: How, when and why? Updates Surg. 2021, 73, 1203–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liao, S.; You, J.; Wu, H. Conversion surgery for initially unresectable pancreatic ductal adenocarcinoma following induction therapy: A systematic review of the published literature. Updates Surg. 2022, 74, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, M.; Barreto, S.G. Surgery for pancreatic cancer: Current controversies and challenges. Future Oncol. 2021, 17, 5135–5162. [Google Scholar] [CrossRef] [PubMed]
- Colucci, G.F.D.C.; Falconi, M.; Giuliani, F.; Parisi, S.; Reni, M. Linee Guida del Carcinoma del Pancreas; AIOM: Milan, Italy, 2009; Volume 1. [Google Scholar]
- Joo, I.; Lee, J.M.; Lee, E.S.; Son, J.Y.; Lee, D.H.; Ahn, S.J.; Chang, W.; Lee, S.M.; Kang, H.J.; Yang, H.K. Preoperative CT Classification of the Resectability of Pancreatic Cancer: Interobserver Agreement. Radiology 2019, 293, 343–349. [Google Scholar] [CrossRef]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef]
- Versteijne, E.; Vogel, J.A.; Besselink, M.G.; Busch, O.R.C.; Wilmink, J.W.; Daams, J.G.; van Eijck, C.H.J.; Groot Koerkamp, B.; Rasch, C.R.N.; van Tienhoven, G. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br. J. Surg. 2018, 105, 946–958. [Google Scholar] [CrossRef]
- Seufferlein, T.; Uhl, W.; Kornmann, M.; Algül, H.; Friess, H.; König, A.; Ghadimi, M.; Gallmeier, E.; Bartsch, D.K.; Lutz, M.P.; et al. Perioperative or only adjuvant gemcitabine plus nab-paclitaxel for resectable pancreatic cancer (NEONAX)-a randomized phase II trial of the AIO pancreatic cancer group. Ann. Oncol. 2023, 34, 91–100. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, J.M. Imaging diagnosis of pancreatic cancer: A state-of-the-art review. World J. Gastroenterol. 2014, 20, 7864–7877. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.B.; Lee, S.S.; Kim, J.H.; Kim, H.J.; Byun, J.H.; Hong, S.M.; Song, K.B.; Kim, S.C. Pancreatic Cancer CT: Prediction of Resectability according to NCCN Criteria. Radiology 2018, 289, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.J.; Winslow, E.; Kooby, D.; Lad, N.L.; Parikh, A.A.; Scoggins, C.R.; Ahmad, S.; Martin, R.C.; Maithel, S.K.; Kim, H.J.; et al. Vein involvement during pancreaticoduodenectomy: Is there a need for redefinition of “borderline resectable disease”? J. Gastrointest. Surg. 2013, 17, 1209–1217, discussion 1217. [Google Scholar] [CrossRef] [PubMed]
- Van Veldhuisen, E.; van den Oord, C.; Brada, L.J.; Walma, M.S.; Vogel, J.A.; Wilmink, J.W.; Del Chiaro, M.; van Lienden, K.P.; Meijerink, M.R.; van Tienhoven, G.; et al. Locally Advanced Pancreatic Cancer: Work-Up, Staging, and Local Intervention Strategies. Cancers 2019, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Giani, A.; Mazzola, M.; Morini, L.; Zironda, A.; Bertoglio, C.L.; De Martini, P.; Magistro, C.; Ferrari, G. Hepatic vascular anomalies during totally laparoscopic pancreaticoduodenectomy: Challenging the challenge. Updates Surg. 2022, 74, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Kawai, N.; Kaga, T.; Ishihara, T.; Hyodo, F.; Kato, H.; Kambadakone, A.R.; Matsuo, M. Vascular involvement and resectability of pancreatic ductal adenocarcinoma on contrast-enhanced MRI: Comparison with pancreatic protocol CT. Abdom. Radiol. 2022, 47, 2835–2844. [Google Scholar] [CrossRef]
- GM, O.K.; Knox, J.J. Locally advanced pancreatic cancer: An emerging entity. Curr. Probl. Cancer 2018, 42, 12–25. [Google Scholar] [CrossRef]
- Huguet, F.; Mukherjee, S.; Javle, M. Locally advanced pancreatic cancer: The role of definitive chemoradiotherapy. Clin. Oncol. 2014, 26, 560–568. [Google Scholar] [CrossRef]
- Maggino, L.; Malleo, G.; Marchegiani, G.; Viviani, E.; Nessi, C.; Ciprani, D.; Esposito, A.; Landoni, L.; Casetti, L.; Tuveri, M.; et al. Outcomes of Primary Chemotherapy for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2019, 154, 932–942. [Google Scholar] [CrossRef]
- Reni, M.; Zanon, S.; Balzano, G.; Nobile, S.; Pircher, C.C.; Chiaravalli, M.; Passoni, P.; Arcidiacono, P.G.; Nicoletti, R.; Crippa, S.; et al. Selecting patients for resection after primary chemotherapy for non-metastatic pancreatic adenocarcinoma. Ann. Oncol. 2017, 28, 2786–2792. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Wu, W.; Tan, Y.; Chen, X.; Duan, Y.; Sun, D.; Lu, Y.; Xu, X. The comparation of short-term outcome between laparoscopic and open pancreaticoduodenectomy: A propensity score matching analysis. Updates Surg. 2021, 73, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Delpero, J.R.; Sauvanet, A. Vascular Resection for Pancreatic Cancer: 2019 French Recommendations Based on a Literature Review From 2008 to 6-2019. Front. Oncol. 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Quero, G.; Pecorelli, N.; Paiella, S.; Fiorillo, C.; Petrone, M.C.; Rosa, F.; Capretti, G.; Laterza, V.; Kauffmann, E.; Nobile, S.; et al. Quantitative assessment of the impact of COVID-19 pandemic on pancreatic surgery: An Italian multicenter analysis of 1423 cases from 10 tertiary referral centers. Updates Surg. 2022, 74, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Shaib, W.L.; Ip, A.; Cardona, K.; Alese, O.B.; Maithel, S.K.; Kooby, D.; Landry, J.; El-Rayes, B.F. Contemporary Management of Borderline Resectable and Locally Advanced Unresectable Pancreatic Cancer. Oncologist 2016, 21, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Chadha, A.S.; Suh, Y.; Chen, H.C.; Rao, A.; Das, P.; Minsky, B.D.; Mahmood, U.; Delclos, M.E.; Sawakuchi, G.O.; et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 755–765. [Google Scholar] [CrossRef] [PubMed]
- De Geus, S.W.L.; Eskander, M.F.; Kasumova, G.G.; Ng, S.C.; Kent, T.S.; Mancias, J.D.; Callery, M.P.; Mahadevan, A.; Tseng, J.F. Stereotactic body radiotherapy for unresected pancreatic cancer: A nationwide review. Cancer 2017, 123, 4158–4167. [Google Scholar] [CrossRef] [PubMed]
- Ermongkonchai, T.; Khor, R.; Muralidharan, V.; Tebbutt, N.; Lim, K.; Kutaiba, N.; Ng, S.P. Stereotactic radiotherapy and the potential role of magnetic resonance-guided adaptive techniques for pancreatic cancer. World J. Gastroenterol. 2022, 28, 745–754. [Google Scholar] [CrossRef]
- Reyngold, M.; Parikh, P.; Crane, C.H. Ablative radiation therapy for locally advanced pancreatic cancer: Techniques and results. Radiat. Oncol. 2019, 14, 95. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef]
- Gemenetzis, G.; Groot, V.P.; Blair, A.B.; Laheru, D.A.; Zheng, L.; Narang, A.K.; Fishman, E.K.; Hruban, R.H.; Yu, J.; Burkhart, R.A.; et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann. Surg. 2019, 270, 340–347. [Google Scholar] [CrossRef]
- Marchegiani, G.; Perri, G.; Bianchi, B.; Esposito, A.; Landoni, L.; Casetti, L.; Tuveri, M.; Malleo, G.; Paiella, S.; Fontana, M.; et al. Pancreatic surgery during COVID-19 pandemic: Major activity disruption of a third-level referral center during 2020. Updates Surg. 2022, 74, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, T.; Girard, E.; Lemoine, C.; Schlienger, G.; Alao, O.; Risse, O.; Berdah, S.; Chirica, M.; Moutardier, V.; Birnbaum, D.J. Intra-pancreatic distal cholangiocarcinoma and pancreatic ductal adenocarcinoma: A common short and long-term prognosis? Updates Surg. 2021, 73, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Abi Jaoude, J.; Kouzy, R.; Nguyen, N.D.; Lin, D.; Noticewala, S.S.; Ludmir, E.B.; Taniguchi, C.M. Radiation therapy for patients with locally advanced pancreatic cancer: Evolving techniques and treatment strategies. Curr. Probl. Cancer 2020, 44, 100607. [Google Scholar] [CrossRef]
- Crippa, S.; Belfiori, G.; Tamburrino, D.; Partelli, S.; Falconi, M. Indications to total pancreatectomy for positive neck margin after partial pancreatectomy: A review of a slippery ground. Updates Surg. 2021, 73, 1219–1229. [Google Scholar] [CrossRef]
- Cassinotto, C.; Sa-Cunha, A.; Trillaud, H. Radiological evaluation of response to neoadjuvant treatment in pancreatic cancer. Diagn. Interv. Imaging 2016, 97, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.K.; Lee, J.M.; Lee, E.S.; Yu, M.H.; Joo, I.; Yoon, J.H.; Jang, J.Y.; Lee, K.B.; Lee, S.H. How to approach pancreatic cancer after neoadjuvant treatment: Assessment of resectability using multidetector CT and tumor markers. Eur. Radiol. 2022, 32, 56–66. [Google Scholar] [CrossRef]
- Wagner, M.; Antunes, C.; Pietrasz, D.; Cassinotto, C.; Zappa, M.; Sa Cunha, A.; Lucidarme, O.; Bachet, J.B. CT evaluation after neoadjuvant FOLFIRINOX chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur. Radiol. 2017, 27, 3104–3116. [Google Scholar] [CrossRef]
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Van der Heijde, N.; Lof, S.; Busch, O.R.; de Hingh, I.; de Kleine, R.H.; Molenaar, I.Q.; Mungroop, T.H.; Stommel, M.W.; Besselink, M.G.; van Eijck, C. Incidence and impact of postoperative pancreatic fistula after minimally invasive and open distal pancreatectomy. Surgery 2022, 171, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Nahm, C.B.; Connor, S.J.; Samra, J.S.; Mittal, A. Postoperative pancreatic fistula: A review of traditional and emerging concepts. Clin. Exp. Gastroenterol. 2018, 11, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, G.; Bassi, C. Prevention, prediction, and mitigation of postoperative pancreatic fistula. Br. J. Surg. 2021, 108, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, E.F.; Napoli, N.; Genovese, V.; Ginesini, M.; Gianfaldoni, C.; Vistoli, F.; Amorese, G.; Boggi, U. Feasibility and safety of robotic-assisted total pancreatectomy: A pilot western series. Updates Surg. 2021, 73, 955–966. [Google Scholar] [CrossRef]
- Giovinazzo, F.; Linneman, R.; Riva, G.V.D.; Greener, D.; Morano, C.; Patijn, G.A.; Besselink, M.G.H.; Nieuwenhuijs, V.B.; Abu Hilal, M.; de Hingh, I.H.; et al. Clinical relevant pancreatic fistula after pancreatoduodenectomy: When negative amylase levels tell the truth. Updates Surg. 2021, 73, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Hackert, T.; Werner, J.; Büchler, M.W. Postoperative pancreatic fistula. Surgeon 2011, 9, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Simon, R. Complications After Pancreaticoduodenectomy. Surg. Clin. N. Am. 2021, 101, 865–874. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years after. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Zhao, G.D.; Zhao, Z.M.; Hu, M.G.; Tan, X.L.; Zhang, X.; Gao, Y.X.; Liu, R. A comparative study of end-to-end pancreatic anastomosis versus pancreaticojejunostomy after robotic central pancreatectomy. Updates Surg. 2021, 73, 967–975. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Z.; Zhang, J.; Che, X. Safety and efficacy of robot-assisted versus open pancreaticoduodenectomy: A meta-analysis of multiple worldwide centers. Updates Surg. 2021, 73, 893–907. [Google Scholar] [CrossRef]
- Lee, J.M.; Yoon, J.H. Dual-Energy CT for Risk of Postoperative Pancreatic Fistula. Radiology 2022, 304, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Andreasi, V.; Partelli, S.; Rancoita, P.M.V.; Mele, S.; Mazza, M.; La Fauci, D.; Pecorelli, N.; Guarneri, G.; Tamburrino, D.; Crippa, S.; et al. Clinical and economic validation of grade B postoperative pancreatic fistula subclassification. Surgery 2022, 171, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Bundred, J.R.; Lin, A.; Halle-Smith, J.; Pande, R.; Sutcliffe, R.; Harrison, E.M.; Roberts, K.J. Systematic review and meta-analysis of factors associated with post-operative pancreatic fistula following pancreatoduodenectomy. ANZ J. Surg. 2021, 91, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Salvia, R.; Lionetto, G.; Perri, G.; Malleo, G.; Marchegiani, G. Total pancreatectomy and pancreatic fistula: Friend or foe? Updates Surg. 2021, 73, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Pande, R.; Halle-Smith, J.M.; Phelan, L.; Thorne, T.; Panikkar, M.; Hodson, J.; Roberts, K.J.; Arshad, A.; Connor, S.; Conlon, K.C.; et al. External validation of postoperative pancreatic fistula prediction scores in pancreatoduodenectomy: A systematic review and meta-analysis. HPB 2022, 24, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, Q.; Wang, C. Postoperative drain amylase predicts pancreatic fistula in pancreatic surgery: A systematic review and meta-analysis. Int. J. Surg. 2015, 22, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Yang, Y.F.; Han, P.; Ye, P.C.; Kong, H. Protein-energy malnutrition worsens hospitalization outcomes of patients with pancreatic cancer undergoing open pancreaticoduodenectomy. Updates Surg. 2022, 74, 1627–1636. [Google Scholar] [CrossRef]
- Callery, M.P.; Pratt, W.B.; Kent, T.S.; Chaikof, E.L.; Vollmer, C.M., Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J. Am. Coll. Surg. 2013, 216, 1–14. [Google Scholar] [CrossRef]
- Chao, Y.J.; Liao, T.K.; Su, P.J.; Wang, C.J.; Shan, Y.S. Impact of body mass index on the early experience of robotic pancreaticoduodenectomy. Updates Surg. 2021, 73, 929–937. [Google Scholar] [CrossRef]
- McMillan, M.T.; Soi, S.; Asbun, H.J.; Ball, C.G.; Bassi, C.; Beane, J.D.; Behrman, S.W.; Berger, A.C.; Bloomston, M.; Callery, M.P.; et al. Risk-adjusted Outcomes of Clinically Relevant Pancreatic Fistula Following Pancreatoduodenectomy: A Model for Performance Evaluation. Ann. Surg. 2016, 264, 344–352. [Google Scholar] [CrossRef]
- Chopra, A.; Nassour, I.; Zureikat, A.; Paniccia, A. Perioperative and oncologic outcomes of open, laparoscopic, and robotic distal pancreatectomy for pancreatic adenocarcinoma. Updates Surg. 2021, 73, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Kolbinger, F.R.; Lambrecht, J.; Leger, S.; Ittermann, T.; Speidel, S.; Weitz, J.; Hoffmann, R.T.; Distler, M.; Kühn, J.P. The image-based preoperative fistula risk score (preFRS) predicts postoperative pancreatic fistula in patients undergoing pancreatic head resection. Sci. Rep. 2022, 12, 4064. [Google Scholar] [CrossRef] [PubMed]
- Zimmitti, G.; Coppola, A.; Ardito, F.; Meniconi, R.; Ettorre, G.M.; Rosso, E.; Manzoni, A.; Colasanti, M.; Clemente, G.; Murazio, M.; et al. Outcomes comparison of Pancreato-Gastrostomy and Isolated Jejunal Loop Pancreato-Jejunostomy following Pancreato-Duodenectomy in patients with soft pancreas and at moderate-high risk for POPF: A retrospective multicenter experience-based analysis. Updates Surg. 2022, 74, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Bonsdorff, A.; Sallinen, V. Prediction of postoperative pancreatic fistula and pancreatitis after pancreatoduodenectomy or distal pancreatectomy: A review. Scand. J. Surg. 2023, 112, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.F.; Kuang, T.T.; Dong, Y.; Zuo, D.; Qiu, Y.J.; Lou, W.H.; Wang, W.P. Prediction of pancreatic fistula after pancreatectomy by virtual touch tissue imaging and quantification (VTIQ) technology. Pancreatology 2021, 21, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Bhasker, N.; Kolbinger, F.R.; Skorobohach, N.; Zwanenburg, A.; Löck, S.; Weitz, J.; Hoffmann, R.T.; Distler, M.; Speidel, S.; Leger, S.; et al. Prediction of clinically relevant postoperative pancreatic fistula using radiomic features and preoperative data. Sci. Rep. 2023, 13, 7506. [Google Scholar] [CrossRef] [PubMed]
- Tajima, Y.; Kawabata, Y.; Hirahara, N. Preoperative imaging evaluation of pancreatic pathologies for the objective prediction of pancreatic fistula after pancreaticoduodenectomy. Surg. Today 2018, 48, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Cleary, S.P.; Gryfe, R.; Guindi, M.; Greig, P.; Smith, L.; Mackenzie, R.; Strasberg, S.; Hanna, S.; Taylor, B.; Langer, B.; et al. Prognostic factors in resected pancreatic adenocarcinoma: Analysis of actual 5-year survivors. J. Am. Coll. Surg. 2004, 198, 722–731. [Google Scholar] [CrossRef]
- Cai, J.; Chen, H.; Lu, M.; Zhang, Y.; Lu, B.; You, L.; Zhang, T.; Dai, M.; Zhao, Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021, 520, 1–11. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, L.; Wang, X.; Zhu, S.; Luo, J.; Zhang, Y.; Tong, Y.; Feng, F.; Kang, Y.; Bi, Q. Nomogram Predicts Risk and Prognostic Factors for Bone Metastasis of Pancreatic Cancer: A Population-Based Analysis. Front. Endocrinol. 2021, 12, 752176. [Google Scholar] [CrossRef]
- Distler, M.; Pilarsky, E.; Kersting, S.; Grützmann, R. Preoperative CEA and CA 19-9 are prognostic markers for survival after curative resection for ductal adenocarcinoma of the pancreas—A retrospective tumor marker prognostic study. Int. J. Surg. 2013, 11, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Shyr, B.U.; Shyr, B.S.; Chen, S.C.; Shyr, Y.M.; Wang, S.E. Robotic and open pancreaticoduodenectomy: Results from Taipei Veterans General Hospital in Taiwan. Updates Surg. 2021, 73, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Attiyeh, M.A.; Chakraborty, J.; Doussot, A.; Langdon-Embry, L.; Mainarich, S.; Gönen, M.; Balachandran, V.P.; D’Angelica, M.I.; DeMatteo, R.P.; Jarnagin, W.R.; et al. Survival Prediction in Pancreatic Ductal Adenocarcinoma by Quantitative Computed Tomography Image Analysis. Ann. Surg. Oncol. 2018, 25, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Bakasa, W.; Viriri, S. Pancreatic Cancer Survival Prediction: A Survey of the State-of-the-Art. Comput. Math. Methods Med. 2021, 2021, 1188414. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Hamada, T.; Higashi, M.; Matsuo, K.; Maemura, K.; Kurahara, H.; Horinouchi, M.; Hiraki, T.; Sugimoto, T.; Akahane, T.; et al. Predicted Prognosis of Patients with Pancreatic Cancer by Machine Learning. Clin. Cancer Res. 2020, 26, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, F.; Sugiura, T.; Okamura, Y.; Ito, T.; Yamamoto, Y.; Ashida, R.; Ohgi, K.; Uesaka, K. The preoperative controlling nutritional status (CONUT) score is an independent prognostic marker for pancreatic ductal adenocarcinoma. Updates Surg. 2021, 73, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, Y.J.; Kim, K.G.; Park, J.S. Preoperative CT texture features predict prognosis after curative resection in pancreatic cancer. Sci. Rep. 2019, 9, 17389. [Google Scholar] [CrossRef]
- Cassinotto, C.; Chong, J.; Zogopoulos, G.; Reinhold, C.; Chiche, L.; Lafourcade, J.P.; Cuggia, A.; Terrebonne, E.; Dohan, A.; Gallix, B. Resectable pancreatic adenocarcinoma: Role of CT quantitative imaging biomarkers for predicting pathology and patient outcomes. Eur. J. Radiol. 2017, 90, 152–158. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, L.; Zhong, X.; Zhu, S.; Zhang, Y.; Ge, M.; Kang, Y.; Bi, Q. Two Novel Nomograms Predicting the Risk and Prognosis of Pancreatic Cancer Patients with Lung Metastases: A Population-Based Study. Front. Public Health 2022, 10, 884349. [Google Scholar] [CrossRef]
- Rigiroli, F.; Hoye, J.; Lerebours, R.; Lyu, P.; Lafata, K.J.; Zhang, A.R.; Erkanli, A.; Mettu, N.B.; Morgan, D.E.; Samei, E.; et al. Exploratory analysis of mesenteric-portal axis CT radiomic features for survival prediction of patients with pancreatic ductal adenocarcinoma. Eur. Radiol. 2023, 33, 5779–5791. [Google Scholar] [CrossRef]
- Rossi, G.; Altabella, L.; Simoni, N.; Benetti, G.; Rossi, R.; Venezia, M.; Paiella, S.; Malleo, G.; Salvia, R.; Guariglia, S.; et al. Computed tomography-based radiomic to predict resectability in locally advanced pancreatic cancer treated with chemotherapy and radiotherapy. World J. Gastrointest. Oncol. 2022, 14, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, D.; Mori, M.; Prato, F.; Crippa, S.; Belfiori, G.; Reni, M.; Mushtaq, J.; Aleotti, F.; Guazzarotti, G.; Cao, R.; et al. Prediction of Early Distant Recurrence in Upfront Resectable Pancreatic Adenocarcinoma: A Multidisciplinary, Machine Learning-Based Approach. Cancers 2021, 13, 4938. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Sham, J.G.; Kawamoto, S.; Blair, A.B.; Rozich, N.; Fouladi, D.F.; Shayesteh, S.; Hruban, R.H.; He, J.; Wolfgang, C.L.; et al. CT Radiomics-Based Preoperative Survival Prediction in Patients with Pancreatic Ductal Adenocarcinoma. Am. J. Roentgenol. 2021, 217, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wei, Y.; Cheng, S.; Lu, Z.; Zhang, K.; Jiang, K.; Xu, Q. Survival prediction after upfront surgery in patients with pancreatic ductal adenocarcinoma: Radiomic, clinic-pathologic and body composition analysis. Pancreatology 2021, 21, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Miranda, E.; Khalvati, F.; Namdar, K.; Deniffel, D.; Dong, X.; Abbas, E.; Wilson, J.M.; O’Kane, G.M.; Knox, J.; Gallinger, S.; et al. Validation of Prognostic Radiomic Features from Resectable Pancreatic Ductal Adenocarcinoma in Patients with Advanced Disease Undergoing Chemotherapy. Can. Assoc. Radiol. J. 2021, 72, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Liu, C.; Gao, F.; Qi, Y.; Lu, H.; Liu, Z.; Zhang, X.; Cai, X.; Ji, R.Y.; Hou, Y.; et al. Prediction of Clinically Relevant Pancreatico-Enteric Anastomotic Fistulas after Pancreatoduodenectomy Using Deep Learning of Preoperative Computed Tomography. Theranostics 2020, 10, 9779–9788. [Google Scholar] [CrossRef] [PubMed]
- Capretti, G.; Bonifacio, C.; De Palma, C.; Nebbia, M.; Giannitto, C.; Cancian, P.; Laino, M.E.; Balzarini, L.; Papanikolaou, N.; Savevski, V.; et al. A machine learning risk model based on preoperative computed tomography scan to predict postoperative outcomes after pancreatoduodenectomy. Updates Surg. 2022, 74, 235–243. [Google Scholar] [CrossRef]
- Alves, N.; Schuurmans, M.; Litjens, G.; Bosma, J.S.; Hermans, J.; Huisman, H. Fully Automatic Deep Learning Framework for Pancreatic Ductal Adenocarcinoma Detection on Computed Tomography. Cancers 2022, 14, 376. [Google Scholar] [CrossRef]
- Petrelli, F.; Inno, A.; Barni, S.; Ghidini, A.; Labianca, R.; Falconi, M.; Reni, M.; Cascinu, S. Borderline resectable pancreatic cancer: More than an anatomical concept. Dig. Liver Dis. 2017, 49, 223–226. [Google Scholar] [CrossRef]
- Matsumoto, I.; Murakami, Y.; Shinzeki, M.; Asari, S.; Goto, T.; Tani, M.; Motoi, F.; Uemura, K.; Sho, M.; Satoi, S.; et al. Proposed preoperative risk factors for early recurrence in patients with resectable pancreatic ductal adenocarcinoma after surgical resection: A multi-center retrospective study. Pancreatology 2015, 15, 674–680. [Google Scholar] [CrossRef]
- Napoli, N.; Kauffmann, E.F.; Vistoli, F.; Amorese, G.; Boggi, U. State of the art of robotic pancreatoduodenectomy. Updates Surg. 2021, 73, 873–880. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, J.C.; Yang, S.Y.; Kim, J.; Hwang, J.H. Neoadjuvant therapy versus upfront surgery in resectable pancreatic cancer according to intention-to-treat and per-protocol analysis: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 15662. [Google Scholar] [CrossRef] [PubMed]
- Al Abbas, A.I.; Zeh Iii, H.J.; Polanco, P.M. State of the art robotic distal pancreatectomy: A review of the literature. Updates Surg. 2021, 73, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Park, M.S.; Hong, H.S.; Kang, C.M.; Choi, J.Y.; Lim, J.S.; Lee, W.J.; Kim, M.J.; Kim, K.W. Effects of neoadjuvant combined chemotherapy and radiation therapy on the CT evaluation of resectability and staging in patients with pancreatic head cancer. Radiology 2009, 250, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Cassinotto, C.; Mouries, A.; Lafourcade, J.P.; Terrebonne, E.; Belleannée, G.; Blanc, J.F.; Lapuyade, B.; Vendrely, V.; Laurent, C.; Chiche, L.; et al. Locally advanced pancreatic adenocarcinoma: Reassessment of response with CT after neoadjuvant chemotherapy and radiation therapy. Radiology 2014, 273, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Limkin, E.J.; Sun, R.; Dercle, L.; Zacharaki, E.I.; Robert, C.; Reuzé, S.; Schernberg, A.; Paragios, N.; Deutsch, E.; Ferté, C. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann. Oncol. 2017, 28, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Pagano, N.; Ricci, C.; Ingaldi, C.; Sadalla, S.; Fabbri, A.; Alberici, L.; Impellizeri, G.; Pallio, S.; Zagari, R.M.; De Leo, A.; et al. Performance of EUS-FNB in solid pancreatic masses: A lesson from 463 consecutive procedures and a practical nomogram. Updates Surg. 2022, 74, 945–952. [Google Scholar] [CrossRef]
- Das, R.; McGrath, K.; Seiser, N.; Smith, K.; Uttam, S.; Brand, R.E.; Fasanella, K.E.; Khalid, A.; Chennat, J.S.; Sarkaria, S.; et al. Tumor Size Differences Between Preoperative Endoscopic Ultrasound and Postoperative Pathology for Neoadjuvant-Treated Pancreatic Ductal Adenocarcinoma Predict Patient Outcome. Clin. Gastroenterol. Hepatol. 2022, 20, 886–897. [Google Scholar] [CrossRef]
- Kang, C.M.; Lee, S.H.; Hwang, H.K.; Yun, M.; Lee, W.J. Preoperative Volume-Based PET Parameter, MTV2.5, as a Potential Surrogate Marker for Tumor Biology and Recurrence in Resected Pancreatic Cancer. Medicine 2016, 95, e2595. [Google Scholar] [CrossRef]
- Abdalla, T.S.A.; Almanfalouti, V.; Effenberger, K.; Uzunoglu, F.G.; Ghadban, T.; Dupreé, A.; Izbicki, J.R.; Pantel, K.; Reeh, M. Evaluation of the Hamburg-Glasgow Classification in Pancreatic Cancer: Preoperative Staging by Combining Disseminated Tumor Load and Systemic Inflammation. Cancers 2021, 13, 5942. [Google Scholar] [CrossRef]
- Rho, S.Y.; Lee, S.G.; Park, M.; Lee, J.; Lee, S.H.; Hwang, H.K.; Lee, M.J.; Paik, Y.K.; Lee, W.J.; Kang, C.M. Developing a preoperative serum metabolome-based recurrence-predicting nomogram for patients with resected pancreatic ductal adenocarcinoma. Sci. Rep. 2019, 9, 18634. [Google Scholar] [CrossRef] [PubMed]
- Tummers, W.S.; Groen, J.V.; Sibinga Mulder, B.G.; Farina-Sarasqueta, A.; Morreau, J.; Putter, H.; van de Velde, C.J.; Vahrmeijer, A.L.; Bonsing, B.A.; Mieog, J.S.; et al. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. Br. J. Surg. 2019, 106, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Feng, Y.; Grant, P.E.; Ou, Y. Segmentation ability map: Interpret deep features for medical image segmentation. Med Image Anal. 2023, 84, 102726. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, S.M.; McIntyre, C.A.; Schilsky, J.B.; Harrington, K.A.; Gerst, S.R.; Flynn, J.R.; Gonen, M.; Capanu, M.; Wong, W.; Lawrence, S.; et al. Preoperative CT predictors of survival in patients with pancreatic ductal adenocarcinoma undergoing curative intent surgery. Abdom. Radiol. 2021, 46, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Barreto, S.G.; Pandanaboyana, S.; Ironside, N.; Windsor, J.A. Does revision of resection margins based on frozen section improve overall survival following pancreatoduodenectomy for pancreatic ductal adenocarcinoma? A meta-analysis. HPB 2017, 19, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Kleive, D.; Labori, K.J.; Line, P.D.; Gladhaug, I.P.; Verbeke, C.S. Pancreatoduodenectomy with venous resection for ductal adenocarcinoma rarely achieves complete (R0) resection. HPB 2020, 22, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.; Satoi, S.; Kon, M. Redefining the R1 resection in patients with pancreatic ductal adenocarcinoma. J. Hepatobiliary Pancreat. Sci. 2016, 23, 523–532. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, Q.; Zhao, Z.; Zhang, X.; Gao, Y.; Tan, X.; Liu, R. The standardized technique and surgical video of robotic pancreaticoduodenectomy at the Chinese PLA General Hospital. Updates Surg. 2022, 74, 245–254. [Google Scholar] [CrossRef]
- Partelli, S.; Cinelli, L.; Andreasi, V.; Rancoita, P.M.V.; Pecorelli, N.; Tamburrino, D.; Crippa, S.; Falconi, M. Evaluation of factors predicting loss of benefit provided by laparoscopic distal pancreatectomy compared to open approach. Updates Surg. 2022, 74, 213–221. [Google Scholar] [CrossRef]
- Lindholm, E.; Bergmann, G.B.; Haugaa, H.; Labori, K.J.; Yaqub, S.; Bjørnbeth, B.A.; Line, P.D.; Grindheim, G.; Kjøsen, G.; Pischke, S.E.; et al. Early detection of anastomotic leakage after pancreatoduodenectomy with microdialysis catheters: An observational Study. HPB 2022, 24, 901–909. [Google Scholar] [CrossRef]
- Malgras, B.; Dokmak, S.; Aussilhou, B.; Pocard, M.; Sauvanet, A. Management of postoperative pancreatic fistula after pancreaticoduodenectomy. J. Visc. Surg. 2023, 160, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Casadei, R.; Ricci, C.; D’Ambra, M.; Marrano, N.; Alagna, V.; Rega, D.; Monari, F.; Minni, F. Laparoscopic versus open distal pancreatectomy in pancreatic tumours: A case-control study. Updates Surg. 2010, 62, 171–174. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Han, I.W.; Cho, K.; Ryu, Y.; Shin, S.H.; Heo, J.S.; Choi, D.W.; Chung, M.J.; Kwon, O.C.; Cho, B.H. Risk prediction platform for pancreatic fistula after pancreatoduodenectomy using artificial intelligence. World J. Gastroenterol. 2020, 26, 4453–4464. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Q.; Yadav, D.K.; Jiang, W.; Hua, Y.F.; Lu, C. Risk Factors for Clinically Relevant Postoperative Pancreatic Fistula (CR-POPF) after Distal Pancreatectomy: A Single Center Retrospective Study. Can. J. Gastroenterol. Hepatol. 2021, 2021, 8874504. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.; Ratnayake, B.; Lee, S.; French, J.J.; Wilson, C.; Roberts, K.J.; Loveday, B.P.T.; Manas, D.; Windsor, J.; White, S.; et al. Systematic review and meta-analysis of risk factors of postoperative pancreatic fistula after distal pancreatectomy in the era of 2016 International Study Group pancreatic fistula definition. HPB 2021, 23, 1139–1151. [Google Scholar] [CrossRef]
- Kanda, M.; Fujii, T.; Suenaga, M.; Takami, H.; Hattori, M.; Inokawa, Y.; Yamada, S.; Nakayama, G.; Sugimoto, H.; Koike, M.; et al. Estimated pancreatic parenchymal remnant volume accurately predicts clinically relevant pancreatic fistula after pancreatoduodenectomy. Surgery 2014, 156, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Oshima, M.; Kakinoki, K.; Yamamoto, N.; Akamoto, S.; Yachida, S.; Hagiike, M.; Kamada, H.; Masaki, T.; Suzuki, Y. Pancreatic thickness as a predictive factor for postoperative pancreatic fistula after distal pancreatectomy using an endopath stapler. Surg. Today 2013, 43, 141–147. [Google Scholar] [CrossRef]
- Kirihara, Y.; Takahashi, N.; Hashimoto, Y.; Sclabas, G.M.; Khan, S.; Moriya, T.; Sakagami, J.; Huebner, M.; Sarr, M.G.; Farnell, M.B. Prediction of pancreatic anastomotic failure after pancreatoduodenectomy: The use of preoperative, quantitative computed tomography to measure remnant pancreatic volume and body composition. Ann. Surg. 2013, 257, 512–519. [Google Scholar] [CrossRef]
- Tranchart, H.; Gaujoux, S.; Rebours, V.; Vullierme, M.P.; Dokmak, S.; Levy, P.; Couvelard, A.; Belghiti, J.; Sauvanet, A. Preoperative CT scan helps to predict the occurrence of severe pancreatic fistula after pancreaticoduodenectomy. Ann. Surg. 2012, 256, 139–145. [Google Scholar] [CrossRef]
- Kakizawa, N.; Noda, H.; Watanabe, F.; Ichida, K.; Suzuki, K.; Rikiyama, T. A High Abdominal Aortic Calcification Score on CT is a Risk Factor for Postoperative Pancreatic Fistula in Elderly Patients Undergoing Pancreaticoduodenectomy. World J. Surg. 2018, 42, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Aiolfi, A.; Lombardo, F.; Bonitta, G.; Danelli, P.; Bona, D. Systematic review and updated network meta-analysis comparing open, laparoscopic, and robotic pancreaticoduodenectomy. Updates Surg. 2021, 73, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Pasculli, A.; Bugiantella, W.; De Rosa, M.; Catena, F.; Rondelli, F.; Costa, G.; Rocca, A.; Longaroni, M.; Testini, M. Minimally invasive laparoscopic and robot-assisted emergency treatment of strangulated giant hiatal hernias: Report of five cases and literature review. World J. Emerg. Surg. 2020, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Cianci, S.; Perrone, E.; Rossitto, C.; Fanfani, F.; Tropea, A.; Biondi, A.; Scambia, G.; Gueli Alletti, S. Percutaneous-assisted vs mini-laparoscopic hysterectomy: Comparison of ultra-minimally invasive approaches. Updates Surg. 2021, 73, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Fabbi, M.; De Pascale, S.; Ascari, F.; Petz, W.L.; Fumagalli Romario, U. Side-to-side esophagogastric anastomosis for minimally invasive Ivor-Lewis esophagectomy: Operative technique and short-term outcomes. Updates Surg. 2021, 73, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Cipriani, F.; Belli, G.; Berti, S.; Boggi, U.; Bottino, V.; Cillo, U.; Cescon, M.; Cimino, M.; Corcione, F.; et al. The Italian Consensus on minimally invasive simultaneous resections for synchronous liver metastasis and primary colorectal cancer: A Delphi methodology. Updates Surg. 2021, 73, 1247–1265. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.A.; Laxague, F.; Schlottmann, F.; Sadava, E.E. Re-laparoscopy for the treatment of complications after laparoscopic appendectomy: Is it possible to maintain the minimally invasive approach? Updates Surg. 2021, 73, 2199–2204. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, C.Y. Postoperative quality of life and cosmetic outcome between minimally invasive video-assisted thyroidectomy and bilateral axillo-breast approach robotic thyroidectomy: A single center retrospective cohort study. Updates Surg. 2021, 73, 1459–1465. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Valeri, M.; Amato, L.; De Rosa, M.; Rondelli, F.; Cappuccio, M.; Gambale, F.E.; Fantozzi, M.; Sciaudone, G.; Avella, P.; et al. Robotic revision surgery after failed Nissen anti-reflux surgery: A single center experience and a literature review. J. Robot. Surg. 2023, 17, 1517–1524. [Google Scholar] [CrossRef]
- Birindelli, A.; Martin, M.; Khan, M.; Gallo, G.; Segalini, E.; Gori, A.; Yetasook, A.; Podda, M.; Giuliani, A.; Tugnoli, G.; et al. Laparoscopic splenectomy as a definitive management option for high-grade traumatic splenic injury when non operative management is not feasible or failed: A 5-year experience from a level one trauma center with minimally invasive surgery expertise. Updates Surg. 2021, 73, 1515–1531. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Rocca, A.; De Rosa, M.; Fontani, A.; Ermili, F.; Andolfi, E.; Bugiantella, W.; Levi Sandri, G.B. Minimally invasive robotic-assisted combined colorectal and liver excision surgery: Feasibility, safety and surgical technique in a pilot series. Updates Surg. 2021, 73, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, F.; Ratti, F.; Fiorentini, G.; Reineke, R.; Aldrighetti, L. Systematic review of perioperative and oncologic outcomes of minimally-invasive surgery for hilar cholangiocarcinoma. Updates Surg. 2021, 73, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Buondonno, A.; Avella, P.; Cappuccio, M.; Scacchi, A.; Vaschetti, R.; Di Marzo, G.; Maida, P.; Luciani, C.; Amato, B.; Brunese, M.C.; et al. A Hub and Spoke Learning Program in Bariatric Surgery in a Small Region of Italy. Front. Surg. 2022, 9, 855527. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, D.; Parini, D.; Marano, L.; Cipriani, F.; Di Marzo, F.; Macrì, A.; D’Ugo, D.; Roviello, F.; Gronchi, A. Surgical management of oncologic patient during and after the COVID-19 outbreak: Practical recommendations from the Italian society of Surgical Oncology. Updates Surg. 2021, 73, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Andolfi, E.; Fontani, A.; Calise, F.; Rocca, A.; Giuliani, A. Robot-assisted liver surgery in a general surgery unit with a “Referral Centre Hub&Spoke Learning Program”. Early outcomes after our first 70 consecutive patients. Minerva Chir. 2018, 73, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Bracale, U.; Podda, M.; Castiglioni, S.; Peltrini, R.; Sartori, A.; Arezzo, A.; Corcione, F.; Agresta, F. Changes in surgical behaviors during the COVID-19 pandemic. The SICE CLOUD19 Study. Updates Surg. 2021, 73, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Avella, P.; Segreto, A.L.; Izzo, M.L.; Buondonno, A.; Coluzzi, M.; Cappuccio, M.; Brunese, M.C.; Vaschetti, R.; Scacchi, A.; et al. Postoperative Outcomes Analysis after Pancreatic Duct Occlusion: A Safe Option to Treat the Pancreatic Stump after Pancreaticoduodenectomy in Low-Volume Centers. Front. Surg. 2021, 8, 804675. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.F.; Kattan, M.W.; Klimstra, D.; Conlon, K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann. Surg. 2004, 240, 293–298. [Google Scholar] [CrossRef]
- Xu, J.; Shi, K.Q.; Chen, B.C.; Huang, Z.P.; Lu, F.Y.; Zhou, M.T. A nomogram based on preoperative inflammatory markers predicting the overall survival of pancreatic ductal adenocarcinoma. J. Gastroenterol. Hepatol. 2017, 32, 1394–1402. [Google Scholar] [CrossRef]
- Ielpo, B.; Sanchez, P.; Grande, L.; Burdio, F. Laparoscopic pancreatoduodenectomy: How we have standardized the technique (with video). Updates Surg. 2022, 74, 1479–1481. [Google Scholar] [CrossRef]
- Puleo, F.; Nicolle, R.; Blum, Y.; Cros, J.; Marisa, L.; Demetter, P.; Quertinmont, E.; Svrcek, M.; Elarouci, N.; Iovanna, J.; et al. Stratification of Pancreatic Ductal Adenocarcinomas Based on Tumor and Microenvironment Features. Gastroenterology 2018, 155, 1999–2013.e1993. [Google Scholar] [CrossRef] [PubMed]
- Bowman, A.W.; Bolan, C.W. MRI evaluation of pancreatic ductal adenocarcinoma: Diagnosis, mimics, and staging. Abdom. Radiol. 2019, 44, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Yun, G.; Kim, Y.H.; Lee, Y.J.; Kim, B.; Hwang, J.H.; Choi, D.J. Tumor heterogeneity of pancreas head cancer assessed by CT texture analysis: Association with survival outcomes after curative resection. Sci. Rep. 2018, 8, 7226. [Google Scholar] [CrossRef] [PubMed]
- McQuerry, J.A.; Chang, J.T.; Bowtell, D.D.L.; Cohen, A.; Bild, A.H. Mechanisms and clinical implications of tumor heterogeneity and convergence on recurrent phenotypes. J. Mol. Med. 2017, 95, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Kröner, P.T.; Engels, M.M.; Glicksberg, B.S.; Johnson, K.W.; Mzaik, O.; van Hooft, J.E.; Wallace, M.B.; El-Serag, H.B.; Krittanawong, C. Artificial intelligence in gastroenterology: A state-of-the-art review. World J. Gastroenterol. 2021, 27, 6794–6824. [Google Scholar] [CrossRef] [PubMed]
- Kenner, B.; Chari, S.T.; Kelsen, D.; Klimstra, D.S.; Pandol, S.J.; Rosenthal, M.; Rustgi, A.K.; Taylor, J.A.; Yala, A.; Abul-Husn, N.; et al. Artificial Intelligence and Early Detection of Pancreatic Cancer: 2020 Summative Review. Pancreas 2021, 50, 251–279. [Google Scholar] [CrossRef]
- Wang, H.; Chetty, R.; Hosseini, M.; Allende, D.S.; Esposito, I.; Matsuda, Y.; Deshpande, V.; Shi, J.; Dhall, D.; Jang, K.T.; et al. Pathologic Examination of Pancreatic Specimens Resected for Treated Pancreatic Ductal Adenocarcinoma: Recommendations from the Pancreatobiliary Pathology Society. Am. J. Surg. Pathol. 2022, 46, 754–764. [Google Scholar] [CrossRef]
- Faur, A.C.; Lazar, D.C.; Ghenciu, L.A. Artificial intelligence as a noninvasive tool for pancreatic cancer prediction and diagnosis. World J. Gastroenterol. 2023, 29, 1811–1823. [Google Scholar] [CrossRef]
- Xie, T.; Wang, X.; Li, M.; Tong, T.; Yu, X.; Zhou, Z. Pancreatic ductal adenocarcinoma: A radiomics nomogram outperforms clinical model and TNM staging for survival estimation after curative resection. Eur. Radiol. 2020, 30, 2513–2524. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Galdiero, R.; Setola, S.V.; Palaia, R.; Belli, A.; Silvestro, L.; Cozzi, D.; Brunese, L.; et al. Pancreatic cancer detection and characterization: State of the art and radiomics. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3684–3699. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, M.; Barat, M.; Dohan, A.; Gaujoux, S.; Coriat, R.; Hoeffel, C.; Cassinotto, C.; Chassagnon, G.; Soyer, P. CT and MRI of pancreatic tumors: An update in the era of radiomics. Jpn. J. Radiol. 2020, 38, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Tovar, D.R.; Rosenthal, M.H.; Maitra, A.; Koay, E.J. Potential of artificial intelligence in the risk stratification for and early detection of pancreatic cancer. Artif. Intell. Surg. 2023, 3, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Yokota, H.; Hoshino, I.; Iwatate, Y.; Wakamatsu, K.; Uno, T.; Suyari, H. Deep learning-based gene selection in comprehensive gene analysis in pancreatic cancer. Sci. Rep. 2021, 11, 16521. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Guo, S.; Jiang, H.; Gao, S.; Shao, C.; Cao, K.; Fang, X.; Li, J.; Wang, L.; Hua, W.; et al. Relationship between Radiomics and Risk of Lymph Node Metastasis in Pancreatic Ductal Adenocarcinoma. Pancreas 2019, 48, 1195–1203. [Google Scholar] [CrossRef]
- Popa, F.; Mihai, P. Geriatric surgery—Present and perspective. J. Med. Life 2008, 1, 239–246. [Google Scholar] [PubMed]
- Chia, C.L.; Tan, K.Y. The Era of Geriatric Surgery. Ann. Acad. Med. Singap. 2019, 48, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Brunese, M.C.; Cappuccio, M.; Scacchi, A.; Martucci, G.; Buondonno, A.; Perrotta, F.M.; Quarto, G.; Avella, P.; Amato, B. Impact of Physical Activity on Disability Risk in Elderly Patients Hospitalized for Mild Acute Diverticulitis and Diverticular Bleeding Undergone Conservative Management. Medicina 2021, 57, 360. [Google Scholar] [CrossRef]
- Aldrighetti, L.; Boggi, U.; Falconi, M.; Giuliante, F.; Cipriani, F.; Ratti, F.; Torzilli, G. Perspectives from Italy during the COVID-19 pandemic: Nationwide survey-based focus on minimally invasive HPB surgery. Updates Surg. 2020, 72, 241–247. [Google Scholar] [CrossRef]
- Guerra, G.; Testa, D.; Montagnani, S.; Tafuri, D.; Salzano, F.A.; Rocca, A.; Amato, B.; Salzano, G.; Dell’Aversana Orabona, G.; Piombino, P.; et al. Surgical management of pleomorphic adenoma of parotid gland in elderly patients: Role of morphological features. Int. J. Surg. 2014, 12 (Suppl. S2), S12–S16. [Google Scholar] [CrossRef]
- Komici, K.; Dello Iacono, A.; De Luca, A.; Perrotta, F.; Bencivenga, L.; Rengo, G.; Rocca, A.; Guerra, G. Adiponectin and Sarcopenia: A Systematic Review With Meta-Analysis. Front. Endocrinol. 2021, 12, 576619. [Google Scholar] [CrossRef] [PubMed]
- Aprea, G.; De Rosa, D.; Milone, M.; Rocca, A.; Bianco, T.; Massa, G.; Compagna, R.; Johnson, L.B.; Sanguinetti, A.; Polistena, A.; et al. Laparoscopic distal pancreatectomy in elderly patients: Is it safe? Aging Clin. Exp. Res. 2017, 29 (Suppl. S1), 41–45. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Porfidia, C.; Russo, R.; Tamburrino, A.; Avella, P.; Vaschetti, R.; Bianco, P.; Calise, F. Neuraxial anesthesia in hepato-pancreatic-bilio surgery: A first western pilot study of 46 patients. Updates Surg. 2023, 75, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Komici, K.; Cappuccio, M.; Scacchi, A.; Vaschetti, R.; Delli Carpini, G.; Picerno, V.; Avella, P.; Brunese, M.C.; Rengo, G.; Guerra, G.; et al. The Prevalence and the Impact of Frailty in Hepato-Biliary Pancreatic Cancers: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1116. [Google Scholar] [CrossRef]
- Luciani, C.; Scacchi, A.; Vaschetti, R.; Di Marzo, G.; Fatica, I.; Cappuccio, M.; Guerra, G.; Ceccarelli, G.; Avella, P.; Rocca, A. The uniportal VATS in the treatment of stage II pleural empyema: A safe and effective approach for adults and elderly patients-a single-center experience and literature review. World J. Emerg. Surg. 2022, 17, 46. [Google Scholar] [CrossRef]
- Caan, B.J.; Cespedes Feliciano, E.M.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of Muscle and Adiposity Measured by Computed Tomography with Survival in Patients with Nonmetastatic Breast Cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Floridi, C.; De Bernardi, I.; Fontana, F.; Muollo, A.; Ierardi, A.M.; Agostini, A.; Fonio, P.; Squillaci, E.; Brunese, L.; Fugazzola, C.; et al. Microwave ablation of renal tumors: State of the art and development trends. Radiol. Med. 2014, 119, 533–540. [Google Scholar] [CrossRef]
- Chianca, V.; Albano, D.; Messina, C.; Gitto, S.; Ruffo, G.; Guarino, S.; Del Grande, F.; Sconfienza, L.M. Sarcopenia: Imaging assessment and clinical application. Abdom. Radiol. 2022, 47, 3205–3216. [Google Scholar] [CrossRef]
- Anconina, R.; Ortega, C.; Metser, U.; Liu, Z.A.; Elimova, E.; Allen, M.; Darling, G.E.; Wong, R.; Taylor, K.; Yeung, J.; et al. Combined 18 F-FDG PET/CT Radiomics and Sarcopenia Score in Predicting Relapse-Free Survival and Overall Survival in Patients with Esophagogastric Cancer. Clin. Nucl. Med. 2022, 47, 684–691. [Google Scholar] [CrossRef]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Battaglini, C.L.; Williams, G.R. Bioelectrical Impedance Analysis for the Assessment of Sarcopenia in Patients with Cancer: A Systematic Review. Oncologist 2020, 25, 170–182. [Google Scholar] [CrossRef]
- Brunese, L.; Greco, B.; Setola, F.R.; Lassandro, F.; Guarracino, M.R.; De Rimini, M.; Piccolo, S.; De Rosa, N.; Muto, R.; Bianco, A.; et al. Non-small cell lung cancer evaluated with quantitative contrast-enhanced CT and PET-CT: Net enhancement and standardized uptake values are related to tumour size and histology. Med. Sci. Monit. 2013, 19, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Birdsell, L.A.; Martin, L.; Baracos, V.E.; Fearon, K.C. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin. Cancer Res. 2009, 15, 6973–6979. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Hyder, O.; Firoozmand, A.; Kneuertz, P.; Schulick, R.D.; Huang, D.; Makary, M.; Hirose, K.; Edil, B.; Choti, M.A.; et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J. Gastrointest. Surg. 2012, 16, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Narasimhan, A.; Silverman, L.M.; Young, A.R.; Shahda, S.; Liu, S.; Wan, J.; Liu, Y.; Koniaris, L.G.; Zimmers, T.A. Sex specificity of pancreatic cancer cachexia phenotypes, mechanisms, and treatment in mice and humans: Role of Activin. J. Cachexia Sarcopenia Muscle 2022, 13, 2146–2161. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Chen, C.Y.; Huang, C.J.; Wang, C.J.; Chao, Y.J.; Chiang, N.J.; Wang, H.C.; Tung, H.L.; Liu, H.C.; Shan, Y.S. The Differential Clinical Impacts of Cachexia and Sarcopenia on the Prognosis of Advanced Pancreatic Cancer. Cancers 2022, 14, 3137. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.; Shin, S.H.; Kim, J.H.; Jeong, W.K.; Park, D.J.; Kim, N.; Heo, J.S.; Choi, D.W.; Han, I.W. The effects of sarcopenia and sarcopenic obesity after pancreaticoduodenectomy in patients with pancreatic head cancer. HPB 2020, 22, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Jaseanchiun, W.; Kato, H.; Hayasaki, A.; Fujii, T.; Iizawa, Y.; Tanemura, A.; Murata, Y.; Azumi, Y.; Kuriyama, N.; Kishiwada, M.; et al. The clinical impact of portal venous patency ratio on prognosis of patients with pancreatic ductal adenocarcinoma undergoing pancreatectomy with combined resection of portal vein following preoperative chemoradiotherapy. Pancreatology 2019, 19, 307–315. [Google Scholar] [CrossRef]
- Song, A.; Liu, F.; Wu, L.; Si, X.; Zhou, Y. Histopathologic tumor invasion of superior mesenteric vein/portal vein is a poor prognostic indicator in patients with pancreatic ductal adenocarcinoma: Results from a systematic review and meta-analysis. Oncotarget 2017, 8, 32600–32607. [Google Scholar] [CrossRef]
- Kim, M.; Kang, T.W.; Cha, D.I.; Kim, Y.K.; Kim, S.H.; Jang, K.T.; Han, I.W.; Sohn, I. Prediction and clinical implications of portal vein/superior mesenteric vein invasion in patients with resected pancreatic head cancer: The significance of preoperative CT parameters. Clin. Radiol. 2018, 73, 564–573. [Google Scholar] [CrossRef]
- Nakai, A.; Kamata, K.; Hyodo, T.; Chikugo, T.; Hara, A.; Otsuka, Y.; Tanaka, H.; Yoshikawa, T.; Ishikawa, R.; Okamoto, A.; et al. Utility of contrast-enhanced harmonic EUS for diagnosis of portal vein invasion by pancreatic cancer. Endosc. Ultrasound 2022, 11, 401–406. [Google Scholar] [CrossRef]
- Meyer, M.; Ronald, J.; Vernuccio, F.; Nelson, R.C.; Ramirez-Giraldo, J.C.; Solomon, J.; Patel, B.N.; Samei, E.; Marin, D. Reproducibility of CT Radiomic Features within the Same Patient: Influence of Radiation Dose and CT Reconstruction Settings. Radiology 2019, 293, 583–591. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Leger, S.; Agolli, L.; Pilz, K.; Troost, E.G.C.; Richter, C.; Löck, S. Assessing robustness of radiomic features by image perturbation. Sci. Rep. 2019, 9, 614. [Google Scholar] [CrossRef]
- Wong, J.; Baine, M.; Wisnoskie, S.; Bennion, N.; Zheng, D.; Yu, L.; Dalal, V.; Hollingsworth, M.A.; Lin, C. Effects of interobserver and interdisciplinary segmentation variabilities on CT-based radiomics for pancreatic cancer. Sci. Rep. 2021, 11, 16328. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lin, X.; Shen, J.; Chen, X.; Chen, J.; Li, Z.P.; Wang, M.; Yuan, C.; Diao, X.F.; Luo, Y.; et al. Accurate and Feasible Deep Learning Based Semi-Automatic Segmentation in CT for Radiomics Analysis in Pancreatic Neuroendocrine Neoplasms. IEEE J. Biomed. Health Inform. 2021, 25, 3498–3506. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kaur Saini, S.; Thakur, N.; Juneja, M. Survey of Denoising, Segmentation and C lassification of Pancreatic Cancer Imaging. Curr. Med. Imaging 2023, 20, e150523216892. [Google Scholar] [CrossRef]
- Nasief, H.; Hall, W.; Zheng, C.; Tsai, S.; Wang, L.; Erickson, B.; Li, X.A. Improving Treatment Response Prediction for Chemoradiation Therapy of Pancreatic Cancer Using a Combination of Delta-Radiomics and the Clinical Biomarker CA19-9. Front. Oncol. 2019, 9, 1464. [Google Scholar] [CrossRef] [PubMed]
- Rigiroli, F.; Hoye, J.; Lerebours, R.; Lafata, K.J.; Li, C.; Meyer, M.; Lyu, P.; Ding, Y.; Schwartz, F.R.; Mettu, N.B.; et al. CT Radiomic Features of Superior Mesenteric Artery Involvement in Pancreatic Ductal Adenocarcinoma: A Pilot Study. Radiology 2021, 301, 610–622. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Zhang, S.L.; Wang, H.; Yan, Y.; Qi, X.; Chen, S.; Chen, F.M. A CT Radiomics-Based Risk Score for Preoperative Estimation of Intraoperative Superior Mesenteric-Portal Vein Involvement in Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2023, 30, 1206–1216. [Google Scholar] [CrossRef]
- Kim, H.; Park, T.; Jang, J.; Lee, S. Comparison of survival prediction models for pancreatic cancer: Cox model versus machine learning models. Genomics Inform. 2022, 20, e23. [Google Scholar] [CrossRef]
- Katzman, J.L.; Shaham, U.; Cloninger, A.; Bates, J.; Jiang, T.; Kluger, Y. DeepSurv: Personalized treatment recommender system using a Cox proportional hazards deep neural network. BMC Med. Res. Methodol. 2018, 18, 24. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, Z.; Keles, E.; Yazici, C.; Tirkes, T.; Bagci, U. A review of deep learning and radiomics approaches for pancreatic cancer diagnosis from medical imaging. Curr. Opin. Gastroenterol. 2023, 39, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.H.; Yu, E.S.; Kim, M.H.; Kim, J.; Byun, J.H.; Lee, S.S.; Hwang, H.J.; Hwang, J.Y.; Lee, M.G. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: Frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology 2010, 257, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Strobel, O.; Hank, T.; Hinz, U.; Bergmann, F.; Schneider, L.; Springfeld, C.; Jäger, D.; Schirmacher, P.; Hackert, T.; Büchler, M.W. Pancreatic Cancer Surgery: The New R-status Counts. Ann. Surg. 2017, 265, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, T.A.; Javed, S.; Sarmadi, T.; Pandol, S.J.; Li, D. Artificial intelligence and imaging for risk prediction of pancreatic cancer: A narrative review. Chin. Clin. Oncol. 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Soyer, P.; Fishman, E.K.; Rowe, S.P.; Patlas, M.N.; Chassagnon, G. Does artificial intelligence surpass the radiologist? Diagn. Interv. Imaging 2022, 103, 445–447. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Mori, M.; Benedetti, G.; Partelli, S.; Broggi, S.; Cattaneo, G.M.; Palumbo, D.; Muffatti, F.; Falconi, M.; De Cobelli, F.; et al. Robustness of CT radiomic features against image discretization and interpolation in characterizing pancreatic neuroendocrine neoplasms. Phys. Med. 2020, 76, 125–133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacella, G.; Brunese, M.C.; D’Imperio, E.; Rotondo, M.; Scacchi, A.; Carbone, M.; Guerra, G. Pancreatic Ductal Adenocarcinoma: Update of CT-Based Radiomics Applications in the Pre-Surgical Prediction of the Risk of Post-Operative Fistula, Resectability Status and Prognosis. J. Clin. Med. 2023, 12, 7380. https://doi.org/10.3390/jcm12237380
Pacella G, Brunese MC, D’Imperio E, Rotondo M, Scacchi A, Carbone M, Guerra G. Pancreatic Ductal Adenocarcinoma: Update of CT-Based Radiomics Applications in the Pre-Surgical Prediction of the Risk of Post-Operative Fistula, Resectability Status and Prognosis. Journal of Clinical Medicine. 2023; 12(23):7380. https://doi.org/10.3390/jcm12237380
Chicago/Turabian StylePacella, Giulia, Maria Chiara Brunese, Eleonora D’Imperio, Marco Rotondo, Andrea Scacchi, Mattia Carbone, and Germano Guerra. 2023. "Pancreatic Ductal Adenocarcinoma: Update of CT-Based Radiomics Applications in the Pre-Surgical Prediction of the Risk of Post-Operative Fistula, Resectability Status and Prognosis" Journal of Clinical Medicine 12, no. 23: 7380. https://doi.org/10.3390/jcm12237380
APA StylePacella, G., Brunese, M. C., D’Imperio, E., Rotondo, M., Scacchi, A., Carbone, M., & Guerra, G. (2023). Pancreatic Ductal Adenocarcinoma: Update of CT-Based Radiomics Applications in the Pre-Surgical Prediction of the Risk of Post-Operative Fistula, Resectability Status and Prognosis. Journal of Clinical Medicine, 12(23), 7380. https://doi.org/10.3390/jcm12237380





