Trabecular Bone Score (TBS) in Individuals with Type 2 Diabetes Mellitus: An Updated Review
Abstract
:1. Introduction
1.1. The Scientific Background of Why the Study Topic Must Be Conducted
1.2. Aim
2. Methods
3. TBS as Practical Tool to Assess the Bone Health of Adults with T2DM
3.1. Sample-Based Study Regarding TBS in Diabetic Bone Disease
3.2. Lower TBS and Higher BMD in T2DM
3.3. Glucose Profile and Diabetes Characteristics: TBS Changes
3.4. Metabolic Components-Associated Impact on Bone Quality
3.5. Bone Turnover Markers in Diabetes-Induced Osteoporosis
3.6. Interventional Approach for Osteoporosis in T2DM Patients
4. Discussions
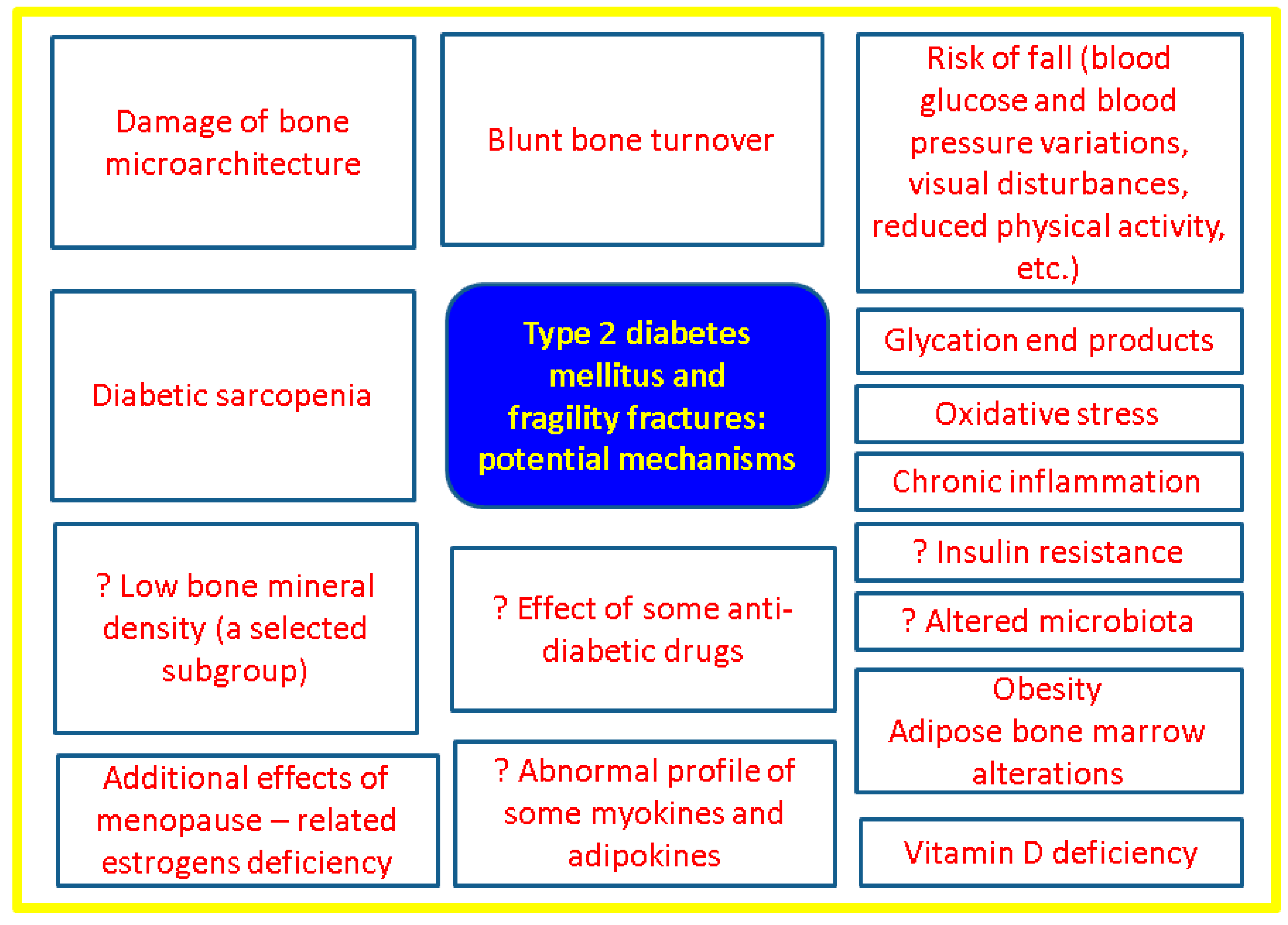
4.1. The Spectrum of “Sweet Bones”
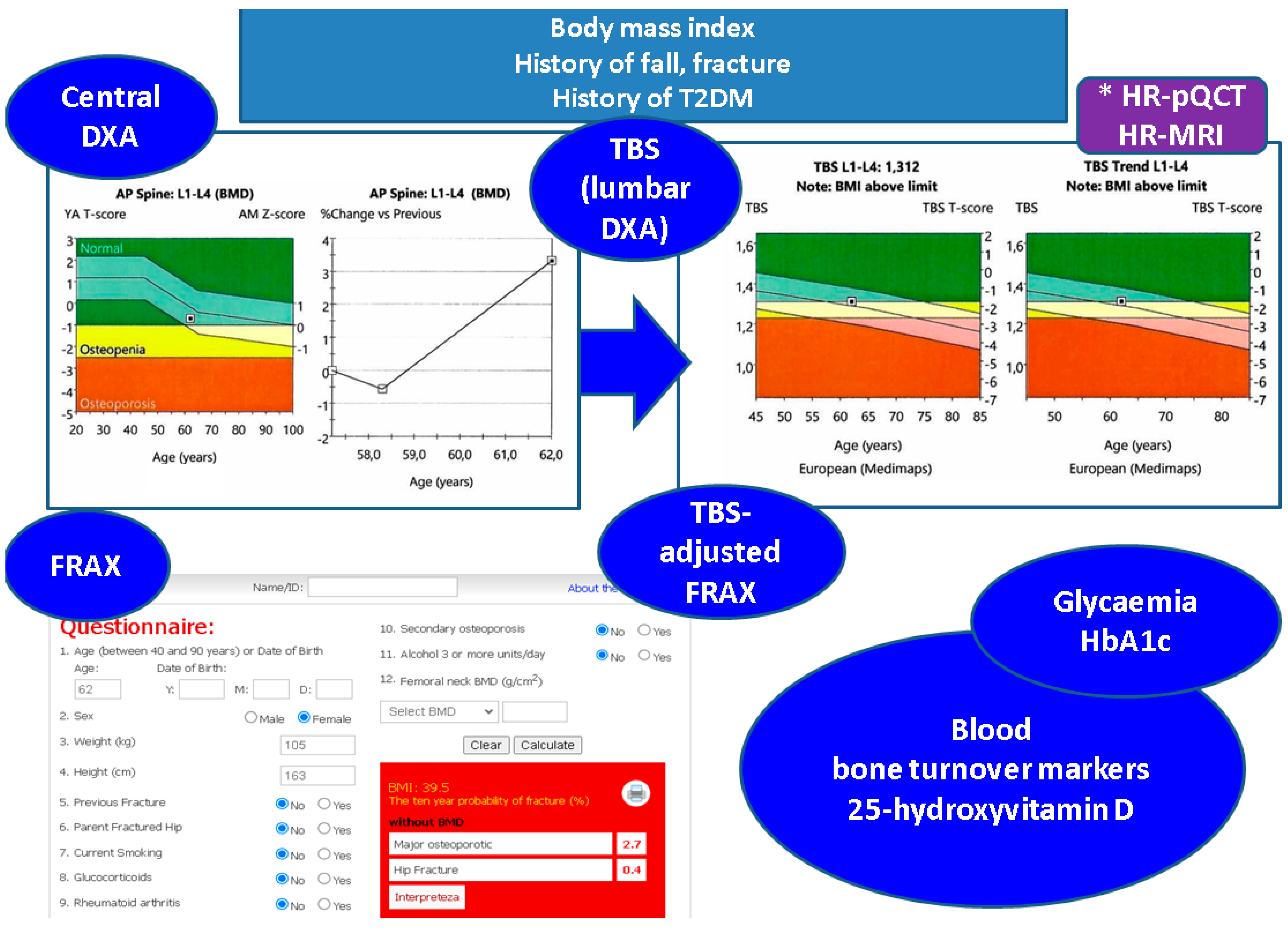
4.2. Integrating TBS to the Panel of Bone Status Assessment in T2DM
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGE | advanced glycation end products |
| BMD | bone mineral density |
| DPP | Dipeptidyl peptidase |
| DXA | Dual-Energy X-ray Absorptiometry |
| DALY | disability-adjusted life year |
| FRAX | Fracture Risk Assessment Tool |
| GLP | glucagon-like peptide |
| HR-pQCT | high-resolution peripheral quantitative computed tomography |
| HR-MRI | high-resolution magnetic resonance imaging |
| HDL | high-density lipoprotein |
| ISCD | The International Society for Clinical Densitometry |
| IGF | Insulin-like Growth Factor |
| N | number of patients |
| OR | odds ratio |
| PTH | parathormone |
| RANKL | receptor activator of NF-κB ligand |
| T1DM | type 1 diabetes mellitus |
| T2DM | type 2 diabetes mellitus |
| TBS | trabecular bone score |
| TSH | Thyroid Stimulating Hormone |
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Mousa, M.; Abdelmannan, D.; Tay, G.; Hassoun, A.; Alsafar, H. Microvascular and macrovascular complications of type 2 diabetes mellitus: Exome wide association analyses. Front. Endocrinol. 2023, 14, 1143067. [Google Scholar] [CrossRef]
- Schousboe, J.T.; Morin, S.N.; Kline, G.A.; Lix, L.M.; Leslie, W.D. Differential risk of fracture attributable to type 2 diabetes mellitus according to skeletal site. Bone 2022, 154, 116220. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Z.; Poundarik, A.A.; Zaki, M.J.; Bockman, R.S.; Glicksberg, B.S.; Nadkarni, G.N.; Vashishth, D. Unmasking Fracture Risk in Type 2 Diabetes: The Association of Longitudinal Glycemic Hemoglobin Level and Medications. J. Clin. Endocrinol. Metab. 2022, 107, e1390–e1401. [Google Scholar] [CrossRef] [PubMed]
- Koromani, F.; Oei, L.; Shevroja, E.; Trajanoska, K.; Schoufour, J.; Muka, T.; Franco, O.H.; Ikram, M.A.; Zillikens, M.C.; Uitterlinden, A.G.; et al. Vertebral Fractures in Individuals with Type 2 Diabetes: More Than Skeletal Complications Alone. Diabetes Care 2020, 43, 137–144. [Google Scholar] [CrossRef]
- Koromani, F.; Ghatan, S.; van Hoek, M.; Zillikens, M.C.; Oei, E.H.G.; Rivadeneira, F.; Oei, L. Type 2 Diabetes Mellitus and Vertebral Fracture Risk. Curr. Osteoporos. Rep. 2021, 19, 50–57. [Google Scholar] [CrossRef]
- Thong, E.P.; Milat, F.; Enticott, J.C.; Joham, A.E.; Ebeling, P.R.; Mishra, G.D.; Teede, H.J. The diabetes-fracture association in women with type 1 and type 2 diabetes is partially mediated by falls: A 15-year longitudinal study. Osteoporos. Int. 2021, 32, 1175–1184. [Google Scholar] [CrossRef]
- Dou, J.; Wang, J.; Zhang, Q. Differences in the roles of types 1 and 2 diabetes in the susceptibility to the risk of fracture: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2021, 13, 84. [Google Scholar] [CrossRef]
- Tebé, C.; Martínez-Laguna, D.; Carbonell-Abella, C.; Reyes, C.; Moreno, V.; Diez-Perez, A.; Collins, G.; Prieto-Alhambra, D. The association between type 2 diabetes mellitus, hip fracture, and post-hip fracture mortality: A multi-state cohort analysis. Osteoporos. Int. 2019, 30, 2407–2415. [Google Scholar] [CrossRef]
- Arceo-Mendoza, R.M.; Camacho, P.M. Postmenopausal Osteoporosis: Latest Guidelines. Endocrinol. Metab. Clin. North Am. 2021, 50, 167–178. [Google Scholar] [CrossRef]
- Hart, N.H.; Newton, R.U.; Tan, J.; Rantalainen, T.; Chivers, P.; Siafarikas, A.; Nimphius, S. Biological basis of bone strength: Anatomy, physiology and measurement. J. Musculoskelet Neuronal Interact. 2020, 20, 347–371. [Google Scholar] [PubMed]
- Sihota, P.; Yadav, R.N.; Dhaliwal, R.; Bose, J.C.; Dhiman, V.; Neradi, D.; Karn, S.; Sharma, S.; Aggarwal, S.; Goni, V.G.; et al. Investigation of Mechanical, Material, and Compositional Determinants of Human Trabecular Bone Quality in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, e2271–e2289. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.; Glüer, C.; Binkley, N.; McCloskey, E.; Brandi, M.-L.; Cooper, C.; Kendler, D.; Lamy, O.; Laslop, A.; Camargos, B.; et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 2015, 78, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.H.; Hong, N.; Kim, J.-W.; Kim, D.Y.; Kim, J.H. Application of the Trabecular Bone Score in Clinical Practice. J. Bone Metab. 2021, 28, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zheng, L.; Theopold, J.; Schleifenbaum, S.; Heyde, C.-E.; Osterhoff, G. Methods for bone quality assessment in human bone tissue: A systematic review. J. Orthop. Surg. Res. 2022, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Mao, M.; Fang, J.; Xie, Y.; Rui, Y. Fracture risk assessment in diabetes mellitus. Front. Endocrinol. 2022, 13, 961761. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Oden, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Barkmann, R.; Boutroy, S.; Brown, J.; Chapurlat, R.; et al. A meta-analysis oftrabecular bone score in fracture risk prediction and its relationship to FRAX. J. Bone Miner. Res. 2016, 31, 940–948. [Google Scholar] [CrossRef]
- Schousboe, J.T.; Vo, T.; Taylor, B.C.; Cawthon, P.M.; Schwartz, A.V.; Bauer, D.C.; Orwoll, E.S.; Lane, N.E.; Barrett-Connor, E.; Ensrud, K.E.; et al. Prediction of Incident Major Osteoporotic and Hip Fractures by Trabecular Bone Score (TBS) and Prevalent Radiographic Vertebral Fracture in Older Men. J. Bone Miner. Res. 2016, 31, 690–697. [Google Scholar] [CrossRef]
- Ali, D.; Tencerova, M.; Figeac, F.; Kassem, M.; Jafari, A. The pathophysiology of osteoporosis in obesity and type 2 diabetes in aging women and men: The mechanisms and roles of increased bone marrow adiposity. Front. Endocrinol. 2022, 13, 981487. [Google Scholar] [CrossRef]
- Available online: https://www.osteoporosis.foundation/policy-makers/burden-osteoporosis (accessed on 6 October 2023).
- Shoback, D.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Eastell, R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J. Clin. Endocrinol. Metab. 2020, 105, dgaa048. [Google Scholar] [CrossRef]
- Eastell, R.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Shoback, D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1595–1622. [Google Scholar] [CrossRef]
- Moayyeri, A.; Warden, J.; Han, S.; Suh, H.; Pinedo-Villanueva, R.; Harvey, N.; Curtis, J.; Silverman, S.; Multani, J.; Yeh, E. Estimating the economic burden of osteoporotic fractures in a multinational study: A real-world data perspective. Osteoporos. Int. 2023, 34, 2121–2132. [Google Scholar] [CrossRef]
- Yeh, E.J.; Rajkovic-Hooley, O.; Silvey, M.; Ambler, W.S.; Milligan, G.; Pinedo-Villanueva, R.; Harvey, N.C.; Moayyeri, A. Impact of fragility fractures on activities of daily living and productivity in community-dwelling women: A multi-national study. Osteoporos. Int. 2023, 34, 1751–1762. [Google Scholar] [CrossRef]
- Harvey, N.C.; Poole, K.E.; Ralston, S.H.; McCloskey, E.V.; Sangan, C.B.; Wiggins, L.; Jones, C.; Gittoes, N.; Compston, J.; Abrahamsen, B.; et al. Towards a cure for osteoporosis: The UK Royal Osteoporosis Society (ROS) Osteoporosis Research Roadmap. Arch. Osteoporos. 2022, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes–Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Leslie, R.D.; Ma, R.C.W.; Franks, P.W.; Nadeau, K.J.; Pearson, E.R.; Redondo, M.J. Understanding diabetes heterogeneity: Key steps towards precision medicine in diabetes. Lancet Diabetes Endocrinol. 2023, 11, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ma, Y.; Luo, Y.; Song, Y.; Xiong, G.; Ma, Y.; Sun, X.; Kan, C. Metabolic diseases and healthy aging: Identifying environmental and behavioral risk factors and promoting public health. Front. Public Health 2023, 11, 1253506. [Google Scholar] [CrossRef] [PubMed]
- Dyrek, N.; Wikarek, A.; Niemiec, M.; Kocełak, P. Selected musculoskeletal disorders in patients with thyroid dysfunction, diabetes, and obesity. Rheumatologia 2023, 61, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Eastell, R.; Pierroz, D.D.; Lane, N.E.; Al-Daghri, N.; Suzuki, A.; Napoli, N.; Mithal, A.; Chakhtoura, M.; Fuleihan, G.E.-H.; et al. Biochemical Markers of Bone Fragility in Patients with Diabetes. J. Clin. Endocrinol. Metab. 2023, 108, dgad255. [Google Scholar] [CrossRef]
- Nistor, C.E.; Ciuche, A.; Cucu, A.P.; Nitipir, C.; Slavu, C.; Serban, B.; Cursaru, A.; Cretu, B.; Cirstoiu, C. Management of Lung Cancer Presenting with Solitary Bone Metastasis. Medicina 2022, 58, 1463. [Google Scholar] [CrossRef]
- Rubin, M.R. Bone Cells and Bone Turnover in Diabetes Mellitus. Curr. Osteoporos. Rep. 2015, 13, 186–191. [Google Scholar] [CrossRef]
- Rubin, M.R. Skeletal fragility in diabetes. Ann. N. Y. Acad. Sci. 2017, 1402, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.D.; Rubin, M.R.; Schwartz, A.V.; Kanis, J.A. Type 2 diabetes and bone. J. Bone Miner. Res. 2012, 27, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Samakkarnthai, P.; Monroe, D.G.; Farr, J.N. Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Cortet, B.; Lucas, S.; Legroux-Gerot, I.; Penel, G.; Chauveau, C.; Paccou, J. Bone disorders associated with diabetes mellitus and its treatments. Jt. Bone Spine 2019, 86, 315–320. [Google Scholar] [CrossRef]
- Lekkala, S.; Taylor, E.A.; Hunt, H.B.; Donnelly, E. Effects of Diabetes on Bone Material Properties. Curr. Osteoporos. Rep. 2019, 17, 455–464. [Google Scholar] [CrossRef]
- Bonaccorsi, G.; Messina, C.; Cervellati, C.; Maietti, E.; Medini, M.; Rossini, M.; Massari, L.; Greco, P. Fracture risk assessment in postmenopausal women with diabetes: Comparison between DeFRA and FRAX tools. Gynecol. Endocrinol. 2018, 34, 404–408. [Google Scholar] [CrossRef]
- El Asri, M.M.; Rodrigo, E.P.; de la Flor, S.D.-S.; Valdivieso, S.P.; Barrón, M.C.R.; Martínez, J.M.O.; Hernández, J.L.H. Trabecular bone score and 25-hydroxyvitamin D levels in microvascular complications of type 2 diabetes mellitus. Med. Clin. 2022, 158, 308–314. [Google Scholar] [CrossRef]
- Ballato, E.; Deepika, F.N.U.; Russo, V.; Fleires-Gutiérrez, A.; Colleluori, G.; Fuenmayor, V.; Chen, R.; Villareal, D.T.; Qualls, C.; Armamento-Villareal, R. One-Year Mean A1c of > 7% is Associated with Poor Bone Microarchitecture and Strength in Men with Type 2 Diabetes Mellitus. Calcif. Tissue Int. 2022, 111, 267–278. [Google Scholar] [CrossRef]
- Fazullina, O.N.; Korbut, A.I.; Klimontov, V.V. Factors associated with trabecular bone score in postmenopausal women with type 2 diabetes and normal bone mineral density. World J. Diabetes 2022, 13, 553–565. [Google Scholar] [CrossRef]
- Gharibzadeh, S.; Goodarzi, G.; Tehrani, S.S.; Fahimfar, N.; Razi, F.; Sanjari, M.; Khalagi, K.; Shafiee, G.; Heshmat, R.; Amini, A.; et al. Bone mass and microarchitecture in T2DM patients and corticosteroids therapy: The Bushehr Elderly Health program. J. Diabetes Metab. Disord. 2022, 21, 717–725. [Google Scholar] [CrossRef]
- Haeri, N.S.; Kotlarczyk, M.P.; Perera, S.; Greenspan, S.L. Diabetes Mellitus is Associated with Poor Bone Microarchitecture in Older Adults Residing in Long-Term Care Facilities. J. Osteoporos. 2022, 2022, 2522014. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.M.; Lim, S.; Kang, M.-I.; Baek, K.-H.; Min, Y.-K. Efficacy of bisphosphonate therapy on postmenopausal osteoporotic women with and without diabetes: A prospective trial. BMC Endocr. Disord. 2022, 22, 99. [Google Scholar] [CrossRef]
- Palomo, T.; Dreyer, P.; Muszkat, P.; Weiler, F.G.; Bonansea, T.C.; Domingues, F.C.; Vieira, J.G.; Silva, B.C.; Brandão, C.M. Effect of soft tissue noise on trabecular bone score in postmenopausal women with diabetes: A cross sectional study. Bone 2022, 157, 116339. [Google Scholar] [CrossRef]
- Dule, S.; Barchetta, I.; Cimini, F.A.; Passarella, G.; Dellanno, A.; Filardi, T.; Venditti, V.; Bleve, E.; Bailetti, D.; Romagnoli, E.; et al. Reduced High-Density Lipoprotein Cholesterol Is an Independent Determinant of Altered Bone Quality in Women with Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 6474. [Google Scholar] [CrossRef]
- Ubago-Guisado, E.; Moratalla-Aranda, E.; González-Salvatierra, S.; Gil-Cosano, J.J.; García-Fontana, B.; García-Fontana, C.; Gracia-Marco, L.; Muñoz-Torres, M. Do patients with type 2 diabetes have impaired hip bone microstructure? A study using 3D modeling of hip dual-energy X-ray absorptiometry. Front. Endocrinol. 2023, 13, 1069224. [Google Scholar] [CrossRef]
- Merugu, C.; Sahoo, J.; Kamalanathan, S.; Ramkumar, G.; Reddy, S.V.B.; Kar, S.S.; Naik, D.; Roy, A.; Narayanan, N.; Patel, D.; et al. Effect of a single dose of zoledronic acid on bone mineral density and trabecular bone score in Indian postmenopausal osteoporotic women with and without type 2 diabetes mellitus—A prospective cohort pilot study. Endocrine 2023, 82, 171–180. [Google Scholar] [CrossRef]
- Naseri, A.; Shojaeefard, E.; Bakhshayeshkaram, M.; Heydari, S.T.; Talezadeh, P.; Farhadi, M.; Nikkhah, A.; Dabbaghmanesh, M.H. Hip structural analysis, trabecular bone score, and bone mineral density in post-menopausal women with type-2 diabetes mellitus: A multi-center cross-sectional study in the south of Iran. Arch. Osteoporos. 2023, 18, 1–8. [Google Scholar] [CrossRef]
- Shevroja, E.; Lamy, O.; Kohlmeier, L.; Koromani, F.; Rivadeneira, F.; Hans, D. Use of Trabecular Bone Score (TBS) as a Complementary Approach to Dual-energy X-ray Absorptiometry (DXA) for Fracture Risk Assessment in Clinical Practice. J. Clin. Densitom. 2017, 20, 334–345. [Google Scholar] [CrossRef]
- Shevroja, E.; Cafarelli, F.P.; Guglielmi, G.; Hans, D. DXA parameters, Trabecular Bone Score (TBS) and Bone Mineral Density (BMD), in fracture risk prediction in endocrine-mediated secondary osteoporosis. Endocrine 2021, 74, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Redondo, L.; Puigoriol, E.; Rodríguez, J.; Peris, P.; Kanterewicz, E. Usefulness of the Trabecular Bone Score for assessing the risk of osteoporotic fracture. Rev. Clin. Esp. 2018, 218, 121–127. [Google Scholar] [CrossRef]
- Jackuliak, P.; Kužma, M.; Killinger, Z.; Payer, J. Good Long-Term Glycemic Compensation is Associated with Better Trabecular Bone Score in Postmenopausal Women with Type 2 Diabetes. Physiol. Res. 2019, 68, S149–S156. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, R.; Cibula, D.; Ghosh, C.; Weinstock, R.S.; Moses, A.M. Bone quality assessment in type 2 diabetes mellitus. Osteoporos. Int. 2014, 25, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Nordklint, A.K.; Almdal, T.P.; Vestergaard, P.; Lundby-Christensen, L.; Boesgaard, T.W.; Breum, L.; Gade-Rasmussen, B.; Sneppen, S.B.; Gluud, C.; Hemmingsen, B.; et al. The effect of metformin versus placebo in combination with insulin analogues on bone mineral density and trabecular bone score in patients with type 2 diabetes mellitus: A randomized placebo-controlled trial. Osteoporos. Int. 2018, 29, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-P.; Kuo, S.-F.; Lin, Y.-C.; Fan, C.-M.; Chen, J.-F. Status of bone strength and factors associated with vertebral fracture in postmenopausal women with type 2 diabetes. Menopause 2019, 26, 182–188. [Google Scholar] [CrossRef]
- Lambrinoudaki, I.; Paschou, S.A.; Armeni, E.; Goulis, D.G. The interplay between diabetes mellitus and menopause: Clinical implications. Nat. Rev. Endocrinol. 2022, 18, 608–622. [Google Scholar] [CrossRef]
- Silva, B.C.; Broy, S.B.; Boutroy, S.; Schousboe, J.T.; Shepherd, J.A.; Leslie, W.D. Fracture Risk Prediction by Non-BMD DXA Measures: The 2015 ISCD Official Positions Part 2: Trabecular Bone Score. J. Clin. Densitom. 2015, 18, 309–330. [Google Scholar] [CrossRef]
- Pothuaud, L.; Barthe, N.; Krieg, M.-A.; Mehsen, N.; Carceller, P.; Hans, D. Evaluation of the Potential Use of Trabecular Bone Score to Complement Bone Mineral Density in the Diagnosis of Osteoporosis: A Preliminary Spine BMD–Matched, Case-Control Study. J. Clin. Densitom. 2009, 12, 170–176. [Google Scholar] [CrossRef]
- Rabier, B.; Héraud, A.; Grand-Lenoir, C.; Winzenrieth, R.; Hans, D. A multicentre, retrospective case–control study assessing the role of trabecular bone score (TBS) in menopausal Caucasian women with low areal bone mineral density (BMDa): Analysing the odds of vertebral fracture. Bone 2010, 46, 176–181. [Google Scholar] [CrossRef]
- Hans, D.; Goertzen, A.L.; Krieg, M.-A.; Leslie, W.D. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: The manitoba study. J. Bone Miner. Res. 2011, 26, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Boutroy, S.; Hans, D.; Sornay-Rendu, E.; Vilayphiou, N.; Winzenrieth, R.; Chapurlat, R. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: The OFELY study. Osteoporos. Int. 2013, 24, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Winzenrieth, R.; Dufour, R.; Pothuaud, L.; Hans, D. A Retrospective Case–Control Study Assessing the Role of Trabecular Bone Score in Postmenopausal Caucasian Women with Osteopenia: Analyzing the Odds of Vertebral Fracture. Calcif. Tissue Int. 2010, 86, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimpur, M.; Sharifi, F.; Nezhad, F.A.; Bagherzadeh, M.; Ostovar, A.; Shafiee, G.; Heshmat, R.; Mehrdad, N.; Razi, F.; Khashayar, P.; et al. Effect of diabetes on BMD and TBS values as determinants of bone health in the elderly: Bushehr Elderly Health program. J. Diabetes Metab. Disord. 2019, 18, 99–106. [Google Scholar] [CrossRef]
- Gani, L.U.; Saripalli, K.R.; Fernandes, K.; Leong, S.F.; Tsai, K.T.; Tan, P.T.; Chong, L.R.; King, T.F.J. Bone mineral density and trabecular bone score in elderly type 2 diabetes Southeast Asian patients with severe osteoporotic hip fractures. PLoS ONE 2020, 15, e0241616. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Montoro, J.I.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. Evaluation of Quality and Bone Microstructure Alterations in Patients with Type 2 Diabetes: A Narrative Review. J. Clin. Med. 2022, 11, 2206. [Google Scholar] [CrossRef]
- Ruaro, B.; Casabella, A.; Paolino, S.; Pizzorni, C.; Alessandri, E.; Seriolo, C.; Botticella, G.; Molfetta, L.; Odetti, P.; Smith, V.; et al. Correlation between bone quality and microvascular damage in systemic sclerosis patients. Rheumatology 2018, 57, 1548–1554. [Google Scholar] [CrossRef]
- Namwongprom, S.; Rojanasthien, S.; Wongboontan, C.; Mangklabruks, A. Contribution of Android and Gynoid Adiposity to Bone Mineral Density in Healthy Postmenopausal Thai Women. J. Clin. Densitom. 2019, 22, 346–350. [Google Scholar] [CrossRef]
- Messina, C.; Buonomenna, C.; Menon, G.; Magnani, S.; Albano, D.; Gitto, S.; Ulivieri, F.M.; Sconfienza, L.M. Fat Mass Does Not Increase the Precision Error of Trabecular Bone Score Measurements. J. Clin. Densitom. 2019, 22, 359–366. [Google Scholar] [CrossRef]
- Stokar, J.; Ben-Porat, T.; Kaluti, D.; Abu-Gazala, M.; Weiss, R.; Mintz, Y.; Elazari, R.; Szalat, A. Trabecular Bone Score Preceding and during a 2-Year Follow-Up after Sleeve Gastrectomy: Pitfalls and New Insights. Nutrients 2023, 15, 3481. [Google Scholar] [CrossRef]
- Aharon-Hananel, G.; Zacay, G.; Tau, N.; Levy-Shraga, Y.; Tirosh, A.; Vered, I.; Tripto-Shkolnik, L. Trabecular Bone Score Change Is Not Predicted by Bone Turnover: Short-term Sequential Follow-up. Isr. Med. Assoc. J. 2023, 25, 438–442. [Google Scholar] [PubMed]
- Jiang, N.; Xia, W. Assessment of bone quality in patients with diabetes mellitus. Osteoporos. Int. 2018, 29, 1721–1736. [Google Scholar] [CrossRef]
- Ferrari, S.; Abrahamsen, B.; Napoli, N.; Akesson, K.; Chandran, M.; Eastell, R.; Fuleihan, G.E.-H.; Josse, R.; Kendler, D.; Kraenzlin, M.; et al. Diagnosis and management of bone fragility in diabetes: An emerging challenge. Osteoporos. Int. 2018, 29, 2585–2596. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.E.; Staden, R.S.; Dumitru, A.V.; Stanciu Găvan, C. A Screening Test for Early Diagnosis of Microcellular Bronchopulmonary Cancer-Pilot Study. J Clin Med. 2019, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Cozadd, A.J.; Schroder, L.K.; Switzer, J.A. Fracture Risk Assessment: An Update. J. Bone Jt. Surg. 2021, 103, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Chen, C. Effects of Bisphosphonate on Osteocyte Proliferation and Bone Formation in Patients with Diabetic Osteoporosis. Comput. Math. Methods Med. 2022, 2022, 2368564. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.V.; Rajan, R.; Cherian, K.; Kapoor, N. Trabecular bone score—An emerging tool in the management of osteoporosis. Indian J. Endocrinol. Metab. 2020, 24, 237–243. [Google Scholar] [CrossRef]
- Alaofè, H.; Amoussa Hounkpatin, W.; Djrolo, F.; Ehiri, J.; Rosales, C. Factors Associated with Quality of Life in Patients with Type 2 Diabetes of South Benin: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 2360. [Google Scholar] [CrossRef]
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Davies, J.; Vollmer, S. Global Economic Burden of Diabetes in Adults: Projections From 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Chan, D.-C.; Lee, J.-K.; Tabu, I.; Alpuerto, B.B. The global burden of fragility fractures—What are the differences, and where are the gaps. Best Pract. Res. Clin. Rheumatol. 2022, 36, 101777. [Google Scholar] [CrossRef]
- Schacter, G.I.; Leslie, W.D. Diabetes and Osteoporosis: Part I, Epidemiology and Pathophysiology. Endocrinol. Metab. Clin. North Am. 2021, 50, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; Cherian, K.E.; Nandyal, M.B.; Jiwanmall, S.A.; Kattula, D.; Paul, T.V.; Kapoor, N. Trabecular Bone Score and Bone Mineral Density in Postmenopausal Women with Morbid Obesity—A Clinical Paradox. Med. Sci. 2021, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Halper-Stromberg, E.; Gallo, T.; Champakanath, A.; Taki, I.; Rewers, M.; Snell-Bergeon, J.; Frohnert, B.I.; Shah, V.N. Bone Mineral Density across the Lifespan in Patients with Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2020, 105, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Syversen, U.; Mosti, M.P.; Mynarek, I.M.; Vedal, T.S.J.; Aasarød, K.; Basso, T.; Reseland, J.E.; Thorsby, P.M.; Asvold, B.O.; Eriksen, E.F.; et al. Evidence of impaired bone quality in men with type 1 diabetes: A cross-sectional study. Endocr. Connect. 2021, 10, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, N.H.-H.; Dal, J.; Kvist, A.V.; Bergh, J.P.v.D.; Jensen, M.H.; Vestergaard, P. Bone parameters in T1D and T2D assessed by DXA and HR-pQCT—A cross-sectional study: The DIAFALL study. Bone 2023, 172, 116753. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, T.; Silverman, E.; Akhter, M.P.; Lyden, E.; Recker, R.R.; Graeff-Armas, L.A. Trabecular bone score and transilial bone trabecular histomorphometry in type 1 diabetes and healthy controls. Bone 2020, 137, 115451. [Google Scholar] [CrossRef]
- Neumann, T.; Lodes, S.; Kästner, B.; Lehmann, T.; Hans, D.; Lamy, O.; Müller, U.A.; Wolf, G.; Sämann, A. Trabecular bone score in type 1 diabetes—A cross-sectional study. Osteoporos. Int. 2016, 27, 127–133. [Google Scholar] [CrossRef]
- Shah, V.N.; Sippl, R.; Joshee, P.; Pyle, L.; Kohrt, W.M.; Schauer, I.E.; Snell-Bergeon, J.K. Trabecular bone quality is lower in adults with type 1 diabetes and is negatively associated with insulin resistance. Osteoporos. Int. 2018, 29, 733–739. [Google Scholar] [CrossRef]
- Carballido-Gamio, J. Imaging techniques to study diabetic bone disease. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 350–360. [Google Scholar] [CrossRef]
- de Araújo, I.M.; Moreira, M.L.M.; de Paula, F.J.A. Diabetes and bone. Arch. Endocrinol. Metab. 2022, 66, 633–641. [Google Scholar] [CrossRef]
- El-Tawdy, A.H.F.; Ibrahim, E.A.H.; Al Sakhawy, E.M.A.; Morsy, T.A. Review on bone disease (osteoporosis) in diabetes mellitus. J. Egypt Soc. Parasitol. 2017, 47, 35–46. [Google Scholar] [CrossRef]
- Palomo, T.; Muszkat, P.; Weiler, F.G.; Dreyer, P.; Brandão, C.M.A.; Silva, B.C. Update on trabecular bone score. Arch. Endocrinol. Metab. 2022, 66, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Yamauchi, M.; Sugimoto, T. Prevalent vertebral fracture is dominantly associated with spinal microstructural deterioration rather than bone mineral density in patients with type 2 diabetes mellitus. PLoS ONE 2019, 14, e0222571. [Google Scholar] [CrossRef] [PubMed]
- Ho-Pham, L.T.; Nguyen, T.V. Association between trabecular bone score and type 2 diabetes: A quantitative update of evidence. Osteoporos. Int. 2019, 30, 2079–2085. [Google Scholar] [CrossRef]
- Vilaca, T.; Schini, M.; Harnan, S.; Sutton, A.; Poku, E.; Allen, I.E.; Cummings, S.R.; Eastell, R. The risk of hip and non-vertebral fractures in type 1 and type 2 diabetes: A systematic review and meta-analysis update. Bone 2020, 137, 115457. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.B.; Kiel, D.P.; Williams, S.A.; Weiss, R.J.; Samelson, E.J. Risk Factors for Incident Fracture in Older Adults with Type 2 Diabetes: The Framingham Heart Study. Diabetes Care 2021, 44, 1547–1555. [Google Scholar] [CrossRef]
- Hunt, H.B.; Torres, A.M.; Palomino, P.M.; Marty, E.; Saiyed, R.; Cohn, M.; Jo, J.; Warner, S.; Sroga, G.E.; King, K.B.; et al. Altered Tissue Composition, Microarchitecture, and Mechanical Performance in Cancellous Bone from Men with Type 2 Diabetes Mellitus. J. Bone Miner. Res. 2019, 34, 1191–1206. [Google Scholar] [CrossRef]
- Asadipooya, K.; Uy, E.M. Advanced Glycation End Products (AGEs), Receptor for AGEs, Diabetes, and Bone: Review of the Literature. J. Endocr. Soc. 2019, 3, 1799–1818. [Google Scholar] [CrossRef] [PubMed]
- Romero-Díaz, C.; Duarte-Montero, D.; Gutiérrez-Romero, S.A.; Mendivil, C.O. Diabetes and Bone Fragility. Diabetes Ther. 2021, 12, 71–86. [Google Scholar] [CrossRef]
- Eller-Vainicher, C.; Cairoli, E.; Grassi, G.; Grassi, F.; Catalano, A.; Merlotti, D.; Falchetti, A.; Gaudio, A.; Chiodini, I.; Gennari, L. Pathophysiology and Management of Type 2 Diabetes Mellitus Bone Fragility. J. Diabetes Res. 2020, 2020, 7608964. [Google Scholar] [CrossRef]
- Zha, K.; Wang, N.; Zhou, Y.; Ying, R.; Gu, T.; Zhao, Y.; Guo, H.; An, Z.; Lu, Y. Novel Associations of Dyslipidaemia with Vitamin D and Bone Metabolism in Elderly Patients with Diabetes: A Cross-Sectional Study. Diabetes Metab. Syndr. Obes. 2023, 16, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Biver, E.; Brennan-Speranza, T.C. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021, 9, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.K.; Leutscher, P.; Sørensen, S. Gut Microbiota in Bone Health and Diabetes. Curr. Osteoporos. Rep. 2021, 19, 462–479. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Busse, B.; Eastell, R.; Ferrari, S.; Frost, M.; Müller, R.; Burden, A.M.; Rivadeneira, F.; Napoli, N.; Rauner, M. Bone fragility in diabetes: Novel concepts and clinical implications. Lancet Diabetes Endocrinol. 2022, 10, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Guo, Q.; Yang, W.; Wang, Y.; Sun, Z.; Wu, H. Mapping Knowledge Landscapes and Emerging Trends of the Links Between Bone Metabolism and Diabetes Mellitus: A Bibliometric Analysis From 2000 to 2021. Front. Public Health 2022, 10, 918483. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, N.; Carsote, M.; Cocolos, A.; Petrova, E.; Olaru, M.; Dumitrache, C.; Ghemigian, A. The Link Between Bone Osteocalcin and Energy Metabolism in a Group of Postmenopausal Women. Curr. Health Sci. J. 2019, 45, 47–51. [Google Scholar] [PubMed]
- Vitale, J.A.; Sansoni, V.; Faraldi, M.; Messina, C.; Verdelli, C.; Lombardi, G.; Corbetta, S. Circulating Carboxylated Osteocalcin Correlates with Skeletal Muscle Mass and Risk of Fall in Postmenopausal Osteoporotic Women. Front. Endocrinol. 2021, 12, 669704. [Google Scholar] [CrossRef]
- Li, J.; Niu, X.; Si, Q.; Song, Q.; Jin, M.; Zhou, R.; Sun, Y.; Li, J.; Wang, Q. Plasma periostin as a biomarker of osteoporosis in postmenopausal women with type 2 diabetes. J. Bone Miner. Metab. 2021, 39, 631–638. [Google Scholar] [CrossRef]
- Kim, B.-J.; Rhee, Y.; Kim, C.H.; Baek, K.H.; Min, Y.-K.; Kim, D.-Y.; Ahn, S.H.; Kim, H.; Lee, S.H.; Lee, S.-Y.; et al. Plasma periostin associates significantly with non-vertebral but not vertebral fractures in postmenopausal women: Clinical evidence for the different effects of periostin depending on the skeletal site. Bone 2015, 81, 435–441. [Google Scholar] [CrossRef]
- Fang, P.; She, Y.; Han, L.; Wan, S.; Shang, W.; Zhang, Z.; Min, W. A promising biomarker of elevated galanin level in hypothalamus for osteoporosis risk in type 2 diabetes mellitus. Mech. Ageing Dev. 2021, 194, 111427. [Google Scholar] [CrossRef]
- Ungureanu, M.-C.; Bilha, S.C.; Hogas, M.; Velicescu, C.; Leustean, L.; Teodoriu, L.C.; Preda, C. Preptin: A New Bone Metabolic Parameter? Metabolites 2023, 13, 991. [Google Scholar] [CrossRef]
- Wang, X.; Hu, T.; Ruan, Y.; Yao, J.; Shen, H.; Xu, Y.; Zheng, B.; Zhang, Z.; Wang, J.; Tan, Q. The Association of Serum Irisin with Bone Mineral Density and Turnover Markers in New-Onset Type 2 Diabetic Patients. Int. J. Endocrinol. 2022, 2022, 7808393. [Google Scholar] [CrossRef]
- Kawao, N.; Kaji, H. Interactions Between Muscle Tissues and Bone Metabolism. J. Cell. Biochem. 2015, 116, 687–695. [Google Scholar] [CrossRef]
- Faienza, M.F.; Pontrelli, P.; Brunetti, G. Type 2 diabetes and bone fragility in children and adults. World J. Diabetes 2022, 13, 900–911. [Google Scholar] [CrossRef]
- Starup-Linde, J.; Lykkeboe, S.; Handberg, A.; Vestergaard, P.; Høyem, P.; Fleischer, J.; Hansen, T.K.; Poulsen, P.L.; Laugesen, E. Glucose variability and low bone turnover in people with type 2 diabetes. Bone 2021, 153, 116159. [Google Scholar] [CrossRef] [PubMed]
- Vigevano, F.; Gregori, G.; Colleluori, G.; Chen, R.; Autemrongsawat, V.; Napoli, N.; Qualls, C.; Villareal, D.T.; Armamento-Villareal, R. In Men with Obesity, T2DM Is Associated with Poor Trabecular Microarchitecture and Bone Strength and Low Bone Turnover. J. Clin. Endocrinol. Metab. 2021, 106, 1362–1376. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Ock, S.Y.; Chung, Y.-S. Trabecular Bone Score (TBS) and TBS-Adjusted Fracture Risk Assessment Tool are Potential Supplementary Tools for the Discrimination of Morphometric Vertebral Fractures in Postmenopausal Women with Type 2 Diabetes. J. Clin. Densitom. 2016, 19, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Conte, C.; Eastell, R.; Ewing, S.K.; Bauer, D.C.; Strotmeyer, E.S.; Black, D.M.; Samelson, E.J.; Vittinghoff, E.; Schwartz, A.V. Bone Turnover Markers Do Not Predict Fracture Risk in Type 2 Diabetes. J. Bone Miner. Res. 2020, 35, 2363–2371. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Wu, J.; Kuo, S.-F.; Cheung, Y.-C.; Sung, C.-M.; Fan, C.-M.; Chen, F.-P.; Mhuircheartaigh, J.N. Vertebral Fractures in Type 2 Diabetes Patients: Utility of Trabecular Bone Score and Relationship with Serum Bone Turnover Biomarkers. J. Clin. Densitom. 2020, 23, 37–43. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, J.; Guo, G.; Lan, Z.; Li, X.; Pan, Z.; Rao, X.; Zheng, Z.; Luo, F.; Lin, L.; et al. Vitamin D Status Is Negatively Correlated with Insulin Resistance in Chinese Type 2 Diabetes. Int. J. Endocrinol. 2016, 2016, 1794894. [Google Scholar] [CrossRef]
- Bao, K.; Jiao, Y.; Xing, L.; Zhang, F.; Tian, F. The role of wnt signaling in diabetes-induced osteoporosis. Diabetol. Metab. Syndr. 2023, 15, 84. [Google Scholar] [CrossRef]
- Mohsin, S.; Kaimala, S.; Sunny, J.J.; Adeghate, E.; Brown, E.M. Type 2 Diabetes Mellitus Increases the Risk to Hip Fracture in Postmenopausal Osteoporosis by Deteriorating the Trabecular Bone Microarchitecture and Bone Mass. J. Diabetes Res. 2019, 2019, 3876957. [Google Scholar] [CrossRef]
- Lui, D.; Lee, C.; Chan, Y.; Chow, W.; Fong, C.; Siu, D.; Tse, H.; Woo, Y.; Lam, K. HbA1c variability, in addition to mean HbA1c, predicts incident hip fractures in Chinese people with type 2 diabetes. Osteoporos. Int. 2020, 31, 1955–1964. [Google Scholar] [CrossRef]
- Eastell, R.; Vittinghoff, E.; Lui, L.; Ewing, S.K.; Schwartz, A.V.; Bauer, D.C.; Black, D.M.; Bouxsein, M.L. Diabetes Mellitus and the Benefit of Antiresorptive Therapy on Fracture Risk. J. Bone Miner. Res. 2022, 37, 2121–2131. [Google Scholar] [CrossRef]
- Carsote, M.; Paduraru, D.N.; Nica, A.E.; Valea, A. Parathyroidectomy: Is vitamin D a player for a good outcome? J. Med. Life 2016, 4, 348–352. [Google Scholar]
- Anastasilakis, A.D.; Tsourdi, E.; Tabacco, G.; Naciu, A.M.; Napoli, N.; Vescini, F.; Palermo, A. The Impact of Antiosteoporotic Drugs on Glucose Metabolism and Fracture Risk in Diabetes: Good or Bad News? J. Clin. Med. 2021, 10, 996. [Google Scholar] [CrossRef]
- Xiong, Z.; Yi, P.; Tang, X.; Shu, L.; Zhang, C. Meta-Analysis of the Efficacy and Safety of Alendronate Combined with Atorvastatin in the Treatment of Osteoporosis in Diabetes Mellitus. BioMed Res. Int. 2022, 2022, 6747469. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gao, L.; Liu, C.; Bao, X.; Tian, Y.; Li, Y. Denosumab improves glycaemic parameters in postmenopausal osteoporosis patients with combined Type 2 diabetes mellitus. Cell. Mol. Biol. 2023, 69, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Tsou, S.-H.; Kuo, C.-Y.; Chen, W.-L.; Wu, K.-W.; Lin, C.-L.; Huang, C.-N. Denosumab Attenuates Glucolipotoxicity-Induced β-Cell Dysfunction and Apoptosis by Attenuating RANK/RANKL Signals. Int. J. Mol. Sci. 2023, 24, 10289. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Lu, M.; Zhang, Y. The combination of linagliptin and metformin rescues bone loss in type 2 diabetic osteoporosis. J. Drug Target. 2023, 31, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Xu, B.; Ye, Z.; Gao, Y.; Fang, H.; Song, J.; Liang, D.; Liu, L.; Hu, Z.; Zhang, M.; et al. Metformin attenuates diabetes-induced osteopenia in rats is associated with down-regulation of the RAGE-JAK2-STAT1 signal axis. J. Orthop. Transl. 2023, 40, 37–48. [Google Scholar] [CrossRef]
- El-Arabey, A.A.; Abdalla, M. GATA3 as an immunomodulator in obesity-related metabolic dysfunction associated with fatty liver disease, insulin resistance, and type 2 diabetes. Chem. Biol. Interact. 2022, 366, 110141. [Google Scholar] [CrossRef]
- Bathina, S.; Armamento-Villareal, R. The complex pathophysiology of bone fragility in obesity and type 2 diabetes mellitus: Therapeutic targets to promote osteogenesis. Front. Endocrinol. 2023, 14, 1168687. [Google Scholar] [CrossRef] [PubMed]
- Drapkina, O.M.; Elkina, A.Y.; Sheptulina, A.F.; Kiselev, A.R. Non-Alcoholic Fatty Liver Disease and Bone Tissue Metabolism: Current Findings and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 8445. [Google Scholar] [CrossRef] [PubMed]
- Sheu, A.; Greenfield, J.R.; White, C.P.; Center, J.R. Contributors to impaired bone health in type 2 diabetes. Trends Endocrinol. Metab. 2023, 34, 34–48. [Google Scholar] [CrossRef]
- Sheu, A.; Greenfield, J.R.; White, C.P.; Center, J.R. Assessment and treatment of osteoporosis and fractures in type 2 diabetes. Trends Endocrinol. Metab. 2022, 33, 333–344. [Google Scholar] [CrossRef]
- Radecka, A.; Lubkowska, A. The Significance of Dual-Energy X-ray Absorptiometry (DXA) Examination in Cushing’s Syndrome—A Systematic Review. Diagnostics 2023, 13, 1576. [Google Scholar] [CrossRef]
- Sandru, F.; Carsote, M.; Dumitrascu, M.C.; Albu, S.E.; Valea, A. Glucocorticoids and Trabecular Bone Score. J. Med. Life 2020, 13, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.E.; Stanciu-Găvan, C.; Vasilescu, F.; Dumitru, A.V.; Ciuche, A. Attitude of the surgical approach in hyperparathyroidism: A retrospective study. Exp. Ther. Med. 2021, 22, 959. [Google Scholar] [CrossRef]
- Punda, M.; Ovčariček, P.P. Bone health evaluation in primary hyperparathyroidism using dual-energy X-ray absorptiometry and trabecular bone score. Q. J. Nucl. Med. Mol. Imaging 2023, 67, 138–144. [Google Scholar] [CrossRef]
- Ságová, I.; Mokáň, M.; Tonhajzerová, I.; Rončáková, M.; Vaňuga, P. Age, body composition parameters and glycaemic control contribute to trabecular bone score deterioration in acromegaly more than disease activity. Front. Endocrinol. 2023, 14, 1197725. [Google Scholar] [CrossRef] [PubMed]
- Cianferotti, L.; Cipriani, C.; Corbetta, S.; Corona, G.; Defeudis, G.; Lania, A.G.; Messina, C.; Napoli, N.; Mazziotti, G. Bone quality in endocrine diseases: Determinants and clinical relevance. J. Endocrinol. Investig. 2023, 46, 1283–1304. [Google Scholar] [CrossRef] [PubMed]
- Brancatella, A.; Marcocci, C. TSH suppressive therapy and bone. Endocr. Connect. 2020, 9, R158–R172. [Google Scholar] [CrossRef] [PubMed]

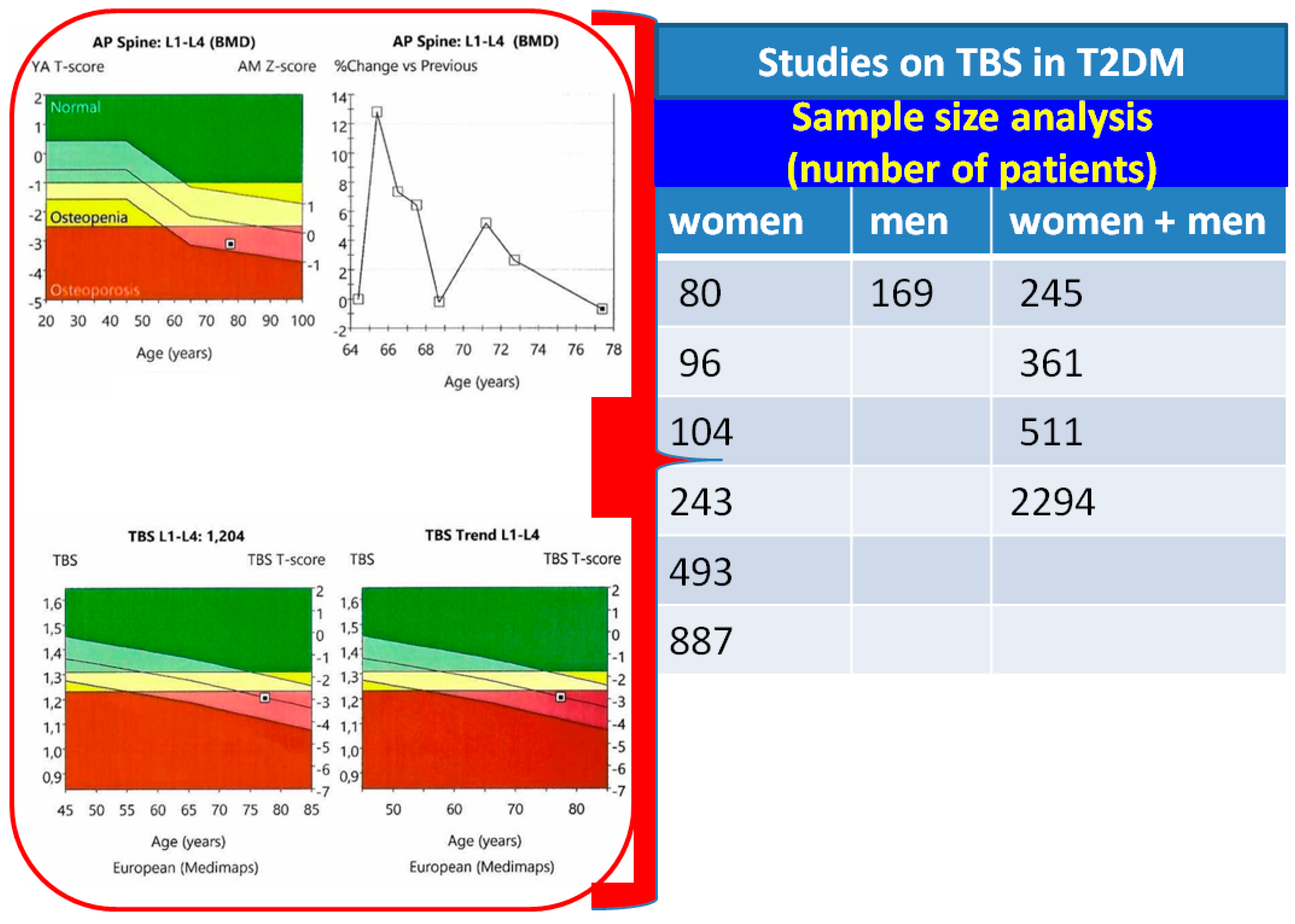
| First Author Year of Publication Reference Number | Study Design Number of Patients Sex ratio (F/M) Age (Years) | Core TBS Findings at Baseline |
|---|---|---|
| Maamar El Asri, 2022 [40] | Cross-sectional study N = 361 with T2DM F/M = 184/177 Mean age: 63.8 y (47–91 y) N1 = 92 with microvascular disease N2 = 269 without microvascular disease | N1: TBS = 1.235 ± 0.1 N2: TBS = 1.287 ± 0.1 Patients with diabetic microvascular disease had a statistically significant lower TBS than the patients without microvascular disease: N1 vs. N2, p = 0.034 |
| Ballato, 2022 [41] | Cross-sectional study N = 169 males Mean age: 51.4 ± 7.5 y (35–65 y) N1 = 91 without T2DM N2 = 26 with HbA1c ≤ 7% N3 = 52 with HbA1c > 7% | N1: TBS = 1.26 ± 0.15 N2: TBS = 1.22 ± 0.12 N3: TBS = 1.21 ± 0.15 No significant differences in TBS among the patients with good vs. poor glycemic control: N1 vs. N2 vs. N3, p = 0.28 |
| Fazullina, 2022 [42] | Cross-sectional study N = 96 postmenopausal females with T2DM and normal BMD Mean age: 64 y (50–75 y) N1 = 53 with TBS > 1.31 N2 = 43 with TBS ≤ 1.31 | N1: TBS = 1.465 (1.39–1.514) N2: TBS = 1.206 (1.127–1.271) Postmenopausal females with T2DM and normal BMD may have impaired bone microarchitecture; a decrease in TBS (≤1.31) was observed in 44.8% of study subjects Prevalence of fractures was higher in N2 group than N1 (32.6% vs. 11.3%, p = 0.02) |
| Gharibzadeh, 2022 [43] | Cross-sectional study N = 2294 F/M = 1182/1112 Mean age: 69.3 ± 6.3 y Females N1 = 412 with T2DM N2 = 770 non-diabetic controls Males N1′ = 314 with T2DM N2′ = 798 non-diabetic controls | Females N1: TBS = 1.23 ± 0.09 N2: TBS = 1.24 ± 0.08 Males N1′: TBS = 1.36 ± 0.09 N2′: TBS = 1.35 ± 0.08 T2DM had a significant effect only in men’s TBS (p = 0.03) |
| Haeri, 2022 [44] | Cohort study N = 511 F/M = 433/78 Mean age females: 80.6 ± 8.0 y Mean age males: 82.4 ± 8.3 y Females N1 = 105 with T2DM N2 = 328 non-diabetic controls Males N1 = 18 with T2DM N2 = 60 non-diabetic controls | Females N1: TBS = 1.211 ± 0.172 N2: TBS = 1.266 ± 0.136 Males N1: TBS = 1.255 ± 0.189 N2: TBS = 1.268 ± 0.132 Diabetic females compared with nondiabetics had lower spine TBS (p = 0.0299), but no differences between groups in males (p = 0.7935) |
| Kim, 2022 [45] | Prospective study N = 104 postmenopausal females N1 = 49 with T2DM Mean age: 73 y (67–79 y) N2 = 55 non-diabetic controls Mean age: 66 y (63–73 y) | N1: TBS = 1.289 ± 0.076 N2: TBS = 1.300 ± 0.058 At baseline, there was no difference in TBS between groups (p = 0.294) |
| Palomo, 2022 [46] | Cross-sectional study N = 493 females Mean age: 71.8 y N1 = 116 with HbA1c ≥ 6.5% N2 = 217 with HbA1c 5.7–6.4% N3 = 160 with HbA1c ≤ 5.6% | N1: TBS = 1.280 ± 0.109 N2: TBS = 1.299 ± 0.093 N3: TBS = 1.314 ± 0.104 TBS was lower in patients with higher HbA1c (p = 0.020) |
| Dule, 2023 [47] | Observational case-control study N = 243 N1 = 126 females with T2DM Mean age: 62.96 ± 6.73 y N2 = 117 non-diabetic controls Mean age: 61.91 ± 5.8 y | N1: TBS = 1.180 ± 0.112 N2: TBS = 1.209 ± 0.120 T2DM was associated with low TBS (OR = 2.47, 95% CI: 1.19–5.16, p = 0.016) in a regression model adjusted for age, menopausal status and BMI |
| Ubago-Guisado, 2023 [48] | Case-control study N = 245 N1 = 111 with T2DM F/M = 48/63 Mean age: 65.4 ± 7.6 y N2 = 134 non-diabetic controls F/M = 65/69 Mean age: 64.7 ± 8.6 y | N1: TBS = 1.074 ± 0.187 N2: TBS = 1.291 ± 0.110 TBS was lower in the T2DM group compared to the controls (p < 0.001) |
| Merugu, 2023 [49] | Prospective cohort study N = 80 N1 = 40 females with T2DM Mean age: 60.5 y (57.2–65 y) N2 = 40 non-diabetic controls Mean age: 57.5 y (53–64.7 y) | N1: TBS = 1.24 ± 0.07 N2: TBS = 1.26 ± 0.08 At baseline, TBS was similar between groups (p = 0.25) |
| Naseri, 2023 [50] | Cross-sectional study N = 887 N1 = 348 postmenopausal females Mean age: 55.13 ± 6.61 y N2 = 539 non-diabetic controls Mean age: 55.13 ± 6.61 y | N1: TBS = 1.280 ± 0.111 N2: TBS = 1.343 ± 0.101 TBS was statistically significantly lower in diabetic subjects than in non-diabetic controls (p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trandafir, A.-I.; Sima, O.-C.; Gheorghe, A.-M.; Ciuche, A.; Cucu, A.-P.; Nistor, C.; Carsote, M. Trabecular Bone Score (TBS) in Individuals with Type 2 Diabetes Mellitus: An Updated Review. J. Clin. Med. 2023, 12, 7399. https://doi.org/10.3390/jcm12237399
Trandafir A-I, Sima O-C, Gheorghe A-M, Ciuche A, Cucu A-P, Nistor C, Carsote M. Trabecular Bone Score (TBS) in Individuals with Type 2 Diabetes Mellitus: An Updated Review. Journal of Clinical Medicine. 2023; 12(23):7399. https://doi.org/10.3390/jcm12237399
Chicago/Turabian StyleTrandafir, Alexandra-Ioana, Oana-Claudia Sima, Ana-Maria Gheorghe, Adrian Ciuche, Anca-Pati Cucu, Claudiu Nistor, and Mara Carsote. 2023. "Trabecular Bone Score (TBS) in Individuals with Type 2 Diabetes Mellitus: An Updated Review" Journal of Clinical Medicine 12, no. 23: 7399. https://doi.org/10.3390/jcm12237399
APA StyleTrandafir, A.-I., Sima, O.-C., Gheorghe, A.-M., Ciuche, A., Cucu, A.-P., Nistor, C., & Carsote, M. (2023). Trabecular Bone Score (TBS) in Individuals with Type 2 Diabetes Mellitus: An Updated Review. Journal of Clinical Medicine, 12(23), 7399. https://doi.org/10.3390/jcm12237399









