Laryngopharyngeal Reflux Scoring in a Pediatric Population
Abstract
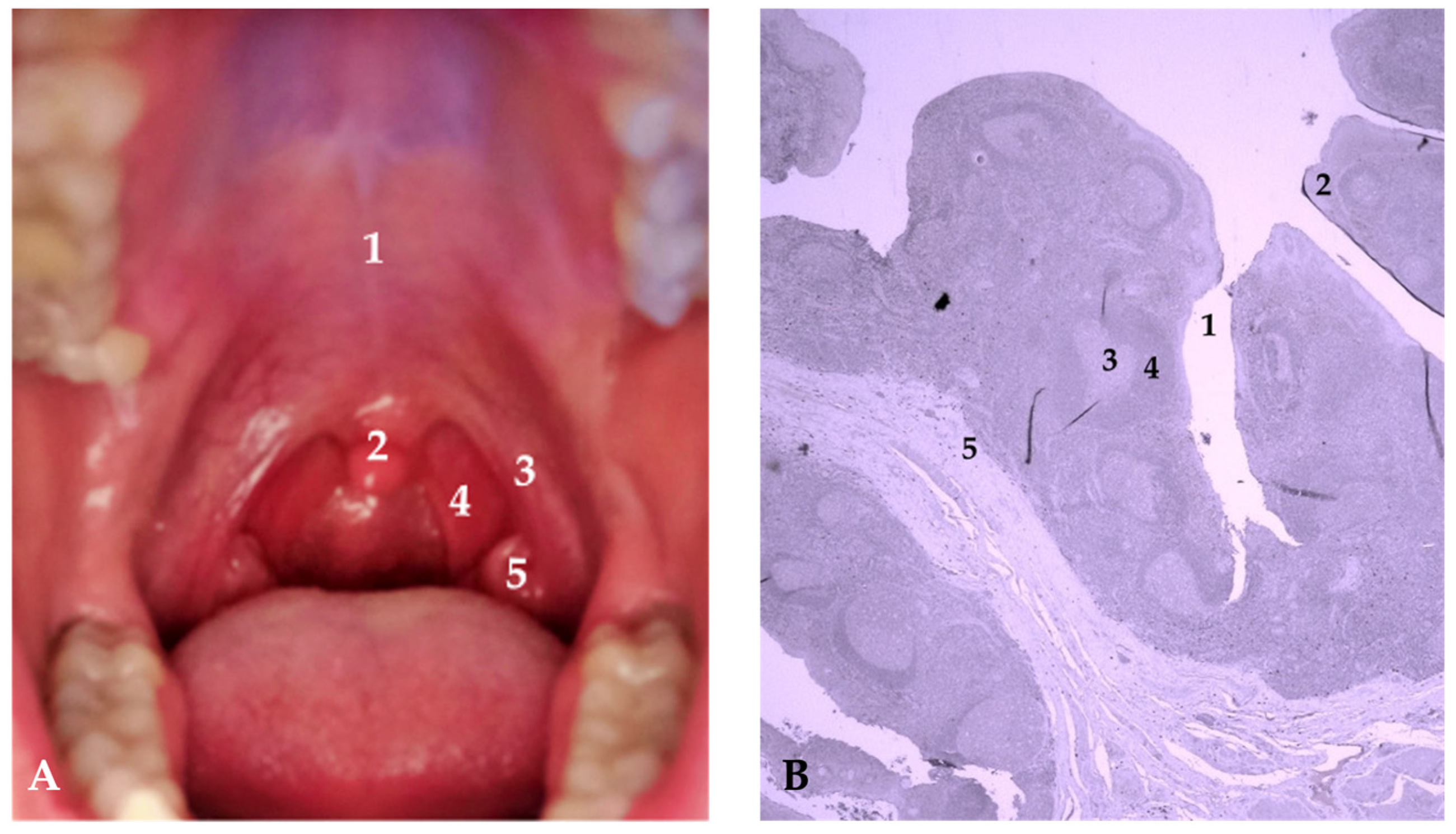
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Subjects and Questionnaires
2.3. Western Blot Analysis of Pepsin A in Salivas
2.4. Immunohistochemical Detection of Pepsin in Tonsillar Tissue
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewin, J.S.; Gillenwater, A.M.; Garrett, J.D.; Bishop-Leone, J.K.; Nguyen, D.D.; Callender, D.L.; Ayers, G.D.; Myers, J.N. Characterization of laryngopharyngeal reflux in patients with premalignant or early carcinomas of the larynx. Cancer 2003, 97, 1010–1014. [Google Scholar] [CrossRef]
- El-Serag, H.B. Time Trends of Gastroesophageal Reflux Disease: A Systematic Review. Clin. Gastroenterol. Hepatol. 2007, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Johnston, N.; Ondrey, F.; Rosen, R.; Hurley, B.P.; Gould, J.; Allen, J.; DelGaudio, J.; Altman, K.W. Airway reflux: Airway reflux. Ann. N. Y. Acad. Sci. 2016, 1381, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Johnston, N.; Yan, J.C.; Hoekzema, C.R.; Samuels, T.L.; Stoner, G.D.; Blumin, J.H.; Bock, J.M. Pepsin promotes proliferation of laryngeal and pharyngeal epithelial cells. Laryngoscope 2012, 122, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Koufman, J.A. The Otolaryngologic Manifestations of Gastroesophageal Reflux Disease (GERD): A Clinical Investigation of 225 Patients Using Ambulatory 24-Hour pH Monitoring and an Experimental Investigation of the Role of Acid and Pepsin in the Development of Laryngeal Injury. Laryngoscope 1991, 101, 1–78. [Google Scholar] [CrossRef] [PubMed]
- Samuels, T.L.; Johnston, N. Pepsin as a Marker of Extraesophageal Reflux. Ann. Otol. Rhinol. Laryngol. 2010, 119, 203–208. [Google Scholar] [CrossRef]
- Native Human Pepsin (PP) Protein Pepsin-27-Creative BioMart [Internet]. Available online: https://www.creativebiomart.net/native-human-pepsin-pp-protein-470424.htm (accessed on 5 November 2023).
- Kageyama, T.; Takahashi, K. The complete amino acid sequence of monkey progastricsin. J. Biol. Chem. 1986, 261, 4406–4419. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Guo, H.; Jiang, W.; Dong, P.; Liang, X. Site-directed mutagenesis of porcine pepsin: Possible role of Asp32, Thr33, Asp215 and Gly217 in maintaining the nuclease activity of pepsin. Enzym. Microb. Technol. 2016, 89, 69–75. [Google Scholar] [CrossRef]
- Kraus, J.; Nártová, E.; Pavlík, E.; Katra, R.; Sterzl, I.; Astl, J. Prevalence of Helicobacter pylori in adenotonsillar hypertrophy in children. Acta Otolaryngol. 2014, 134, 88–92. [Google Scholar] [CrossRef]
- Bardhan, K.D.; Strugala, V.; Dettmar, P.W. Reflux Revisited: Advancing the Role of Pepsin. Int. J. Otolaryngol. 2012, 2012, 646901. [Google Scholar] [CrossRef]
- Gong, X.; Wang, X.-Y.; Yang, L.; Sun, M.-J.; Du, J.; Zhang, W. Detecting Laryngopharyngeal Reflux by Immunohistochemistry of Pepsin in the Biopsies of Vocal Fold Leukoplakia. J. Voice 2018, 32, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.A.; Zavanela, A.R.; Cavallini, A.F.; Freitas, G.S.; Garcia, F.E.; Nunes, H.S. Comparison between the Reflux Finding Score and the Reflux Symptom Index in the Practice of Otorhinolaryngology. Int. Arch. Otorhinolaryngol. 2016, 20, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Sereg-Bahar, M.; Jerin, A.; Hocevar-Boltezar, I. Higher levels of total pepsin and bile acids in the saliva as a possible risk factor for early laryngeal cancer. Radiol. Oncol. 2015, 49, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Belafsky, P.C.; Rees, C.J. Laryngopharyngeal reflux: The value of otolaryngology examination. Curr. Gastroenterol. Rep. 2008, 10, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Belafsky, P.C.; Postma, G.N.; Koufman, J.A. Validity and Reliability of the Reflux Symptom Index (RSI). J. Voice 2002, 16, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Belafsky, P.C.; Postma, G.N.; Koufman, J.A. The Validity and Reliability of the Reflux Finding Score (RFS). Laryngoscope 2001, 111, 1313–1317. [Google Scholar] [CrossRef]
- Hutnik, R.; Zlatopolsky, A.; Mehraban-Far, S.; Alrassi, J.; McMillan, N.; Amadi, C.; Fujita, K.; Mortensen, M. Laryngopharyngeal reflux: Comparing improvements in reflux symptom index with reflux finding score. Am. J. Otolaryngol. 2020, 41, 102730. [Google Scholar] [CrossRef]
- Richardson, M.A. Sore throat, tonsillitis, and adenoiditis. Med. Clin. N. Am. 1999, 83, 75–83. [Google Scholar] [CrossRef]
- Chen, L.; Al-Kzayer, L.F.Y.; Liu, Y.; Liu, T. B-cell lymphomas involving Waldeyer’s ring characterized by distinctive clinical and histopathological features: A comparison of pediatric to adult patients. Oncotarget 2017, 8, 11544–11554. [Google Scholar] [CrossRef]
- Anderson, J.; Paterek, E. Tonsillitis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK544342/ (accessed on 11 October 2022).
- Van Kempen, M.; Rijkers, G.; van Cauwenberge, P. The Immune Response in Adenoids and Tonsils. Int. Arch. Allergy Immunol. 2000, 122, 8–19. [Google Scholar] [CrossRef]
- Zalzal, G.H.; Tran, L.P. Pediatric gastroesophageal reflux and laryngopharyngeal reflux. Otolaryngol. Clin. N. Am. 2000, 33, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Hori, S.; Osamura, R.Y.; Tsutsumi, Y. Reticular crypt epithelium and intra-epithelial lymphoid cells in the hyperplastic human palatine tonsil: An immunohistochemical analysis. Pathol. Int. 1995, 45, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jeong, H.S.; Kim, K.M.; Lee, Y.J.; Jung, M.H.; Park, J.J.; Kim, J.P.; Woo, S.H. Extra-Esophageal Pepsin from Stomach Refluxate Promoted Tonsil Hypertrophy. PLoS ONE 2016, 11, e0152336. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jang, S.J.; Yun, J.W.; Jung, M.H.; Woo, S.H. Effects of pepsin and pepstatin on reflux tonsil hypertrophy in vitro. PLoS ONE 2018, 13, e0207090. [Google Scholar] [CrossRef]
- Brodsky, L.; Moore, L.; Stanievich, J.F. A Comparison of Tonsillar Size and Oropharyngeal Dimensions in Children with Obstructive Adenotonsillar Hypertrophy. Int. J. Pediatr. Otorhinolaryngol. 1987, 13, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Magaki, S.; Hojat, S.A.; Wei, B.; So, A.; Yong, W.H. An Introduction to the Performance of Immunohistochemistry. Methods Mol. Biol. 2019, 1897, 289–298. [Google Scholar] [PubMed]
- Scotch, M.; Duggal, M.; Brandt, C.; Lin, Z.; Shiffman, R. Use of statistical analysis in the biomedical informatics literature. J. Am. Med. Inform. Assoc. 2010, 17, 3–5. [Google Scholar] [CrossRef]
- Marino, M.J. Chapter 3—Statistical Analysis in Preclinical Biomedical Research. In Research in the Biomedical Sciences [Internet]; Williams, M., Curtis, M.J., Mullane, K., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 107–144. Available online: https://www.sciencedirect.com/science/article/pii/B9780128047255000033 (accessed on 13 October 2023).
- Meyer, R.; Vandenplas, Y.; Lozinsky, A.C.; Vieira, M.C.; Canani, R.B.; Dupont, C.; Uysal, P.; Cavkaytar, O.; Knibb, R.; Fleischer, D.M.; et al. Diagnosis and management of food allergy-associated gastroesophageal reflux disease in young children—EAACI position paper. Pediatr. Allergy Immunol. 2022, 33, e13856. [Google Scholar] [CrossRef]
- Venkatesan, N.N.; Pine, H.S.; Underbrink, M. Laryngopharyngeal Reflux Disease in Children. Pediatr. Clin. N. Am. 2013, 60, 865–878. [Google Scholar] [CrossRef]
- Saniasiaya, J.; Kulasegarah, J. Dysphonia and reflux in children: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2020, 139, 110473. [Google Scholar] [CrossRef]
- Lechien, J.R. Pediatric Laryngopharyngeal Reflux: An Evidence-Based Review. Children 2023, 10, 583. [Google Scholar] [CrossRef]
- Altman, K.W.; Stephens, R.M.; Lyttle, C.S.; Weiss, K.B.; Ba, R.M.S.; Ma, C.S.L. Changing Impact of Gastroesophageal Reflux in Medical and Otolaryngology Practice. Laryngoscope 2005, 115, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Branski, R.C.; Bhattacharyya, N.; Shapiro, J. The Reliability of the Assessment of Endoscopic Laryngeal Findings Associated With Laryngopharyngeal Reflux Disease. Laryngoscope 2002, 112, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.F. Gastroesophageal Reflux Disease and the Larynx. J. Clin. Gastroenterol. 2003, 36, 198–203. [Google Scholar] [CrossRef]
- Jiang, A.; Liang, M.; Su, Z.; Chai, L.; Lei, W.; Wang, Z.; Wang, A.; Wen, W.; Chen, M. Immunohistochemical detection of pepsin in laryngeal mucosa for diagnosing laryngopharyngeal reflux. Laryngoscope 2011, 121, 1426–1430. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.; Lively, M.O.; Johnston, N.; Dettmar, P.W.; Koufman, J.A. Sensitive Pepsin Immunoassay for Detection of Laryngopharyngeal Reflux. Laryngoscope 2005, 115, 1473–1478. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Ren, J.; Xu, Y. Pepsin in saliva as a diagnostic biomarker in laryngopharyngeal reflux: A meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Henríquez, C.; Ruano-Ravina, A.; Vaamonde, P.; Martínez-Capoccioni, G.; Martín-Martín, C. Is Pepsin a Reliable Marker of Laryngopharyngeal Reflux? A Systematic Review. Otolaryngol. Head Neck Surg. 2017, 157, 385–391. [Google Scholar] [CrossRef]
- Divakaran, S.; Rajendran, S.; Thomas, R.M.; Jacob, J.; Kurien, M. Laryngopharyngeal Reflux: Symptoms, Signs, and Presence of Pepsin in Saliva-A Reliable Diagnostic Triad. Int. Arch. Otorhinolaryngol. 2020, 25, e273–e278. [Google Scholar] [CrossRef]
- Bobin, F.; Journe, F.; Lechien, J.R. Saliva pepsin level of laryngopharyngeal reflux patients is not correlated with reflux episodes. Laryngoscope 2019, 130, 1278–1281. [Google Scholar] [CrossRef]
- Na, S.Y.; Kwon, O.E.; Lee, Y.C.; Eun, Y. Optimal timing of saliva collection to detect pepsin in patients with laryngopharyngeal reflux. Laryngoscope 2016, 126, 2770–2773. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, J.E.; D’Agostino, R.B.; Lively, M.O. Pepsin in saliva as a biomarker for oropharyngeal reflux compared with 24-hour esophageal impedance/pH monitoring in pediatric patients. Neurogastroenterol. Motil. 2017, 29, e12936. [Google Scholar] [CrossRef] [PubMed]
- Spyridoulias, A.; Lillie, S.; Vyas, A.; Fowler, S.J. Detecting laryngopharyngeal reflux in patients with upper airways symptoms: Symptoms, signs or salivary pepsin? Respir. Med. 2015, 109, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.R.; Kwon, O.E.; Park, J.M.; Dong, S.H.; Jung, S.Y.; Lee, Y.C.; Eun, Y.-G. Association Between Pepsin in the Saliva and the Subjective Symptoms in Patients with Laryngopharyngeal Reflux. J. Voice 2019, 33, 150–154. [Google Scholar] [CrossRef]
- Sereg-Bahar, M.; Jerin, A.; Jansa, R.; Stabuc, B.; Hocevar-Boltezar, I. Pepsin and bile acids in saliva in patients with laryngopharyngeal reflux–a prospective comparative study. Clin. Otolaryngol. 2015, 40, 234–239. [Google Scholar] [CrossRef]




| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Gender | Count | Age Average (Years) | Age −95% CI 1 | Age +95% CI | Two-Way ANOVA Results | |
|---|---|---|---|---|---|---|
| Male | IHC negative | 18 | 6.88 | 5.54 | 8.23 | F(1,72) = 0.08602; p = 0.7701 |
| IHC positive | 23 | 7.87 | 6.45 | 9.29 | ||
| Female | IHC negative | 17 | 7.00 | 5.42 | 8.57 | |
| IHC positive | 18 | 7.55 | 5.86 | 9.25 | ||
| IHC Finding | Count or % Relative to RFS Result | |||
|---|---|---|---|---|
| RFS Below 7 | RFS 7 and Above | Total | p 1 | |
| IHC negative | 21 (27.63%) | 14 (18.42%) | 35 (46.05%) | 0.49 |
| IHC positive | 21 (27.63%) | 20 (26.32%) | 41 (53.95%) | |
| Total | 42 (55.26%) | 34 (44.74%) | 76 (100%) | |
| IHC Finding | Count or % Relative to RSI Result | |||
|---|---|---|---|---|
| RSI Below 13 | RSI 13 and Above | Total | p 1 | |
| IHC negative | 28 (36.84%) | 7 (9.21%) | 35 (46.05%) | 0.02 |
| IHC positive | 22 (28.95%) | 19 (25%) | 41 (53.95%) | |
| Total | 50 (65.79%) | 26 (34.21%) | 76 (100%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abičić, I.; Čović, M.; Zjalić, M.; Bakula, M.; Marjanović, K.; Šestak, A.; Dmitrović, B.; Mendeš, T.; Smolić, M.; Wu, G.Y.; et al. Laryngopharyngeal Reflux Scoring in a Pediatric Population. J. Clin. Med. 2023, 12, 7425. https://doi.org/10.3390/jcm12237425
Abičić I, Čović M, Zjalić M, Bakula M, Marjanović K, Šestak A, Dmitrović B, Mendeš T, Smolić M, Wu GY, et al. Laryngopharyngeal Reflux Scoring in a Pediatric Population. Journal of Clinical Medicine. 2023; 12(23):7425. https://doi.org/10.3390/jcm12237425
Chicago/Turabian StyleAbičić, Ivan, Marina Čović, Milorad Zjalić, Marina Bakula, Ksenija Marjanović, Anamarija Šestak, Branko Dmitrović, Tihana Mendeš, Martina Smolić, George Y. Wu, and et al. 2023. "Laryngopharyngeal Reflux Scoring in a Pediatric Population" Journal of Clinical Medicine 12, no. 23: 7425. https://doi.org/10.3390/jcm12237425
APA StyleAbičić, I., Čović, M., Zjalić, M., Bakula, M., Marjanović, K., Šestak, A., Dmitrović, B., Mendeš, T., Smolić, M., Wu, G. Y., Mihalj, H., Zubčić, Ž., & Včeva, A. (2023). Laryngopharyngeal Reflux Scoring in a Pediatric Population. Journal of Clinical Medicine, 12(23), 7425. https://doi.org/10.3390/jcm12237425






