Ventilatory Management of Patients with Acute Respiratory Distress Syndrome Due to SARS-CoV-2
Abstract
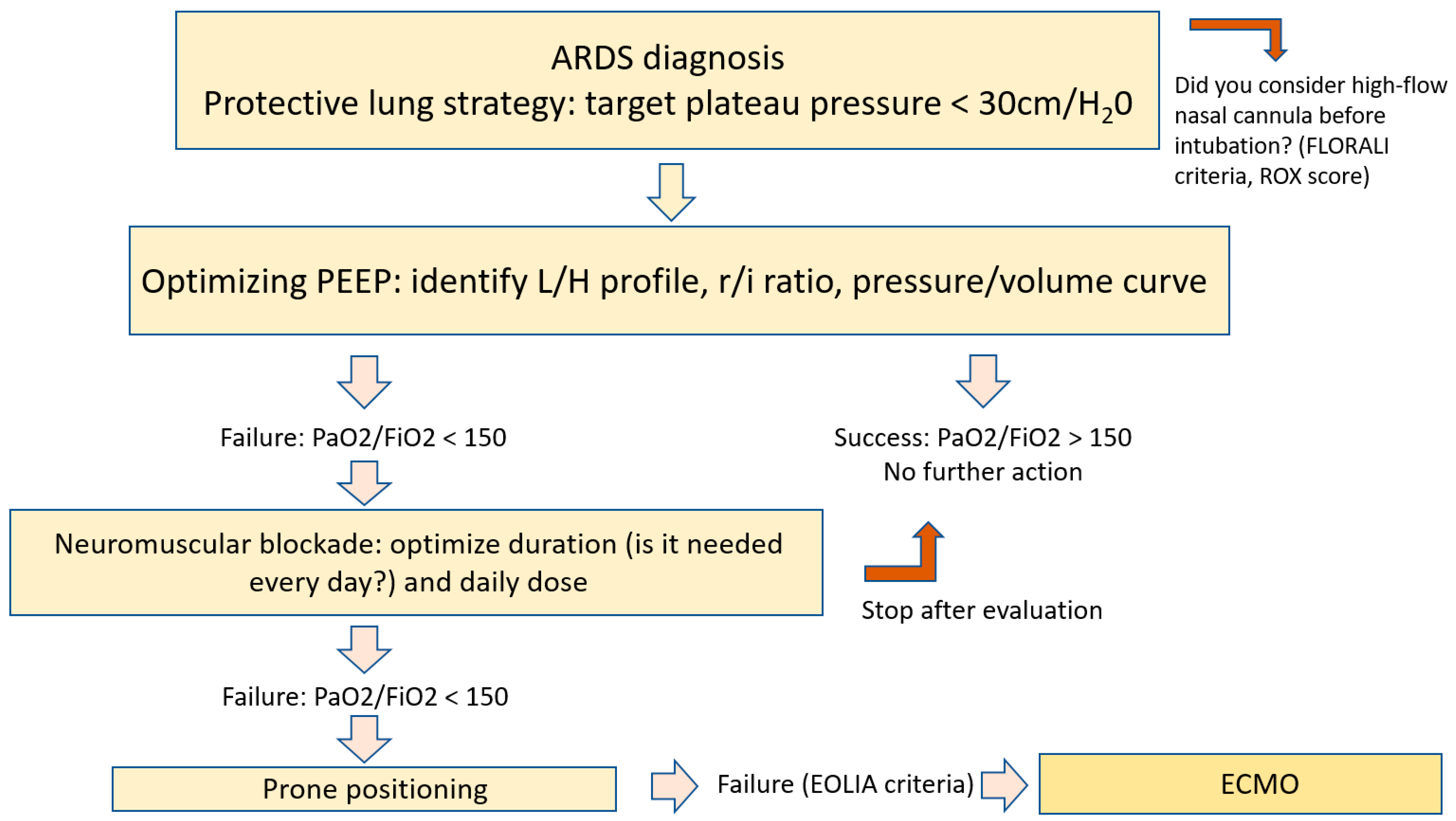
:1. Introduction
2. Pathophysiology of ARDS Due to SARS-CoV-2
3. Ventilatory Management of Respiratory Failure Caused by SARS-CoV-2 Infection
4. Neuromuscular Blockade
5. Prone Positioning
6. Inhaled Nitric Oxide
7. Extracorporeal Assistance
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response. JAMA 2020, 323, 1545–1546. [Google Scholar] [CrossRef]
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef]
- Chu, D.K.; Kim, L.H.; Young, P.J.; Zamiri, N.; Almenawer, S.A.; Jaeschke, R.; Szczeklik, W.; Schunemann, H.J.; Neary, J.D.; Alhazzani, W. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): A systematic review and meta-analysis. Lancet 2018, 391, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- The ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Conservative Oxygen Therapy during Mechanical Ventilation in the ICU. N. Engl. J. Med. 2020, 382, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Schjorring, O.L.; Klitgaard, T.L.; Perner, A.; Wetterslev, J.; Lange, T.; Siegemund, M.; Backlund, M.; Keus, F.; Laake, J.H.; Morgan, M.; et al. Lower or Higher Oxygenation Targets for Acute Hypoxemic Respiratory Failure. N. Engl. J. Med. 2021, 384, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, C.H.; Semler, M.W.; Brower, R.G. Oxygen Toxicity in Critically Ill Adults. Am. J. Respir. Crit. Care Med. 2021, 204, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Frat, J.P.; Thille, A.W.; Mercat, A.; Girault, C.; Ragot, S.; Perbet, S.; Prat, G.; Boulain, T.; Morawiec, E.; Cottereau, A.; et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015, 372, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Delclaux, C.; L’Her, E.; Alberti, C.; Mancebo, J.; Abroug, F.; Conti, G.; Guerin, C.; Schortgen, F.; Lefort, Y.; Antonelli, M.; et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: A randomized controlled trial. JAMA 2000, 284, 2352–2360. [Google Scholar] [CrossRef]
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; Hess, D.; Hill, N.S.; Nava, S.; Navalesi, P.; Antonelli, M.; Brozek, J.; Conti, G.; et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017, 50, 1602426. [Google Scholar] [CrossRef]
- Kang, B.J.; Koh, Y.; Lim, C.M.; Huh, J.W.; Baek, S.; Han, M.; Seo, H.S.; Suh, H.J.; Seo, G.J.; Kim, E.Y.; et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015, 41, 623–632. [Google Scholar] [CrossRef]
- Gattinoni, L.; Marini, J.J.; Collino, F.; Maiolo, G.; Rapetti, F.; Tonetti, T.; Vasques, F.; Quintel, M. The future of mechanical ventilation: Lessons from the present and the past. Crit. Care 2017, 21, 183. [Google Scholar] [CrossRef]
- Brochard, L.; Slutsky, A.; Pesenti, A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am. J. Respir. Crit. Care Med. 2017, 195, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Robert, R.; Kentish-Barnes, N.; Boyer, A.; Laurent, A.; Azoulay, E.; Reignier, J. Ethical dilemmas due to the COVID-19 pandemic. Ann. Intensive Care 2020, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.J.; Laghi, F.; Jubran, A. Why COVID-19 Silent Hypoxemia Is Baffling to Physicians. Am. J. Respir. Crit. Care Med. 2020, 202, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA 2020, 323, 1612–1614. [Google Scholar] [CrossRef]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef]
- Bonny, V.; Maillard, A.; Mousseaux, C.; Placais, L.; Richier, Q. COVID-19: Pathogenesis of a multi-faceted disease. Rev. Med. Interne 2020, 41, 375–389. [Google Scholar] [CrossRef]
- Ards Definition Task Force. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Sinha, P.; Calfee, C.S. Phenotypes in acute respiratory distress syndrome: Moving towards precision medicine. Curr. Opin. Crit. Care 2019, 25, 12–20. [Google Scholar] [CrossRef]
- Guerin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Arabi, Y.; Arroliga, A.C.; Bernard, G.; Bersten, A.D.; Brochard, L.J.; Calfee, C.S.; Combes, A.; Daniel, B.M.; Ferguson, N.D.; et al. A New Global Definition of Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Tonetti, T.; Navalesi, P.; Nava, S.; Antonelli, M.; Pesenti, A.; Grasselli, G.; Grieco, D.L.; Menga, L.S.; Pisani, L.; et al. High-Flow Nasal Oxygen for Severe Hypoxemia: Oxygenation Response and Outcome in Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2022, 205, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Calligaro, G.L.; Lalla, U.; Audley, G.; Gina, P.; Miller, M.G.; Mendelson, M.; Dlamini, S.; Wasserman, S.; Meintjes, G.; Peter, J.; et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: A multi-centre prospective observational study. EClinicalMedicine 2020, 28, 100570. [Google Scholar] [CrossRef]
- Gershengorn, H.B.; Hu, Y.; Chen, J.T.; Hsieh, S.J.; Dong, J.; Gong, M.N.; Chan, C.W. The Impact of High-Flow Nasal Cannula Use on Patient Mortality and the Availability of Mechanical Ventilators in COVID-19. Ann. Am. Thorac. Soc. 2021, 18, 623–631. [Google Scholar] [CrossRef]
- Riviello, E.D.; Kiviri, W.; Twagirumugabe, T.; Mueller, A.; Banner-Goodspeed, V.M.; Officer, L.; Novack, V.; Mutumwinka, M.; Talmor, D.S.; Fowler, R.A. Hospital Incidence and Outcomes of the Acute Respiratory Distress Syndrome Using the Kigali Modification of the Berlin Definition. Am. J. Respir. Crit. Care Med. 2016, 193, 52–59. [Google Scholar] [CrossRef]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Hayden, D.L.; Schoenfeld, D.A.; Ware, L.B. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007, 132, 410–417. [Google Scholar] [CrossRef]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe COVID-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef]
- Gattinoni, L.; Coppola, S.; Cressoni, M.; Busana, M.; Rossi, S.; Chiumello, D. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 201, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020, 191, 148–150. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with COVID-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef]
- Patel, B.V.; Arachchillage, D.J.; Ridge, C.A.; Bianchi, P.; Doyle, J.F.; Garfield, B.; Ledot, S.; Morgan, C.; Passariello, M.; Price, S.; et al. Pulmonary Angiopathy in Severe COVID-19: Physiologic, Imaging, and Hematologic Observations. Am. J. Respir. Crit. Care Med. 2020, 202, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Diehl, J.L.; Peron, N.; Chocron, R.; Debuc, B.; Guerot, E.; Hauw-Berlemont, C.; Hermann, B.; Augy, J.L.; Younan, R.; Novara, A.; et al. Respiratory mechanics and gas exchanges in the early course of COVID-19 ARDS: A hypothesis-generating study. Ann. Intensive Care 2020, 10, 95. [Google Scholar] [CrossRef]
- Huang, S.; Vignon, P.; Mekontso-Dessap, A.; Tran, S.; Prat, G.; Chew, M.; Balik, M.; Sanfilippo, F.; Banauch, G.; Clau-Terre, F.; et al. Echocardiography findings in COVID-19 patients admitted to intensive care units: A multi-national observational study (the ECHO-COVID study). Intensive Care Med. 2022, 48, 667–678. [Google Scholar] [CrossRef]
- Huang, S.; Vieillard-Baron, A.; Evrard, B.; Prat, G.; Chew, M.S.; Balik, M.; Clau-Terre, F.; De Backer, D.; Mekontso Dessap, A.; Orde, S.; et al. Echocardiography phenotypes of right ventricular involvement in COVID-19 ARDS patients and ICU mortality: Post-hoc (exploratory) analysis of repeated data from the ECHO-COVID study. Intensive Care Med. 2023, 49, 946–956. [Google Scholar] [CrossRef]
- Archer, S.L.; Sharp, W.W.; Weir, E.K. Differentiating COVID-19 Pneumonia From Acute Respiratory Distress Syndrome and High Altitude Pulmonary Edema: Therapeutic Implications. Circulation 2020, 142, 101–104. [Google Scholar] [CrossRef]
- Nasa, P.; Azoulay, E.; Khanna, A.K.; Jain, R.; Gupta, S.; Javeri, Y.; Juneja, D.; Rangappa, P.; Sundararajan, K.; Alhazzani, W.; et al. Expert consensus statements for the management of COVID-19-related acute respiratory failure using a Delphi method. Crit. Care 2021, 25, 106. [Google Scholar] [CrossRef]
- Papazian, L.; Aubron, C.; Brochard, L.; Chiche, J.D.; Combes, A.; Dreyfuss, D.; Forel, J.M.; Guerin, C.; Jaber, S.; Mekontso-Dessap, A.; et al. Formal guidelines: Management of acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Dantzker, D.R.; Lynch, J.P.; Weg, J.G. Depression of cardiac output is a mechanism of shunt reduction in the therapy of acute respiratory failure. Chest 1980, 77, 636–642. [Google Scholar] [CrossRef]
- Grasso, S.; Mirabella, L.; Murgolo, F.; Di Mussi, R.; Pisani, L.; Dalfino, L.; Spadaro, S.; Rauseo, M.; Lamanna, A.; Cinnella, G. Effects of Positive End-Expiratory Pressure in “High Compliance” Severe Acute Respiratory Syndrome Coronavirus 2 Acute Respiratory Distress Syndrome. Crit. Care Med. 2020, 48, e1332–e1336. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, S.; Fogagnolo, A.; Campo, G.; Zucchetti, O.; Verri, M.; Ottaviani, I.; Tunstall, T.; Grasso, S.; Scaramuzzo, V.; Murgolo, F.; et al. Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID-19 ICU patients. Crit. Care 2021, 25, 74. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzo, G.; Karbing, D.S.; Fogagnolo, A.; Mauri, T.; Spinelli, E.; Mari, M.; Turrini, C.; Montanaro, F.; Volta, C.A.; Rees, S.E.; et al. Heterogeneity of Ventilation/Perfusion Mismatch at Different Levels of PEEP and in Mechanical Phenotypes of COVID-19 ARDS. Respir. Care 2022, 68, 188–198. [Google Scholar] [CrossRef]
- Lesieur, O.; Quenot, J.P.; Cohen-Solal, Z.; David, R.; De Saint Blanquat, L.; Elbaz, M.; Gaillard Le Roux, B.; Goulenok, C.; Lavoue, S.; Lemiale, V.; et al. Admission criteria and management of critical care patients in a pandemic context: Position of the Ethics Commission of the French Intensive Care Society, update of April 2021. Ann. Intensive Care 2021, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef]
- Grasselli, G.; Calfee, C.S.; Camporota, L.; Poole, D.; Amato, M.B.P.; Antonelli, M.; Arabi, Y.M.; Baroncelli, F.; Beitler, J.R.; Bellani, G.; et al. ESICM guidelines on acute respiratory distress syndrome: Definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023, 49, 727–759. [Google Scholar] [CrossRef]
- Constantin, J.M.; Jabaudon, M.; Lefrant, J.Y.; Jaber, S.; Quenot, J.P.; Langeron, O.; Ferrandiere, M.; Grelon, F.; Seguin, P.; Ichai, C.; et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): A multicentre, single-blind, randomised controlled trial. Lancet Respir. Med. 2019, 7, 870–880. [Google Scholar] [CrossRef]
- Nishimura, M. High-flow nasal cannula oxygen therapy in adults. J. Intensive Care 2015, 3, 15. [Google Scholar] [CrossRef]
- Parke, R.; McGuinness, S.; Eccleston, M. Nasal high-flow therapy delivers low level positive airway pressure. Br. J. Anaesth. 2009, 103, 886–890. [Google Scholar] [CrossRef]
- Roca, O.; Caralt, B.; Messika, J.; Samper, M.; Sztrymf, B.; Hernandez, G.; Garcia-de-Acilu, M.; Frat, J.P.; Masclans, J.R.; Ricard, J.D. An Index Combining Respiratory Rate and Oxygenation to Predict Outcome of Nasal High-Flow Therapy. Am. J. Respir. Crit. Care Med. 2019, 199, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Vest, M.T.; Caplan, R.; Fawcett, M.; Deitchman, A.R.; Valentino, D.; Gajera, M.; Jurkovitz, C.T. Intubation Timing in COVID-19 Based on ROX Index and Association With Patient Outcomes. Respir. Care 2022, 67, 1291–1299. [Google Scholar] [CrossRef]
- Nair, P.R.; Haritha, D.; Behera, S.; Kayina, C.A.; Maitra, S.; Anand, R.K.; Ray, B.R.; Soneja, M.; Subramaniam, R.; Baidya, D.K. Comparison of High-Flow Nasal Cannula and Noninvasive Ventilation in Acute Hypoxemic Respiratory Failure Due to Severe COVID-19 Pneumonia. Respir. Care 2021, 66, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Grieco, D.L.; Menga, L.S.; Cesarano, M.; Rosa, T.; Spadaro, S.; Bitondo, M.M.; Montomoli, J.; Falo, G.; Tonetti, T.; Cutuli, S.L.; et al. Effect of Helmet Noninvasive Ventilation vs High-Flow Nasal Oxygen on Days Free of Respiratory Support in Patients With COVID-19 and Moderate to Severe Hypoxemic Respiratory Failure: The HENIVOT Randomized Clinical Trial. JAMA 2021, 325, 1731–1743. [Google Scholar] [CrossRef]
- Gattinoni, L.; Caironi, P.; Cressoni, M.; Chiumello, D.; Ranieri, V.M.; Quintel, M.; Russo, S.; Patroniti, N.; Cornejo, R.; Bugedo, G. Lung recruitment in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2006, 354, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Eronia, N.; Mauri, T.; Maffezzini, E.; Gatti, S.; Bronco, A.; Alban, L.; Binda, F.; Sasso, T.; Marenghi, C.; Grasselli, G.; et al. Bedside selection of positive end-expiratory pressure by electrical impedance tomography in hypoxemic patients: A feasibility study. Ann. Intensive Care 2017, 7, 76. [Google Scholar] [CrossRef]
- Chen, L.; Del Sorbo, L.; Grieco, D.L.; Junhasavasdikul, D.; Rittayamai, N.; Soliman, I.; Sklar, M.C.; Rauseo, M.; Ferguson, N.D.; Fan, E.; et al. Potential for Lung Recruitment Estimated by the Recruitment-to-Inflation Ratio in Acute Respiratory Distress Syndrome. A Clinical Trial. Am. J. Respir. Crit. Care Med. 2020, 201, 178–187. [Google Scholar] [CrossRef]
- Papazian, L.; Forel, J.M.; Gacouin, A.; Penot-Ragon, C.; Perrin, G.; Loundou, A.; Jaber, S.; Arnal, J.M.; Perez, D.; Seghboyan, J.M.; et al. Neuromuscular blockers in early acute respiratory distress syndrome. N. Engl. J. Med. 2010, 363, 1107–1116. [Google Scholar] [CrossRef]
- Leatherman, J.W.; Fluegel, W.L.; David, W.S.; Davies, S.F.; Iber, C. Muscle weakness in mechanically ventilated patients with severe asthma. Am. J. Respir. Crit. Care Med. 1996, 153, 1686–1690. [Google Scholar] [CrossRef] [PubMed]
- Hraiech, S.; Forel, J.M.; Guervilly, C.; Rambaud, R.; Lehingue, S.; Adda, M.; Sylla, P.; Valera, S.; Carvelli, J.; Gainnier, M.; et al. How to reduce cisatracurium consumption in ARDS patients: The TOF-ARDS study. Ann. Intensive Care 2017, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Courcelle, R.; Gaudry, S.; Serck, N.; Blonz, G.; Lascarrou, J.B.; Grimaldi, D.; on behalf the COVADIS Study Group. Neuromuscular blocking agents (NMBA) for COVID-19 acute respiratory distress syndrome: A multicenter observational study. Crit. Care 2020, 24, 446. [Google Scholar] [CrossRef]
- Ego, A.; Halenarova, K.; Creteur, J.; Taccone, F.S. How to Manage Withdrawal of Sedation and Analgesia in Mechanically Ventilated COVID-19 Patients? J. Clin. Med. 2021, 10, 4917. [Google Scholar] [CrossRef]
- Ho, A.T.N.; Patolia, S.; Guervilly, C. Neuromuscular blockade in acute respiratory distress syndrome: A systematic review and meta-analysis of randomized controlled trials. J. Intensive Care 2020, 8, 12. [Google Scholar] [CrossRef]
- Payen, J.F.; Chanques, G.; Futier, E.; Velly, L.; Jaber, S.; Constantin, J.M. Sedation for critically ill patients with COVID-19: Which specificities? One size does not fit all. Anaesth. Crit. Care Pain Med. 2020, 39, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Brault, C.; Zerbib, Y.; Kontar, L.; Fouquet, U.; Carpentier, M.; Metzelard, M.; Soupison, T.; De Cagny, B.; Maizel, J.; Slama, M. COVID-19- versus non-COVID-19-related Acute Respiratory Distress Syndrome: Differences and Similarities. Am. J. Respir. Crit. Care Med. 2020, 202, 1301–1304. [Google Scholar] [CrossRef]
- Ehrmann, S.; Li, J.; Ibarra-Estrada, M.; Perez, Y.; Pavlov, I.; McNicholas, B.; Roca, O.; Mirza, S.; Vines, D.; Garcia-Salcido, R.; et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: A randomised, controlled, multinational, open-label meta-trial. Lancet Respir. Med. 2021, 9, 1387–1395. [Google Scholar] [CrossRef]
- Papazian, L.; Schmidt, M.; Hajage, D.; Combes, A.; Petit, M.; Lebreton, G.; Rilinger, J.; Giani, M.; Le Breton, C.; Duburcq, T.; et al. Effect of prone positioning on survival in adult patients receiving venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Intensive Care Med. 2022, 48, 270–280. [Google Scholar] [CrossRef]
- Alhazzani, W.; Parhar, K.K.S.; Weatherald, J.; Al Duhailib, Z.; Alshahrani, M.; Al-Fares, A.; Buabbas, S.; Cherian, S.V.; Munshi, L.; Fan, E.; et al. Effect of Awake Prone Positioning on Endotracheal Intubation in Patients With COVID-19 and Acute Respiratory Failure: A Randomized Clinical Trial. JAMA 2022, 327, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Nay, M.A.; Hindre, R.; Perrin, C.; Clement, J.; Plantier, L.; Seve, A.; Druelle, S.; Morrier, M.; Laine, J.B.; Colombain, L.; et al. Prone position versus usual care in hypoxemic COVID-19 patients in medical wards: A randomised controlled trial. Crit. Care 2023, 27, 240. [Google Scholar] [CrossRef]
- Cheema, H.A.; Siddiqui, A.; Ochani, S.; Adnan, A.; Sukaina, M.; Haider, R.; Shahid, A.; Rehman, M.E.U.; Awan, R.U.; Singh, H.; et al. Awake Prone Positioning for Non-Intubated COVID-19 Patients with Acute Respiratory Failure: A Meta-Analysis of Randomised Controlled Trials. J. Clin. Med. 2023, 12, 926. [Google Scholar] [CrossRef] [PubMed]
- Weatherald, J.; Parhar, K.K.S.; Al Duhailib, Z.; Chu, D.K.; Granholm, A.; Solverson, K.; Lewis, K.; Moller, M.H.; Alshahrani, M.; Belley-Cote, E.; et al. Efficacy of awake prone positioning in patients with COVID-19 related hypoxemic respiratory failure: Systematic review and meta-analysis of randomized trials. BMJ 2022, 379, e071966. [Google Scholar] [CrossRef] [PubMed]
- Brunelle, T.; Prud’homme, E.; Alphonsine, J.E.; Baumstarck, K.; Sanz, C.; Salmi, S.; Peres, N.; Forel, J.M.; Papazian, L.; Hraiech, S.; et al. Awake prone position in COVID-19 acute respiratory failure: A randomised crossover study using electrical impedance tomography. ERJ Open Res. 2023, 9, 00509-2022. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzo, G.; Gamberini, L.; Tonetti, T.; Zani, G.; Ottaviani, I.; Mazzoli, C.A.; Capozzi, C.; Giampalma, E.; Bacchi Reggiani, M.L.; Bertellini, E.; et al. Sustained oxygenation improvement after first prone positioning is associated with liberation from mechanical ventilation and mortality in critically ill COVID-19 patients: A cohort study. Ann. Intensive Care 2021, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Pan, D.; Koeckerling, D.; Baldwin, A.J.; West, R. Effect of serial awake prone positioning on oxygenation in patients admitted to intensive care with COVID-19. Postgrad. Med. J. 2022, 98, 360–364. [Google Scholar] [CrossRef]
- Zhu, L.; Ni, Z.; Zhang, Y.; Zhan, Y.; Lan, M.; Zhao, R. Barriers and facilitators of adherence to awake prone positioning: A qualitative study using the COM-B model. BMC Pulm. Med. 2023, 23, 267. [Google Scholar] [CrossRef]
- Godoy, D.A.; Longhitano, Y.; Fazzini, B.; Robba, C.; Battaglini, D. High flow nasal oxygen and awake prone positioning—Two allies against COVID-19: A systematic review. Respir. Physiol. Neurobiol. 2023, 310, 104015. [Google Scholar] [CrossRef]
- Longobardo, A.; Montanari, C.; Shulman, R.; Benhalim, S.; Singer, M.; Arulkumaran, N. Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome. Br. J. Anaesth. 2021, 126, e44–e46. [Google Scholar] [CrossRef]
- Bagate, F.; Tuffet, S.; Masi, P.; Perier, F.; Razazi, K.; de Prost, N.; Carteaux, G.; Payen, D.; Mekontso Dessap, A. Rescue therapy with inhaled nitric oxide and almitrine in COVID-19 patients with severe acute respiratory distress syndrome. Ann. Intensive Care 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoue, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Giani, M.; Redaelli, S.; Siragusa, A.; Fumagalli, B.; Rona, R.; Foti, G. Extracorporeal Gas Exchange for Acute Respiratory Distress Syndrome: Open Questions, Controversies and Future Directions. Membranes 2021, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, G.; Zhao, W.; Peng, W. Anticoagulation strategies in patients with extracorporeal membrane oxygenation: A network meta-analysis and systematic review. Pharmacotherapy 2023, 43, 1084–1093. [Google Scholar] [CrossRef]
- Sanfilippo, F.; La Via, L.; Murabito, P.; Pappalardo, F.; Astuto, M. More evidence available for the use of Bivalirudin in patients supported by extracorporeal membrane oxygenation. Thromb. Res. 2022, 211, 148–149. [Google Scholar] [CrossRef]
- Teijeiro-Paradis, R.; Gannon, W.D.; Fan, E. Complications Associated With Venovenous Extracorporeal Membrane Oxygenation-What Can Go Wrong? Crit. Care Med. 2022, 50, 1809–1818. [Google Scholar] [CrossRef]
- Navaei, A.; Kostousov, V.; Teruya, J. Is it time to switch to bivalirudin for ECMO anticoagulation? Front. Med. 2023, 10, 1237601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacquier, M.; Labruyère, M.; Ecarnot, F.; Roudaut, J.-B.; Andreu, P.; Voizeux, P.; Save, Q.; Pedri, R.; Rigaud, J.-P.; Quenot, J.-P. Ventilatory Management of Patients with Acute Respiratory Distress Syndrome Due to SARS-CoV-2. J. Clin. Med. 2023, 12, 7509. https://doi.org/10.3390/jcm12247509
Jacquier M, Labruyère M, Ecarnot F, Roudaut J-B, Andreu P, Voizeux P, Save Q, Pedri R, Rigaud J-P, Quenot J-P. Ventilatory Management of Patients with Acute Respiratory Distress Syndrome Due to SARS-CoV-2. Journal of Clinical Medicine. 2023; 12(24):7509. https://doi.org/10.3390/jcm12247509
Chicago/Turabian StyleJacquier, Marine, Marie Labruyère, Fiona Ecarnot, Jean-Baptiste Roudaut, Pascal Andreu, Pierre Voizeux, Quentin Save, Romain Pedri, Jean-Philippe Rigaud, and Jean-Pierre Quenot. 2023. "Ventilatory Management of Patients with Acute Respiratory Distress Syndrome Due to SARS-CoV-2" Journal of Clinical Medicine 12, no. 24: 7509. https://doi.org/10.3390/jcm12247509




