Feature-Tracking-Derived Strain Analysis for Identification of Subendocardium-Involved Late Gadolinium Enhancement in Load-Induced Left Ventricular Hypertrophy: A Multicenter Study of Cardiac Magnetic Resonance Data
Abstract
:1. Introduction
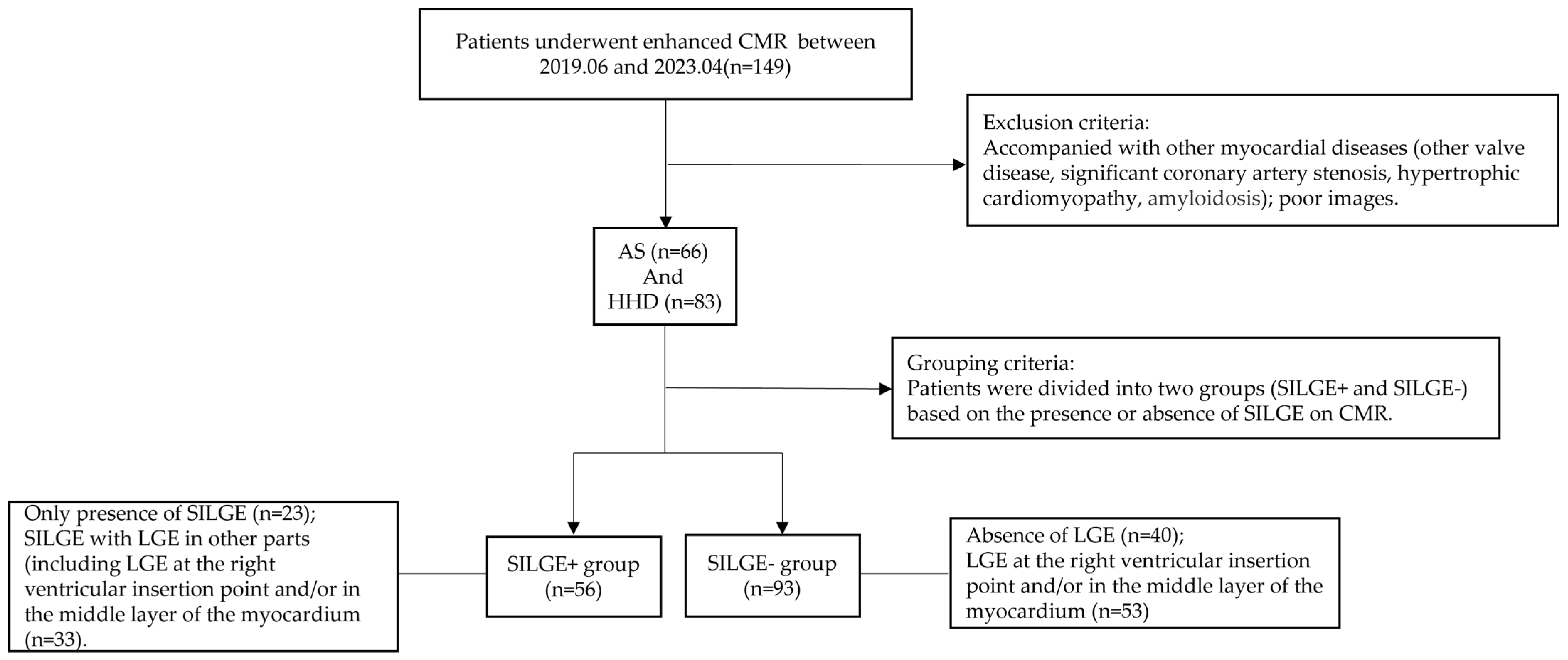
2. Materials and Methods
2.1. Study Cohort
2.2. CMR Imaging
2.3. Image Analysis and Post-Processing
2.4. Measurement of LV Systolic Pressure (LVSP) and LV End-Diastolic Pressure (LVEDP)
2.5. Statistical Analysis
3. Results
3.1. Baseline Demographic Characteristics
3.2. CMR Parameters of LV Remodeling and Function
3.3. NT-proBNP and CMR-FT-Derived Strain Parameters
3.4. LVP in Load-Induced LVH Patients with and without SILGE in Research Center 1
3.5. Logistic Regression Model for Predicting SILGE
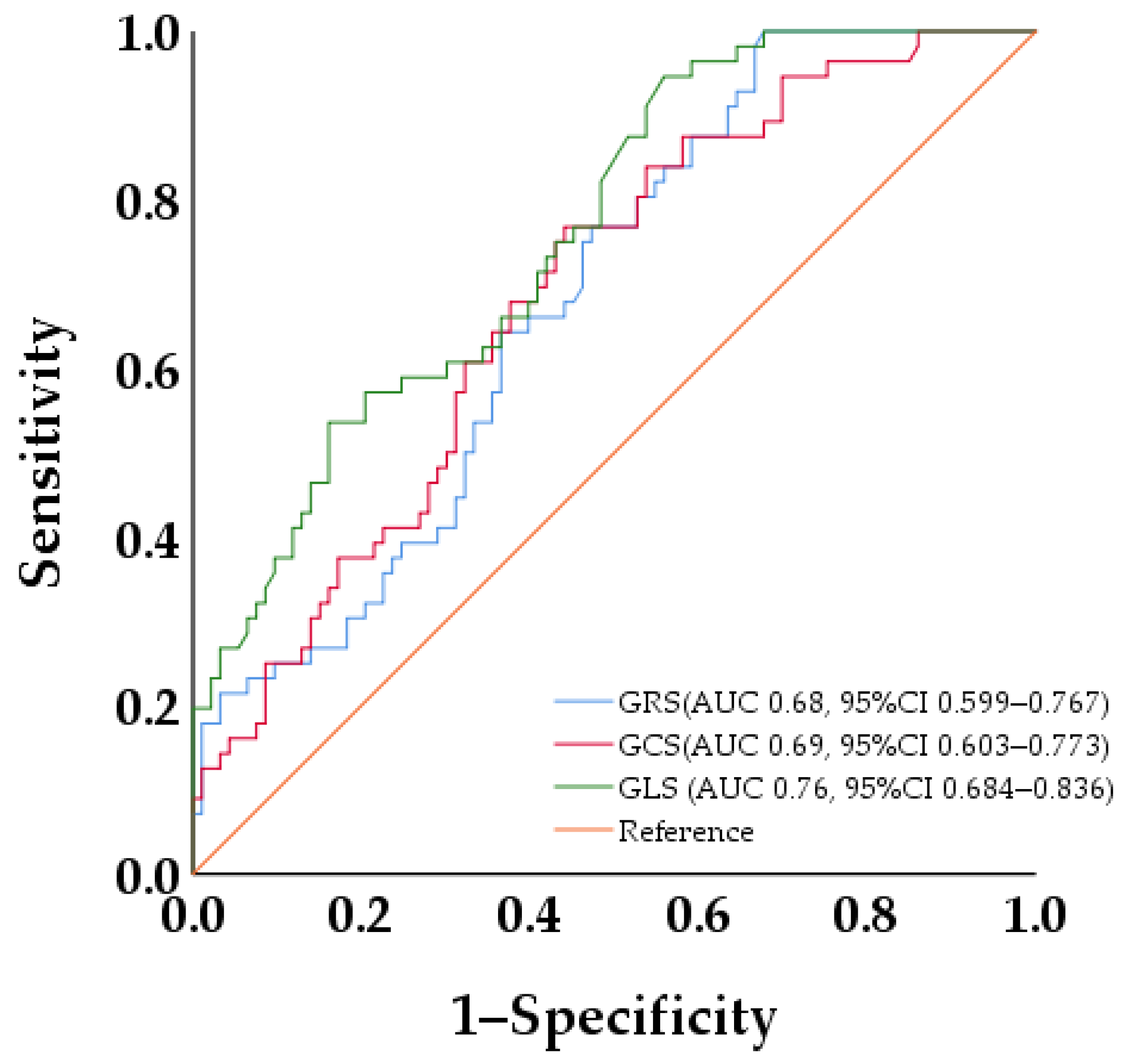
3.6. ROC Curve Analysis of LV Strains for Discriminating SILGE
3.7. Inter- and Intra-Observer Reproducibility of CMR-FT-Derived Strain Parameters
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grossman, W.; Jones, D.; McLaurin, L. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Investig. 1975, 56, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Carabello, B.A.; Paulus, W.J. Aortic stenosis. Lancet 2009, 373, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Musa, T.A.; Treibel, T.A.; Vassiliou, V.S.; Captur, G.; Singh, A.; Chin, C.; Dobson, L.E.; Pica, S.; Loudon, M.; Malley, T.; et al. Myocardial Scar and Mortality in Severe Aortic Stenosis. Circulation 2018, 138, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, K.; Ishibashi, Y.; Shimada, T.; Murakami, Y.; Inoue, S.; Sano, K. Subendocardial enhancement in gadolinium-diethylene-triamine-pentaacetic acid-enhanced magnetic resonance imaging in aortic stenosis. Am. J. Cardiol. 1999, 83, 1443–1446. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, K.; Yang, K.; Song, J.; Yu, S.; Wang, J.; Dong, Z.; Ma, X.; Yin, G.; Li, J.; et al. Subendocardial Involvement as an Underrecognized LGE Subtype Related to Adverse Outcomes in Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2023, 14, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Barone-Rochette, G.; Piérard, S.; De Meester de Ravenstei, C.; Seldrum, S.; Melchior, J.; Maes, F.; Pouleur, A.C.; Vancraeynes, D.; Pasquet, A.; Vanoverschelde, J.L.; et al. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J. Am. Coll. Cardiol. 2014, 64, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Bidani, A.K.; Griffin, K.A. Pathophysiology of hypertensive renal damage: Implications for therapy. Hypertension 2004, 44, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, Y.; Candellier, A.; Diouf, M.; Rusinaru, D.; Altes, A.; Pasquet, A.; Maréchaux, S.; Vanoverschelde, J.L.; Tribouilloy, C. Severe Aortic Stenosis and Chronic Kidney Disease: Outcomes and Impact of Aortic Valve Replacement. J. Am. Heart Assoc. 2020, 9, e017190. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial strain imaging: How useful is it in clinical decision making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef]
- Støylen, A. Strain and Strain Rate: Different Preload Dependence? J. Am. Soc. Echocardiogr. 2018, 31, 843. [Google Scholar] [CrossRef]
- Donal, E.; Bergerot, C.; Thibault, H.; Ernande, L.; Loufou, J.; Augeul, L.; Ovize, M.; Derumeaux, G. Influence of afterload on left ventricular radial and longitudinal systolic functions: A two-dimensional strain imaging study. Eur. J. Echocardiogr. 2009, 10, 914–921. [Google Scholar] [CrossRef] [PubMed]
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Bonow, R.O.; Carabello, B.A.; Chatterjee, K.; de Leon, A.C., Jr.; Faxon, D.P.; Freed, M.D.; Gaasch, W.H.; et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease). J. Am. Coll. Cardiol. 2006, 48, e1–e148. [Google Scholar] [PubMed]
- Olivotto, I.; Maron, M.S.; Autore, C.; Lesser, J.R.; Rega, L.; Casolo, G.; De Santis, M.; Quarta, G.; Nistri, S.; Cecchi, F.; et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2008, 52, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Dardeer, A.M.; Moody, W.E.; Hayer, M.K.; Baig, S.; Price, A.M.; Leyva, F.; Edwards, N.C.; Steeds, R.P. Reference ranges for three-dimensional feature tracking cardiac magnetic resonance: Comparison with two-dimensional methodology and relevance of age and gender. Int. J. Cardiovasc. Imaging 2018, 34, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F. Echocardiographic assessment of left ventricular relaxation and cardiac filling pressures. Curr. Heart Fail. Rep. 2009, 6, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.J.; Fieno, D.S.; Parrish, T.B.; Harris, K.; Chen, E.L.; Simonetti, O.; Bundy, J.; Finn, J.P.; Klocke, F.J.; Judd, R.M. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999, 100, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.J.; Albert, T.S.; Wible, J.H.; Elliott, M.D.; Allen, J.C.; Lee, J.C.; Parker, M.; Napoli, A.; Judd, R.M. Gadoversetamide Myocardial Infarction Imaging Investigators. Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: An international, multicenter, double-blinded, randomized trial. Circulation 2008, 117, 629–637. [Google Scholar]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Xu, J.; Yang, W.; Zhao, S.; Lu, M. State-of-the-art myocardial strain by CMR feature tracking: Clinical applications and future perspectives. Eur. Radiol. 2022, 32, 5424–5435. [Google Scholar] [CrossRef]
- Henein, M.Y.; Gibson, D.G. Long axis function in disease. Heart 1999, 81, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.; Ugander, M.; Mosen, H.; Buhre, T.; Arheden, H. Atrioventricular plane displacement is the major contributor to left ventricular pumping in healthy adults, athletes, and patients with dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1452–H1459. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.D.; Fixler, D.E.; Archie, J.P.; Hoffman, J.I. Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ. Res. 1972, 30, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Kirk, E.S.; Honig, C.R. An experimental and theoretical analysis of myocardial tissue pressure. Am. J. Physiol. 1964, 207, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, A.L.; Vignes, H.; Vermot, J.; Chow, R. Mechanotransduction in cardiovascular morphogenesis and tissue engineering. Curr. Opin. Genet. Dev. 2019, 57, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Treibel, T.A.; López, B.; González, A.; Menacho, K.; Schofield, R.S.; Ravassa, S.; Fontana, M.; White, S.K.; DiSalvo, C.; Roberts, N.; et al. Reappraising myocardial fibrosis in severe aortic stenosis: An invasive and non-invasive study in 133 patients. Eur. Heart J. 2018, 39, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Moore-Morris, T.; Guimarães-Camboa, N.; Banerjee, I.; Zambon, A.C.; Kisseleva, T.; Velayoudon, A.; Stallcup, W.B.; Gu, Y.; Dalton, N.D.; Cedenilla, M.; et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J. Clin. Investig. 2014, 124, 2921–2934. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Ali, S.R.; Ranjbarvazir, S.; Talkhabi, M.; Zhao, P.; Subat, A.; Hojjat, A.; Kamra, P.; Müller, A.M.; Volz, K.S.; Tang, Z.; et al. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ. Res. 2014, 115, 625–635. [Google Scholar] [CrossRef]


| Variable | Overall n = 149 | SILGE+ Group n = 56 | SILGE− Group n = 93 | p Value |
|---|---|---|---|---|
| Clinical data | ||||
| Age, y | 61 (21) | 60 (16) | 62 (25) | 0.980 |
| Male, sex | 106 (71%) | 45 (80%) | 61 (66%) | 0.054 |
| Smoking history | 51 (34%) | 24 (43%) | 27 (29%) | 0.085 |
| Hypertension | 106 (71%) | 36 (64%) | 70 (75%) | 0.152 |
| Diabetes | 36 (23%) | 11 (20%) | 25 (27%) | 0.317 |
| Coronary artery disease | 0 | 0 | 0 | - |
| NT-proBNP, (pg/mL) | 1111.5 (2704.6) | 1651.0 (2873.4) | 956.5 (2029.4) | 0.010 |
| AS | 66 (44%) | 30 (54%) | 36 (39%) | 0.077 |
| HHD | 83 (56%) | 26 (46%) | 57 (61%) | 0.077 |
| CMR | ||||
| Cardiac function | ||||
| LVEDV, (mL) | 183.89 (123.55) | 184.42 (113.10) | 178.93 (127.43) | 0.207 |
| LVESV, (mL) | 103.54 (129.92) | 111.52 (119.61) | 91.76 (136.99) | 0.144 |
| LVSV, (mL) | 75.06 (35.19) | 77.81 (42.61) | 77.09 ± 27.39 | 0.503 |
| LVEF, (%) | 45.45 (36.16) | 42.21 ± 18.15 | 49.63 (38.56) | 0.235 |
| LVMI, (g/m2) | 93.60 (38.83) | 101.10 ± 30.72 | 91.96 ± 33.15 | 0.096 |
| Strain and strain rate | ||||
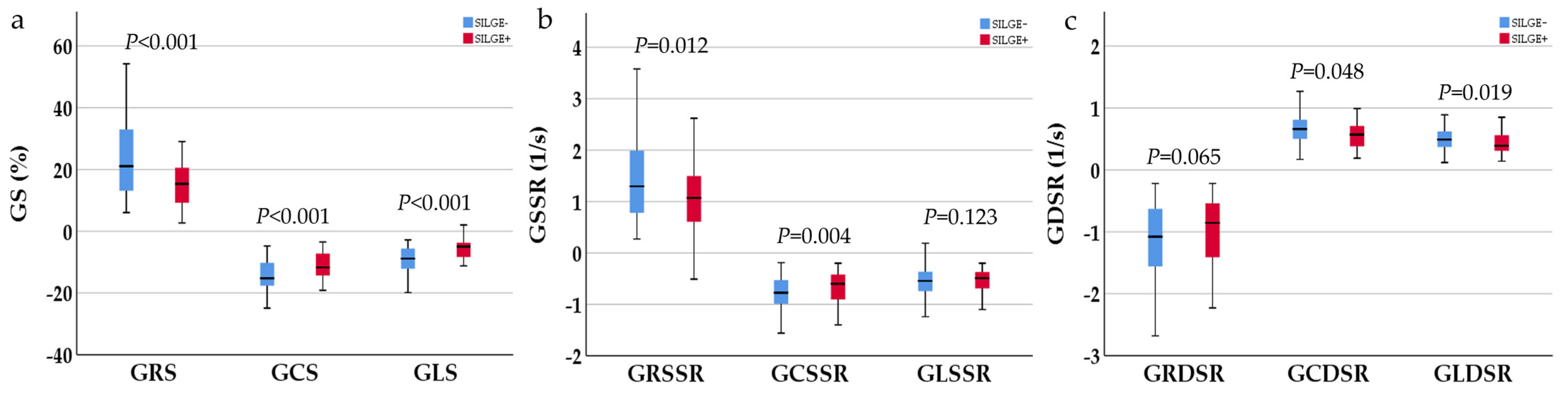
| GRS, (%) | 18.46 (16.37) | 15.59 ± 7.45 | 21.17 (21.2) | <0.001 |
| GCS, (%) | −13.15 ± 5.04 | −11.09 ± 4.37 | −14.39 ± 5.03 | <0.001 |
| GLS, (%) | −7.24 (5.54) | −5.01 ± 3.84 | −9.40 ± 4.52 | <0.001 |
| GRSSR, (1/s) | 1.19 (1.23) | 1.12 ± 0.69 | 1.30 (1.44) | 0.012 |
| GCSSR, (1/s) | −0.71 (0.53) | −0.70 ± 0.37 | −0.81 (0.50) | 0.040 |
| GLSSR, (1/s) | −0.50 (0.41) | −0.46 (0.43) | −0.55 (0.40) | 0.123 |
| GRDSR, (1/s) | −1.08 (1.00) | −0.92 (0.93) | −1.14 (1.09) | 0.065 |
| GCDSR, (1/s) | 0.61 (0.37) | 0.57 (0.38) | 0.66 (0.40) | 0.048 |
| GLDSR, (1/s) | 0.45 (0.29) | 0.39 (0.34) | 0.49 (0.29) | 0.019 |
| Variable | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age, y | 1.005 (0.983–1.029) | 0.643 | ||
| Male, sex | 2.146 (0.978–4.709) | 0.057 | ||
| Smoking history | 1.833 (0.917–3.667) | 0.087 | ||
| Hypertension | 0.525 (0.253–1.091) | 0.084 | ||
| Diabetes | 0.665 (0.298–1.484) | 0.319 | ||
| Disease type | 1.827 (0.934–3.573) | 0.078 | ||
| LVEDV, (mL) | 1.002 (0.998–1.005) | 0.304 | ||
| LVESV, (mL) | 1.002 (0.998–1.005) | 0.382 | ||
| LVSV, (mL) | 1.004 (0.992–1.015) | 0.539 | ||
| LVEF, (%) | 0.989 (0.972–1.007) | 0.227 | ||
| LVMI, (g/m2) | 1.009 (0.998–1.019) | 0.100 | ||
| GRS, (%) | 0.928 (0.894–0.964) | <0.001 | ||
| GCS, (%) | 1.153 (1.070–1.243) | <0.001 | ||
| GLS, (%) | 1.325 (1.180–1.487) | <0.001 | 1.325 (1.180–1.487) | <0.001 |
| Overall | ||
|---|---|---|
| Intra-Observer | Inter-Observer | |
| ICC (95% CI) | ICC (95% CI) | |
| GRS, % | 0.992 (0.987–0.995) | 0.988 (0.981–0.993) |
| GCS, % | 0.988 (0.979–0.993) | 0.975 (0.959–0.985) |
| GLS, % | 0.983 (0.971–0.990) | 0.970 (0.950–0.982) |
| Research center 1 | ||
| Intra-observer | Inter-observer | |
| ICC (95% CI) | ICC (95% CI) | |
| GRS, % | 0.994 (0.984–0.997) | 0.990 (0.974–0.996) |
| GCS, % | 0.982 (0.955–0.993) | 0.963 (0.910–0.985) |
| GLS, % | 0.988 (0.970–0.995) | 0.975 (0.937–0.990) |
| Research center 2 | ||
| Intra-observer | Inter-observer | |
| ICC (95% CI) | ICC (95% CI) | |
| GRS, % | 0.990 (0.976–0.996) | 0.982 (0.955–0.993) |
| GCS, % | 0.989 (0.972–0.996) | 0.974 (0.935–0.990) |
| GLS, % | 0.983 (0.957–0.993) | 0.968 (0.920–0.987) |
| Research center 3 | ||
| Intra-observer | Inter-observer | |
| ICC (95% CI) | ICC (95% CI) | |
| GRS, % | 0.992 (0.979–0.997) | 0.989 (0.972–0.996) |
| GCS, % | 0.990 (0.974–0.996) | 0.980 (0.951–0.992) |
| GLS, % | 0.984 (0.960–0.994) | 0.976 (0.941–0.990) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Long, Q.; Zeng, M.; Wu, L.; Guo, L.; Wang, G. Feature-Tracking-Derived Strain Analysis for Identification of Subendocardium-Involved Late Gadolinium Enhancement in Load-Induced Left Ventricular Hypertrophy: A Multicenter Study of Cardiac Magnetic Resonance Data. J. Clin. Med. 2023, 12, 7543. https://doi.org/10.3390/jcm12247543
Zhong Y, Long Q, Zeng M, Wu L, Guo L, Wang G. Feature-Tracking-Derived Strain Analysis for Identification of Subendocardium-Involved Late Gadolinium Enhancement in Load-Induced Left Ventricular Hypertrophy: A Multicenter Study of Cardiac Magnetic Resonance Data. Journal of Clinical Medicine. 2023; 12(24):7543. https://doi.org/10.3390/jcm12247543
Chicago/Turabian StyleZhong, Ying, Qian Long, Mu Zeng, Lianming Wu, Liang Guo, and Guan Wang. 2023. "Feature-Tracking-Derived Strain Analysis for Identification of Subendocardium-Involved Late Gadolinium Enhancement in Load-Induced Left Ventricular Hypertrophy: A Multicenter Study of Cardiac Magnetic Resonance Data" Journal of Clinical Medicine 12, no. 24: 7543. https://doi.org/10.3390/jcm12247543





