Predictive Factors of Response to Streptozotocin in Neuroendocrine Pancreatic Neoplasms
Abstract
:1. Introduction
2. Materials and Methods
3. Results
- Patient-related features
- Age
- Performance Status
- Blood biomarkers
- Associated genetic syndromes
- Tumor-related features
- Tumor grade
- Ki 67 index
- Anatomic primary tumor site
- Primary tumor size
- Tumor stage
- Site of metastasis origin
- Liver tumor burden
- Extrahepatic spread
- Functional status
- Somatostatin receptors expression
- Mechanisms of DNA repair
- Treatment-related factors
- Line of therapy
- Response to prior treatment
3.1. Patient-Related Features
3.1.1. Age
3.1.2. Performance Status
3.1.3. Blood Markers
3.1.4. Associated Genetic Syndromes
3.2. Tumor-Related Features
3.2.1. Tumor Grade
3.2.2. Ki-67 Index
3.2.3. Anatomic Primary Tumor Site
3.2.4. Primary Tumor Size
3.2.5. Tumor Stage
3.2.6. Site of Metastasis Origin
3.2.7. Liver Tumor Burden
3.2.8. Extrahepatic Spread
3.2.9. Functional Status
3.2.10. Somatostatin Receptors Expression
3.2.11. Mechanisms of DNA Repair
3.3. Treatment-Related Factors
3.3.1. Line of Therapy
3.3.2. Response to Prior Treatments
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konukiewitz, B.; Jesinghaus, M.; Kasajima, A.; Klöppel, G. Neuroendocrine neoplasms of the pancreas: Diagnosis and pitfalls. Virchows Arch. 2022, 480, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.T.; Bodei, L.; Capdevila, J.; Couvelard, A.; Falconi, M.; Glasberg, S.; Kloppel, G.; Lamberts, S.; Peeters, M.; Rindi, G.; et al. Unmet Needs in Functional and Nonfunctional Pancreatic Neuroendocrine Neoplasms. Neuroendocrinology 2019, 108, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Y.; Gong, Y.F.; Zhuang, H.K.; Zhou, Z.X.; Huang, S.Z.; Zou, Y.P.; Huang, B.W.; Sun, Z.H.; Zhang, C.Z.; Tang, Y.Q.; et al. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J. Gastroenterol. 2020, 26, 2305–2322. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Klöppel, G.; et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Ducreux, M.; García Carbonero, R.; Grande, E.; Halfdanarson, T.; Pavel, M.; Tafuto, S.; Welin, S.; Valentí, V.; Salazar, R. Streptozotocin, 1982–2022: Forty Years from the FDA’s Approval to Treat Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2022, 112, 1155–1167. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Baig, M.A.; Panchal, S.S. Streptozotocin-Induced Diabetes Mellitus in Neonatal Rats: An Insight into its Applications to Induce Diabetic Complications. Curr. Diabetes Rev. 2019, 16, 26–39. [Google Scholar] [CrossRef]
- Oberg, K. Chemotherapy and biotherapy in the treatment of neuroendocrine tumours. Ann. Oncol. 2001, 12 (Suppl. S2), S111–S114. [Google Scholar] [CrossRef]
- Kiesewetter, B.; Raderer, M. How I treat neuroendocrine tumours. ESMO Open 2020, 5, e000811. [Google Scholar] [CrossRef]
- Broder, L.E.; Carter, S.K. Pancreatic islet cell carcinoma. II. Results of therapy with streptozotocin in 52 patients. Ann. Intern. Med. 1973, 79, 108–118. [Google Scholar] [CrossRef]
- Moertel, C.G.; Hanley, J.A.; Johnson, L.A. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N. Engl. J. Med. 1980, 303, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.G.; Lefkopoulo, M.; Lipsitz, S.; Hahn, R.G.; Klaassen, D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N. Engl. J. Med. 1992, 326, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Sorbye, H.; Baudin, E.; Raymond, E.; Wiedenmann, B.; Niederle, B.; Sedlackova, E.; Toumpanakis, C.; Anlauf, M.; Cwikla, J.B.; et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology 2016, 103, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A.; ESMO Guidelines Committee. Electronic address: [email protected]. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Ito, T.; Masui, T.; Komoto, I.; Doi, R.; Osamura, R.Y.; Sakurai, A.; Ikeda, M.; Takano, K.; Igarashi, H.; Shimatsu, A.; et al. JNETS clinical practice guidelines for gastroenteropancreatic neuroendocrine neoplasms: Diagnosis, treatment, and follow-up: A synopsis. J. Gastroenterol. 2021, 56, 1033–1044. [Google Scholar] [CrossRef]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef]
- Rogers, J.E.; Lam, M.; Halperin, D.M.; Dagohoy, C.G.; Yao, J.C.; Dasari, A. Fluorouracil, Doxorubicin with Streptozocin and Subsequent Therapies in Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2022, 112, 34–42. [Google Scholar] [CrossRef]
- Schrader, J.; Henes, F.O.; Blaeker, M.; Zimmermann-Fraedrich, K.; Pace, A.; Perez, D.; Izbicki, J.R.; Lohse, A.W.; Benten, D. Extended cycle streptozotocin/5-FU chemotherapy for maintenance therapy in pancreatic neuroendocrine tumors. Endocrine 2019, 65, 460–467. [Google Scholar] [CrossRef]
- Kouvaraki, M.A.; Ajani, J.A.; Hoff, P.; Wolff, R.; Evans, D.B.; Lozano, R.; Yao, J.C. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J. Clin. Oncol. 2005, 22, 4762–4771, Erratum in J. Clin. Oncol. 2005, 23, 248. [Google Scholar] [CrossRef] [PubMed]
- Lahner, H.; Mathew, A.; Klocker, A.L.; Unger, N.; Theysohn, J.; Rekowski, J.; Jöckel, K.H.; Theurer, S.; Schmid, K.W.; Herrmann, K.; et al. Streptozocin/5-fluorouracil chemotherapy of pancreatic neuroendocrine tumours in the era of targeted therapy. Endocrine 2022, 75, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Dilz, L.M.; Denecke, T.; Steffen, I.G.; Prasad, V.; von Weikersthal, L.F.; Pape, U.F.; Wiedenmann, B.; Pavel, M. Streptozocin/5-fluorouracil chemotherapy is associated with durable response in patients with advanced pancreatic neuroendocrine tumours. Eur. J. Cancer 2015, 51, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Clewemar Antonodimitrakis, P.; Sundin, A.; Wassberg, C.; Granberg, D.; Skogseid, B.; Eriksson, B. Streptozocin and 5-Fluorouracil for the Treatment of Pancreatic Neuroendocrine Tumors: Efficacy, Prognostic Factors and Toxicity. Neuroendocrinology 2016, 103, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Kudo, A.; Akahoshi, K.; Ogura, T.; Ogawa, K.; Ban, D.; Tanaka, S.; Tanabe, M. Combination of weekly streptozocin and oral S-1 treatment for patients of unresectable or metastatic pancreatic neuroendocrine neoplasms. J. Cancer Res. Clin. Oncol. 2020, 146, 793–799. [Google Scholar] [CrossRef]
- Shibuya, H.; Hijioka, S.; Sakamoto, Y.; Ito, T.; Ueda, K.; Komoto, I.; Kobayashi, N.; Kudo, A.; Yasuda, H.; Miyake, H.; et al. Multi-center clinical evaluation of streptozocin-based chemotherapy for advanced pancreatic neuroendocrine tumors in Japan: Focus on weekly regimens and monotherapy. Cancer Chemother. Pharmacol. 2018, 82, 661–668. [Google Scholar] [CrossRef]
- Reher, D.; Fehrenbach, U.; Kayser, A.; Pape, U.F.; Henes, F.O.; Cremer, B.; Hörsch, D.; Izbicki, J.; Lohse, A.W.; Rinke, A.; et al. Localization Defines Streptozotocin/5-FU Response in Primary Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2022, 112, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Hijioka, S.; Sakuma, K.; Aoki, M.; Mizuno, N.; Kuwahara, T.; Okuno, N.; Hara, K.; Yatabe, Y. Clinical and in vitro studies of the correlation between MGMT and the effect of streptozocin in pancreatic NET. Cancer Chemother. Pharmacol. 2019, 83, 43–52. [Google Scholar] [CrossRef]
- Delaunoit, T.; Ducreux, M.; Boige, V.; Dromain, C.; Sabourin, J.C.; Duvillard, P.; Schlumberger, M.; de Baere, T.; Rougier, P.; Ruffie, P.; et al. The doxorubicin-streptozotocin combination for the treatment of advanced well-differentiated pancreatic endocrine carcinoma; a judicious option? Eur. J. Cancer 2004, 40, 515–520. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- He, C.B.; Zhang, Y.; Cai, Z.Y.; Lin, X.J. The impact of surgery in metastatic pancreatic neuroendocrine tumors: A competing risk analysis. Endocr. Connect. 2019, 8, 239–251. [Google Scholar] [CrossRef]
- Sharpe, S.M.; In, H.; Winchester, D.J.; Talamonti, M.S.; Baker, M.S. Surgical resection provides an overall survival benefit for patients with small pancreatic neuroendocrine tumors. J. Gastrointest. Surg. 2015, 19, 117–123. [Google Scholar] [CrossRef]
- Teo, R.; Goh, B.K.P.; Tai, D.W.M.; Allen, J.C.; Lim, T.K.H.; Hwang, J.S.G.; Tan, D.M.; Lee, S.Y.; Chan, C.Y.; Cheow, P.C.; et al. Validation and comparison between current prognostication systems for pancreatic neuroendocrine neoplasms: A single-institution experience with 176 patients. Surgery 2017, 161, 1235–1245. [Google Scholar] [CrossRef]
- Chawla, A.; Williams, R.T.; Sich, N.; Clancy, T.; Wang, J.; Ashley, S.; Pezzi, C.; Swanson, R. Pancreaticoduodenectomy and metastasectomy for metastatic pancreatic neuroendocrine tumors. J. Surg. Oncol. 2018, 118, 983–990. [Google Scholar] [CrossRef]
- Oh, T.G.; Chung, M.J.; Park, J.Y.; Bang, S.M.; Park, S.W.; Chung, J.B.; Song, S.Y. Prognostic factors and characteristics of pancreatic neuroendocrine tumors: Single center experience. Yonsei Med. J. 2012, 53, 944–951. [Google Scholar] [CrossRef]
- Kurita, Y.; Kobayashi, N.; Hara, K.; Mizuno, N.; Kuwahara, T.; Okuno, N.; Haba, S.; Tokuhisa, M.; Hasegawa, S.; Sato, T.; et al. Effectiveness and Prognostic Factors of Everolimus in Patients with Pancreatic Neuroendocrine Neoplasms. Intern. Med. 2023, 62, 159–167. [Google Scholar] [CrossRef]
- Lawrence, B.; Gustafsson, B.I.; Kidd, M.; Pavel, M.; Svejda, B.; Modlin, I.M. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol. Metab. Clin. N. Am. 2011, 40, 111–134. [Google Scholar] [CrossRef]
- Pulvirenti, A.; Rao, D.; Mcintyre, C.A.; Gonen, M.; Tang, L.H.; Klimstra, D.S.; Fleisher, M.; Ramanathan, L.V.; Reidy-Lagunes, D.; Allen, P.J. Limited role of Chromogranin A as clinical biomarker for pancreatic neuroendocrine tumors. HPB 2019, 21, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, C.; Tang, M.; Xu, X.; Liu, L.; Ji, Y.; Pan, B.; Lou, W. The value of serum chromogranin A as a predictor of tumor burden, therapeutic response, and nomogram-based survival in well-moderate nonfunctional pancreatic neuroendocrine tumors with liver metastases. Eur. J. Gastroenterol. Hepatol. 2015, 27, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T. Significance of chromogranin A and synaptophysin in pancreatic neuroendocrine tumors. Bosn J. Basic Med. Sci. 2020, 20, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Yang, Q.C.; Lin, Y.; Xue, L.; Chen, M.H.; Chen, J. Chromogranin A as a marker for diagnosis, treatment, and survival in patients with gastroenteropancreatic neuroendocrine neoplasm. Medicine 2014, 93, e247. [Google Scholar] [CrossRef]
- Yang, M.; Ke, N.W.; Zhang, Y.; Zeng, L.; Tan, C.L.; Zhang, H.; Mai, G.; Tian, B.L.; Liu, X.B. Survival Analyses for Patients With Surgically Resected Pancreatic Neuroendocrine Tumors by World Health Organization 2010 Grading Classifications and American Joint Committee on Cancer 2010 Staging Systems. Medicine 2016, 94, e2156, Erratum in Medicine 2016, 95, e507d. [Google Scholar] [CrossRef] [PubMed]
- Vilar, E.; Salazar, R.; Pérez-García, J.; Cortes, J.; Oberg, K.; Tabernero, J. Chemotherapy and role of the proliferation marker Ki-67 in digestive neuroendocrine tumors. Endocr. Relat. Cancer 2007, 14, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Welin, S.; Langer, S.W.; Vestermark, L.W.; Holt, N.; Osterlund, P.; Dueland, S.; Hofsli, E.; Guren, M.G.; Ohrling, K.; et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. 2013, 24, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Milione, M.; Maisonneuve, P.; Spada, F.; Pellegrinelli, A.; Spaggiari, P.; Albarello, L.; Pisa, E.; Barberis, M.; Vanoli, A.; Buzzoni, R.; et al. The Clinicopathologic Heterogeneity of Grade 3 Gastroenteropancreatic Neuroendocrine Neoplasms: Morphological Differentiation and Proliferation Identify Different Prognostic Categories. Neuroendocrinology 2017, 104, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.; Boch, M.; Daniel, H.; Nimphius, W.; Müller, D.; Michl, P.; Rinke, A.; Gress, T.M. Streptozocin-Based Chemotherapy in Patients with Advanced Neuroendocrine Neoplasms—Predictive and Prognostic Markers for Treatment Stratification. PLoS ONE 2015, 10, e0143822. [Google Scholar] [CrossRef]
- Zhou, B.; Duan, J.; Yan, S.; Zhou, J.; Zheng, S. Prognostic Factors of Long−Term Outcome in Surgically Resectable Pancreatic Neuroendocrine Tumors: A 12−Year Experience From a Single Center. Oncol. Lett. 2017, 13, 1157–1164. [Google Scholar] [CrossRef]
- Jin, K.; Luo, G.; Xu, J.; Zhang, B.; Liu, C.; Yu, X. Clinical Outcomes and Prognostic Factors of Resected Pancreatic Neuroendocrine Neoplasms: A Single-Center Experience in China. Oncol. Lett. 2017, 13, 3163–3168. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Wiseman, J.T.; Pawlik, T.M. Surgical management of pancreatic neuroendocrine liver metastases. J. Gastrointest. Oncol. 2020, 11, 590–600. [Google Scholar] [CrossRef]
- Nigri, G.; Petrucciani, N.; Debs, T.; Mangogna, L.M.; Crovetto, A.; Moschetta, G.; Persechino, R.; Aurello, P.; Ramacciato, G. Treatment options for PNET liver metastases: A systematic review. World J. Surg. Oncol. 2018, 16, 142. [Google Scholar] [CrossRef]
- Madeira, I.; Terris, B.; Voss, M.; Denys, A.; Sauvanet, A.; Flejou, J.F.; Vilgrain, V.; Belghiti, J.; Bernades, P.; Ruszniewski, P. Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut 1998, 43, 422–427. [Google Scholar] [CrossRef]
- Yu, F.; Venzon, D.J.; Serrano, J.; Goebel, S.U.; Doppman, J.L.; Gibril, F.; Jensen, R.T. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J. Clin. Oncol. 1999, 17, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Que, F.G.; Nagorney, D.M.; Batts, K.P.; Linz, L.J.; Kvols, L.K. Hepatic resection for metastatic neuroendocrine carcinomas. Am. J. Surg. 1995, 169, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Lebtahi, R.; Cadiot, G.; Delahaye, N.; Genin, R.; Daou, D.; Peker, M.C.; Chosidow, D.; Faraggi, M.; Mignon, M.; Le Guludec, D. Detection of bone metastases in patients with endocrine gastroenteropancreatic tumors: Bone scintigraphy compared with somatostatin receptor scintigraphy. J. Nucl. Med. 1999, 40, 1602–1608. [Google Scholar]
- Mathew, A.; Fendler, W.P.; Theysohn, J.; Herrmann, K.; Führer, D.; Lahner, H. Bone Metastases in Patients with Pancreatic NETs: Prevalence and Prognosis. Horm Metab. Res. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Van Loon, K.; Zhang, L.; Keiser, J.; Carrasco, C.; Glass, K.; Ramirez, M.T.; Bobiak, S.; Nakakura, E.K.; Venook, A.P.; Shah, M.H.; et al. Bone metastases and skeletal-related events from neuroendocrine tumors. Endocr. Connect. 2015, 4, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Di Dato, C.; Martini, C.; Sciammarella, C.; Di Sarno, A.; Colao, A.; Faggiano, A.; NIKE Group. Bone Metastases in Neuroendocrine Neoplasms: From Pathogenesis to Clinical Management. Cancers 2019, 11, 1332. [Google Scholar] [CrossRef]
- Sabet, A.; Khalaf, F.; Haslerud, T.; Al-Zreiqat, A.; Sabet, A.; Simon, B.; Pöppel, T.D.; Biersack, H.J.; Ezziddin, S. Bone metastases in GEP-NET: Response and long-term outcome after PRRT from a follow-up analysis. Am. J. Nucl. Med. Mol. Imaging 2013, 3, 437–445. [Google Scholar]
- Ezziddin, S.; Sabet, A.; Heinemann, F.; Yong-Hing, C.J.; Ahmadzadehfar, H.; Guhlke, S.; Höller, T.; Willinek, W.; Boy, C.; Biersack, H.J. Response and long-term control of bone metastases after peptide receptor radionuclide therapy with (177)Lu-octreotate. J. Nucl. Med. 2011, 52, 1197–1203. [Google Scholar] [CrossRef]
- Eriksson, B.; Skogseid, B.; Lundqvist, G.; Wide, L.; Wilander, E.; Oberg, K. Medical treatment and long-term survival in a prospective study of 84 patients with endocrine pancreatic tumors. Cancer 1990, 65, 1883–1890. [Google Scholar] [CrossRef]
- Walter, T.; van Brakel, B.; Vercherat, C.; Hervieu, V.; Forestier, J.; Chayvialle, J.A.; Molin, Y.; Lombard-Bohas, C.; Joly, M.O.; Scoazec, J.Y. O6-Methylguanine-DNA methyltransferase status in neuroendocrine tumours: Prognostic relevance and association with response to alkylating agents. Br. J. Cancer 2015, 112, 523–531. [Google Scholar] [CrossRef]
- Yagi, K.; Ono, H.; Kudo, A.; Kinowaki, Y.; Asano, D.; Watanabe, S.; Ishikawa, Y.; Ueda, H.; Akahoshi, K.; Tanaka, S.; et al. MGMT is frequently inactivated in pancreatic NET-G2 and is associated with the therapeutic activity of STZ-based regimens. Sci. Rep. 2023, 13, 7535. [Google Scholar] [CrossRef] [PubMed]
- de Mestier, L.; Couvelard, A.; Blazevic, A.; Hentic, O.; de Herder, W.W.; Rebours, V.; Paradis, V.; Ruszniewski, P.; Hofland, L.J.; Cros, J. Critical appraisal of MGMT in digestive NET treated with alkylating agents. Endocr. Relat. Cancer 2020, 27, R391–R405. [Google Scholar] [CrossRef]
- Lemelin, A.; Barritault, M.; Hervieu, V.; Payen, L.; Péron, J.; Couvelard, A.; Cros, J.; Scoazec, J.Y.; Bin, S.; Villeneuve, L.; et al. O6-methylguanine-DNA methyltransferase (MGMT) status in neuroendocrine tumors: A randomized phase II study (MGMT-NET). Dig. Liver Dis. 2019, 51, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, C.; Perrier, M.; Hentic, O.; Brixi, H.; De Rycke, O.; Cros, J.; Rebours, V.; Cadiot, G.; Ruszniewski, P.; de Mestier, L. FOLFOX-bevacizumab chemotherapy in patients with metastatic neuroendocrine tumors. J. Neuroendocrinol. 2023, 35, e13227. [Google Scholar] [CrossRef] [PubMed]
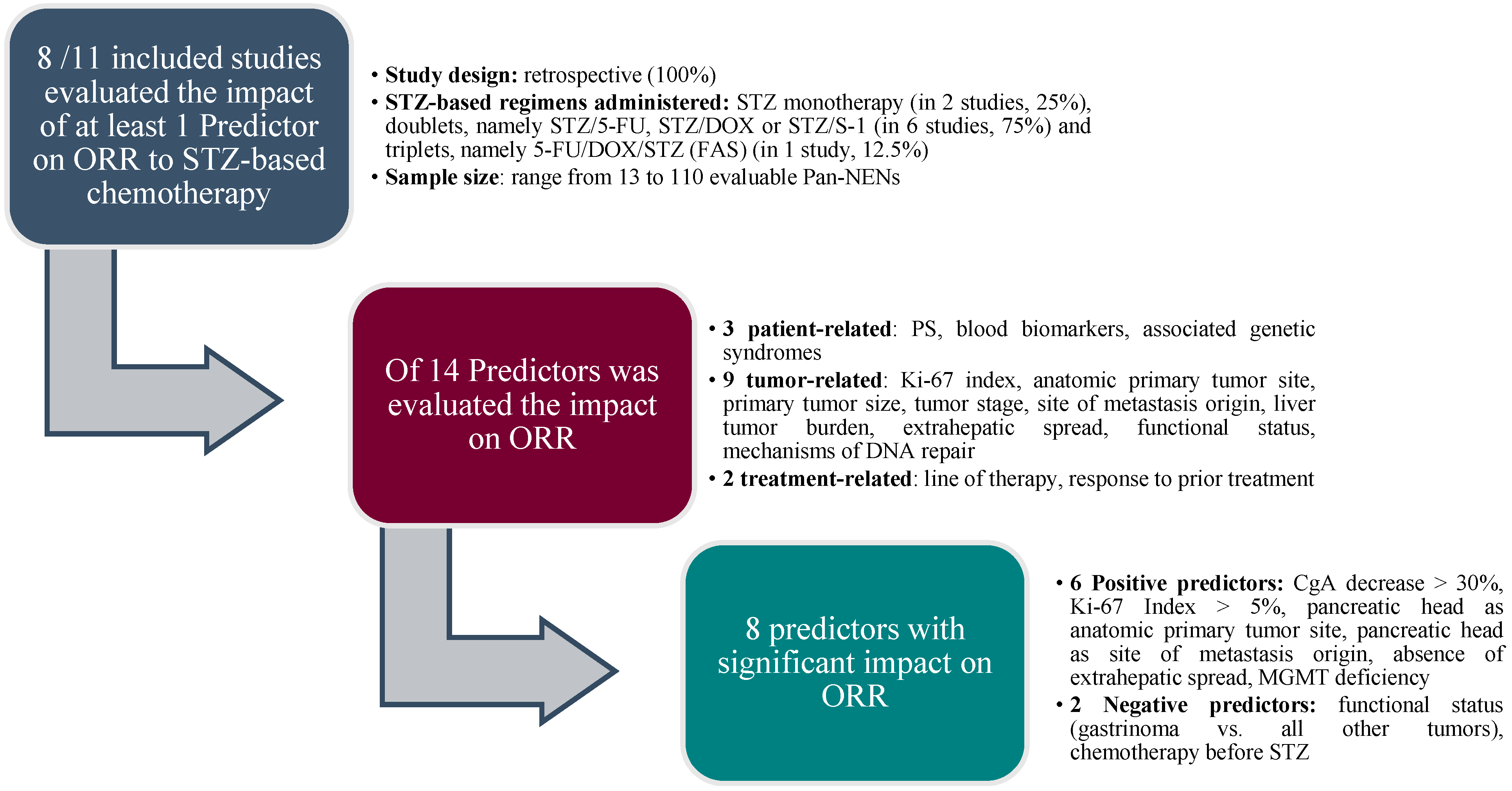
| (a)Patient-related features: age, performance status, blood biomarkers, associated genetic syndromes | ||||||||
| Predictor | Subgroups compared | End-point evaluated | Predictors’ Significance | n evaluable Pan-NENs patients | Treatments | Type of study | First Author | Year |
| Age | >55 vs. ≤55 | OS | Negative | 243 | 5-FU/DOX/STZ (FAS) | retrospective | Rogers JE [19] | 2022 |
| Age | >55 vs. ≤55 | PFS | Not significant | 220 | 5-FU/DOX/STZ (FAS) | retrospective | Rogers JE [19] | 2022 |
| Age | >65 vs. ≤65 | PFS | Not significant | 28 | STZ/5-FU | retrospective | Schrader J [20] | 2019 |
| Age | <54 vs. ≥54 | 2-Year PFS, PFS | Negative | 84 | 5-FU/DOX/STZ (FAS) | retrospective | Kouvaraki MA [21] | 2004 |
| Age | <54 vs. ≥54 | 2-Year OS | Not significant | 84 | 5-FU/DOX/STZ (FAS) | retrospective | Kouvaraki MA [21] | 2004 |
| Performance Status | ≤1 vs. >1 | ORR | Not significant | 50 | STZ/5-FU | retrospective | Lahner H [22] | 2022 |
| Blood biomarkers | CgA decrease > 30% | ORR | Positive | 50 | STZ/5-FU | retrospective | Lahner H [22] | 2022 |
| Blood biomarkers | CgA decrease > 50% | OS | Not significant | 243 | 5-FU/DOX/STZ (FAS) | retrospective | Rogers JE [19] | 2022 |
| Blood biomarkers | CgA decrease > 50% | PFS | Not significant | 220 | 5-FU/DOX/STZ (FAS) | retrospective | Rogers JE [19] | 2022 |
| Blood biomarkers | CgA decrease > 30% | ORR | Positive | 96 | STZ/5-FU | retrospective | Dilz DM [23] | 2015 |
| Blood biomarkers | CgA decrease > 30% | TTP, OS | Not significant | 96 | STZ/5-FU | retrospective | Dilz DM [23] | 2015 |
| Blood biomarkers | CgA decrease > 30% | ORR | Positive | 51 | 5-FU/DOX/STZ (FAS) | retrospective | Kouvaraki MA [21] | 2004 |
| Blood biomarkers | normal vs. increased upon normal values prior to treatment | 2-Year PFS, 2-Year OS | Not significant | 60 | 5-FU/DOX/STZ (FAS) | retrospective | Kouvaraki MA [21] | 2004 |
| Associated genetic syndromes | Presence vs. absence of MEN1 | OS | Not significant | 133 | STZ/5-FU | retrospective | Antonodimitrakis P [24] | 2016 |
| Associated genetic syndromes | Presence vs. absence of MEN1 | PFS, ORR | Not significant | 100 | STZ/5-FU | retrospective | Antonodimitrakis P [24] | 2016 |
| Associated genetic syndromes | Presence vs. absence of MEN1 | OS | Not significant | 100 | STZ/5-FU | retrospective | Antonodimitrakis P [24] | 2016 |
| (b) Tumor-related features: tumor grade, Ki-67 index, anatomic primary tumor site,primary tumor size | ||||||||
| Predictor | Subgroups compared | End-point evaluated | Predictors’ Significance | n evaluable Pan-NENs patients | Treatments | Type of study | First Author | Year |
| Tumor Grade | NET G1/G2 vs. NET G3/NEC G3 | PFS | Not significant | 20 | STZ/S-1 | retrospective | Ono H [25] | 2020 |
| Tumor Grade | G3 vs. G1/G2 | OS | Negative | 133 | STZ/5-FU | retrospective | Antonodimitrakis P [24] | 2016 |
| Tumor Grade | G3 vs. G1/G2 | PFS | Negative | 100 | STZ/5-FU | retrospective | Antonodimitrakis P [24] | 2016 |
| Tumor Grade | Low vs. high | 2-Year PFS | Positive | 30 | 5-FU/DOX/STZ (FAS) | retrospective | Kouvaraki MA [21] | 2004 |
| Tumor Grade | Low vs. high | 2-Year OS | Not significant | 30 | 5-FU/DOX/STZ (FAS) | retrospective | Kouvaraki MA [21] | 2004 |
| Ki 67 Index | ≤ 15% vs. >15% | ORR | Not significant | 50 | STZ/5-FU | retrospective | Lahner H [22] | 2022 |
| Ki 67 Index | <5 vs. >5%, <10% vs. >10, <15% vs. >15% | PFS | Not significant | 20 | STZ/S-1 | retrospective | Ono H [25] | 2020 |
| Ki-67 Index | ≤10% vs. >10% | PFS | Not significant | 25 | STZ/5-FU | retrospective | Schrader J [20] | 2019 |
| Ki-67 Index | >5% vs. ≤5% | ORR | Positive | 110 | STZ monotherapy; STZ/5-FU-S1; STZ/DOX | retrospective | Shibuya H [26] | 2018 |
| Ki-67 Index | >15% vs. ≤15% | ORR | Not significant | 96 | STZ/5-FU | retrospective | Dilz DM [23] | 2015 |
| Ki-67 Index | >15% vs. ≤15% | TTP, OS | Negative | 96 | STZ/5-FU | retrospective | Dilz DM [23] | 2015 |
| Anatomic primary tumor site | Head vs. body vs. tail | ORR | Positive | 63 | STZ/5-FU | retrospective | Reher D [27] | 2022 |
| Anatomic primary tumor site | Head vs. body vs. tail | OS | Not significant | 72 | STZ/5-FU | retrospective | Reher D [27] | 2022 |
| Anatomic primary tumor site | Head vs. body vs. tail | PFS | Not significant | 67 | STZ/5-FU | retrospective | Reher D [27] | 2022 |
| Primary tumor size | ≤50 mm vs. > 50 mm | ORR, OS, PFS | Not significant | 73 | STZ/5-FU | retrospective | Reher D [27] | 2022 |
| Primary tumor size | ≤50 mm vs. > 50 mm | PFS | Not significant | 20 | STZ/S-1 | retrospective | Ono H [25] | 2020 |
| (c) Tumor-related features: tumor stage, site of metastasis origin, liver tumor burden, extrahepatic spread | ||||||||
| Predictor | Subgroups compared | End-point evaluated | Predictors’ Significance | n evaluable Pan-NENs patients | Treatments | Type of study | First Author | Year |
| Tumour stage | Metastatic vs. locally advanced | OS | Not significant | 243 | 5-FU/DOX/STZ (FAS) | retrospective | Rogers JE [19] | 2022 |
| Tumour stage | Metastatic vs. locally advanced | PFS | Not significant | 220 | 5-FU/DOX/STZ (FAS) | retrospective | Rogers JE [19] | 2022 |
| Tumour stage | Stage 4 vs. others | OS | Not significant | 133 | STZ/5-FU | retrospective | Antonodimitrakis P [24] | 2016 |
| Tumour stage | Stage 4 vs. others | PFS | Negative | 100 | STZ/5-FU | retrospective | Antonodimitrakis P [24] | 2016 |
| Tumour stage | Locally advanced vs. metastatic | ORR | Not significant | 33 | 5-FU/DOX/STZ (FAS) | retrospective | Kouvaraki MA [21] | 2004 |
| Site of metastasis origin | Head vs. body vs. tail | ORR | Positive | Not Specified | STZ/5-FU | retrospective | Reher D [27] | 2022 |
| Liver tumour burden | 10, 25, and 50% as cut-off values | ORR | Not significant | 108 | STZ; STZ/5-FU-S1; STZ/DOX | retrospective | Shibuya H [26] | 2018 |
| Liver tumour burden | ≤10% vs. >10% | ORR | Not significant | 84 | STZ/5-FU | retrospective | Dilz DM [23] | 2015 |
| Liver tumour burden | >10% vs. ≤10% | TTP (univariate and multivariate analysis), OS (multivariate analysis) | Not significant | 84 | STZ/5-FU | retrospective | Dilz DM [23] | 2015 |
| Liver tumour burden | >10% vs. ≤10% | OS (univariate analysis) | Negative | 84 | STZ/5-FU | retrospective | Dilz DM [23] | 2015 |
| Liver tumour burden | ≤75% vs. >75% | ORR | Not significant | 73 | 5-FU/DOX/STZ (FAS) | retrospective | Kouvaraki MA [21] | 2004 |
| Liver tumour burden | ≤75% vs. >75% | 2-years PFS, 2-years OS | Positive | 73 | 5-FU/DOX/STZ (FAS) | retrospective | Kouvaraki MA [21] | 2004 |
| Extrahepatic spread | ≥2 distant metastatic sites vs. <2 | ORR | Not significant | 50 | STZ/5-FU | retrospective | Lahner H [22] | 2022 |
| Extrahepatic spread | Presence of bone metastases | ORR | Not significant | 50 | STZ/5-FU | retrospective | Lahner H [22] | 2022 |
| Extrahepatic spread | Presence of bone metastases | OS | Negative | 50 | STZ/5-FU | retrospective | Lahner H [22] | 2022 |
| Extrahepatic spread | Presence of bone metastases | PFS | Not significant | 50 | STZ/5-FU | retrospective | Lahner H [22] | 2022 |
| Extrahepatic spread | Liver only vs. other sites ± liver | ORR | Positive | 76 | 5-FU/DOX/STZ (FAS) | retrospective | Kouvaraki MA [21] | 2004 |
| (d) Tumor-related features: functional status, somatostatin receptors expression, mechanisms of DNA repair | ||||||||
| Predictor | Subgroups compared | End-point evaluated | Predictors’ Significance | n evaluable Pan-NENs patients | Treatments | Type of study | First Author | Year |
| Functional status | Functioning vs. NF | ORR | Not significant | 50 | STZ/5-FU | retrospective | Lahner H [22] | 2022 |
| Functional status | Functioning vs. NF | OS (univariate analysis) | Positive | 243 | 5-FU/DOX/STZ (FAS) | retrospective | Rogers JE [19] | 2022 |
| Functional status | Functioning vs. NF | OS (multivariate analysis], PFS | Not significant | 243 | 5-FU/DOX/STZ (FAS) | retrospective | Rogers JE [19] | 2022 |
| Functional status | Functioning vs. NF | PFS (univariate analysis) | Positive | 100 | STZ/5-FU | retrospective | Antonodimitrakis P [24] | 2016 |
| Functional status | Functioning vs. NF | PFS (multivariate analysis) | Not significant | 100 | STZ/5-FU | retrospective | Antonodimitrakis P [24] | 2016 |
| Functional status | Functioning vs. NF | ORR | Not significant | 96 | STZ/5-FU | retrospective | Dilz DM [23] | 2015 |
| Functional status | Gastrinoma vs. all other PEC | ORR | Negative | 84 | 5-FU/DOX/STZ (FAS) | retrospective | Kouvaraki MA [21] | 2004 |
| Functional status | Gastrinoma vs. all other PEC | 2-years PFS, 2-years OS, PFS | Not significant | 84 | 5-FU/DOX/STZ (FAS) | retrospective | Kouvaraki MA [21] | 2004 |
| Somatostatin receptors expression | Positive vs. negative Octreoscan | PFS | Not significant | 28 | STZ/5-FU | retrospective | Schrader J [20] | 2019 |
| Mechanisms of DNA repair | MGMT deficiency vs. non-deficiency | ORR | Positive | 13 | STZ; STZ/5-FU | retrospective | Hijioka S [28] | 2019 |
| (e) Treatment-related factors: line of therapy and response to prior treatment | ||||||||
| Predictor | Subgroups compared | End-point evaluated | Predictors’ Significance | n evaluable Pan-NENs patients | Treatments | Type of study | First Author | Year |
| Line of therapy | First vs. > first line | ORR | Not significant | 50 | STZ/5-FU | retrospective | Lahner H [22] | 2022 |
| Line of therapy | First vs. > first line | OS | Positive | 50 | STZ/5-FU | retrospective | Lahner H [22] | 2022 |
| Line of therapy | First vs. second line | ORR | Not significant | 110 | STZ; STZ/5-FU-S1; STZ/DOX | retrospective | Shibuya H [26] | 2018 |
| Line of therapy | First vs. second line | 2-Year PFS, OS | Not significant | 84 | 5-FU/DOX/STZ (FAS) | retrospective | Kouvaraki MA [21] | 2004 |
| Line of therapy | First vs. second line | PFS | Positive | 84 | 5-FU/DOXSTZ (FAS) | retrospective | Kouvaraki MA [21] | 2004 |
| Response to prior treatment | Chemotherapy before STZ: yes vs. not | ORR | Negative | 45 | STZ/DOX | retrospective | Delaunoit T [29] | 2004 |
| Response to prior treatment | Chemotherapy before STZ: yes vs. not | OS | Negative | 45 | STZ/DOX | retrospective | Delaunoit T [29] | 2004 |
| Response to prior treatment | Chemoembolization before STZ: yes vs. not | OS | Negative | 45 | STZ/DOX | retrospective | Delaunoit T [29] | 2004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanciulli, G.; La Salvia, A.; Di Molfetta, S.; Cannavale, G.; Puliani, G.; Verrico, M.; Campolo, F.; Colao, A.; Faggiano, A.; NIKE Group. Predictive Factors of Response to Streptozotocin in Neuroendocrine Pancreatic Neoplasms. J. Clin. Med. 2023, 12, 7557. https://doi.org/10.3390/jcm12247557
Fanciulli G, La Salvia A, Di Molfetta S, Cannavale G, Puliani G, Verrico M, Campolo F, Colao A, Faggiano A, NIKE Group. Predictive Factors of Response to Streptozotocin in Neuroendocrine Pancreatic Neoplasms. Journal of Clinical Medicine. 2023; 12(24):7557. https://doi.org/10.3390/jcm12247557
Chicago/Turabian StyleFanciulli, Giuseppe, Anna La Salvia, Sergio Di Molfetta, Giuseppe Cannavale, Giulia Puliani, Monica Verrico, Federica Campolo, Annamaria Colao, Antongiulio Faggiano, and NIKE Group. 2023. "Predictive Factors of Response to Streptozotocin in Neuroendocrine Pancreatic Neoplasms" Journal of Clinical Medicine 12, no. 24: 7557. https://doi.org/10.3390/jcm12247557






