Assessing the Assisted Six-Minute Cycling Test as a Measure of Endurance in Non-Ambulatory Patients with Spinal Muscular Atrophy (SMA)
Abstract
:1. Introduction
1.1. SMA: Overview of Clinical Outcome Assessments
1.2. Current Evaluations for Endurance Capacity in SMA
1.3. Assisted 6-Minute Cycling Test Use in Other Populations
1.4. Study Objective
2. Materials and Methods
2.1. Inclusion/Exclusion
2.2. Performance Outcome (PerfO) of Endurance Using the A6MCT
2.3. Clinical Assessments of Motor Function (RULM, ATEND)
2.4. Perceived Fatigue and Effort (FSS, OMNI RPE)
2.5. Patient-Reported Outcome (EK2)
2.6. Statistical Analysis
3. Results
3.1. Demographics
3.1.1. Clinical Characteristics
3.1.2. Descriptive Statistics of A6MCT Primary and Secondary Variables
3.2. A6MCT Associations to Clinical Characteristics and Secondary Outcomes
3.3. Longitudinal Associations
4. Discussion
4.1. Feasibility
4.2. Relevance to Clinical Outcomes: Convergent Validity
4.3. Relevance to Clinical Characteristics and Change over Time
4.4. Limitations
4.5. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darras, B.T.; De Vivo, D.C. Precious SMA natural history data. Neurology 2018, 91, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S.J.; Coffey, C.S.; Yankey, J.W.; Krosschell, K.; Arnold, W.D.; Rutkove, S.B.; Swoboda, K.J.; Reyna, S.P.; Sakonju, A.; Darras, B.T.; et al. Natural history of infantile-onset spinal muscular atrophy. Ann. Neurol. 2017, 82, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Nusinersen: First Global Approval. Drugs 2017, 77, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Ratni, H.; Ebeling, M.; Baird, J.; Bendels, S.; Bylund, J.; Chen, K.S.; Denk, N.; Feng, Z.; Green, L.; Guerard, M.; et al. Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 (SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA). J. Med. Chem. 2018, 61, 6501–6517. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Trundell, D.; Le Scouiller, S.; Le Goff, L.; Gorni, K.; Vuillerot, C. Assessment of the validity and reliability of the 32-item Motor Function Measure in individuals with Type 2 or non-ambulant Type 3 spinal muscular atrophy. PLoS ONE 2020, 15, e0238786. [Google Scholar] [CrossRef] [PubMed]
- Stolte, B.; Bois, J.; Bolz, S.; Kizina, K.; Totzeck, A.; Schlag, M.; Kleinschnitz, C.; Hagenacker, T. Minimal clinically important differences in functional motor scores in adults with spinal muscular atrophy. Eur. J. Neurol. 2020, 27, 2586–2594. [Google Scholar] [CrossRef]
- Mazzone, E.S.; Mayhew, A.; Montes, J.; Ramsey, D.; Fanelli, L.; Young, S.D.; Salazar, R.; De Sanctis, R.; Pasternak, A.; Glanzman, A.; et al. Revised upper limb module for spinal muscular atrophy: Development of a new module. Muscle Nerve 2017, 55, 869–874. [Google Scholar] [CrossRef]
- Sivo, S.; Mazzone, E.; Antonaci, L.; De Sanctis, R.; Fanelli, L.; Palermo, C.; Montes, J.; Pane, M.; Mercuri, E. Upper limb module in non-ambulant patients with spinal muscular atrophy: 12 month changes. Neuromuscul. Disord. 2014, 25, 212–215. [Google Scholar] [CrossRef]
- Pera, M.C.; Coratti, G.; Mazzone, E.S.; Montes, J.; Scoto, M.; De Sanctis, R.; Main, M.; Mayhew, A.; Lofra, R.M.; Young, S.D.; et al. Revised upper limb module for spinal muscular atrophy: 12 month changes. Muscle Nerve 2019, 59, 426–430. [Google Scholar] [CrossRef]
- Main, M.; Kairon, H.; Mercuri, E.; Muntoni, F. The Hammersmith Functional Motor Scale for Children with Spinal Muscular Atrophy: A Scale to Test Ability and Monitor Progress in Children with Limited Ambulation. Eur. J. Paediatr. Neurol. 2003, 7, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Messina, S.; Battini, R.; Berardinelli, A.; Boffi, P.; Bono, R.; Bruno, C.; Carboni, N.; Cini, C.; Colitto, F.; et al. Reliability of the Hammersmith functional motor scale for spinal muscular atrophy in a multicentric study. Neuromuscul. Disord. 2006, 16, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Coratti, G.; Pera, M.C.; Montes, J.; Scoto, M.; Pasternak, A.; Bovis, F.; Sframeli, M.; D’Amico, A.; Pane, M.; Albamonte, E.; et al. Revised upper limb module in type II and III spinal muscular atrophy: 24-month changes. Neuromuscul. Disord. 2021, 32, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Hagenacker, T.; Wurster, C.D.; Günther, R.; Schreiber-Katz, O.; Osmanovic, A.; Petri, S.; Weiler, M.; Ziegler, A.; Kuttler, J.; Koch, J.C.; et al. Nusinersen in adults with 5q spinal muscular atrophy: A non-interventional, multicentre, observational cohort study. Lancet Neurol. 2020, 19, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Coratti, G.; Cutrona, C.; Pera, M.C.; Bovis, F.; Ponzano, M.; Chieppa, F.; Antonaci, L.; Sansone, V.; Finkel, R.; Pane, M.; et al. Motor function in type 2 and 3 SMA patients treated with Nusinersen: A critical review and meta-analysis. Orphanet J. Rare Dis. 2021, 16, 430. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Costa, J.F.; Povedano, M.; Nascimiento-Osorio, A.E.; Escribano, A.M.; Garcia, S.K.; Dominguez, R.; Exposito, J.M.; González, L.; Marco, C.; Castillo, J.M.; et al. Validation of motor and functional scales for the evaluation of adult patients with 5q spinal muscular atrophy. Eur. J. Neurol. 2022, 29, 3666–3675. [Google Scholar] [CrossRef] [PubMed]
- Montes, J.; Glanzman, A.M.; Mazzone, E.S.; Martens, W.B.; Dunaway, S.; Pasternak, A.; O Riley, S.; Quigley, J.; Pandya, S.; De Vivo, D.C.; et al. Spinal muscular atrophy functional composite score: A functional measure in spinal muscular atrophy. Muscle Nerve 2015, 52, 942–947. [Google Scholar] [CrossRef] [PubMed]
- McGraw, S.; Qian, Y.; Henne, J.; Jarecki, J.; Hobby, K.; Yeh, W.-S. A qualitative study of perceptions of meaningful change in spinal muscular atrophy. BMC Neurol. 2017, 17, 68. [Google Scholar] [CrossRef]
- Kruse, T.; Shamai, S.; Leflerovà, D.; Wirth, B.; Heller, R.; Schloss, N.; Lehmann, H.C.; Brakemeier, S.; Hagenacker, T.; Braumann, B.; et al. Objective measurement of oral function in adults with spinal muscular atrophy. Orphanet J. Rare Dis. 2023, 18, 103. [Google Scholar] [CrossRef]
- Girard, O.; Millet, G.P.; Micallef, J.-P.; Racinais, S. Alteration in neuromuscular function after a 5 km running time trial. Eur. J. Appl. Physiol. 2012, 112, 2323–2330. [Google Scholar] [CrossRef]
- Jones, H.S.; Williams, E.L.; Marchant, D.; Sparks, S.A.; Bridge, C.A.; Midgley, A.W.; Mc Naughton, L.R. Improvements in Cycling Time Trial Performance Are Not Sustained Following the Acute Provision of Challenging and Deceptive Feedback. Front. Physiol. 2016, 7, 399. [Google Scholar] [CrossRef] [PubMed]
- Smits, B.L.M.; Polman, R.C.J.; Otten, B.; Pepping, G.-J.; Hettinga, F.J. Cycling in the Absence of Task-Related Feedback: Effects on Pacing and Performance. Front. Physiol. 2016, 7, 348. [Google Scholar] [CrossRef] [PubMed]
- Kujala, U.M.; Vaara, J.P.; Kainulainen, H.; Vasankari, T.; Vaara, E.; Kyröläinen, H. Associations of Aerobic Fitness and Maximal Muscular Strength with Metabolites in Young Men. JAMA Netw. Open 2019, 2, e198265. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Grgic, J.; Van Every, D.W.; Plotkin, D.L. Loading Recommendations for Muscle Strength, Hypertrophy, and Local Endurance: A Re-Examination of the Repetition Continuum. Sports 2021, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, M.R.; Kraemer, W.J. Performance and physiologic adaptations to resistance training. Am. J. Phys. Med. Rehabil. 2002, 81, S3–S16. [Google Scholar] [CrossRef] [PubMed]
- Young, S.D.; Montes, J.; Kramer, S.S.; Marra, J.; Salazar, R.; Cruz, R.; Chiriboga, C.A.; Garber, C.E.; De Vivo, D.C. Six-minute walk test is reliable and valid in spinal muscular atrophy. Muscle Nerve 2016, 54, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Montes, J.; Young, S.D.; Mazzone, E.S.; Pasternak, A.; Glanzman, A.M.; Finkel, R.S.; Darras, B.T.; Muntoni, F.; Mercuri, E.; De Vivo, D.C.; et al. “Nusinersen improves walking distance and reduces fatigue in later-onset spinal muscular atrophy. Muscle Nerve 2019, 60, 409–414. [Google Scholar] [CrossRef]
- Bartels, B.; Habets, L.E.; Stam, M.; Wadman, R.I.; Wijngaarde, C.A.; Schoenmakers, M.A.G.C.; Takken, T.; Hulzebos, E.H.; van der Pol, W.L.; de Groot, J.F. Assessment of fatigability in patients with spinal muscular atrophy: Development and content validity of a set of endurance ests. BMC Neurol. 2019, 19, 21. [Google Scholar] [CrossRef]
- Bartels, B.; de Groot, J.F.; Habets, L.E.; Wijngaarde, C.A.; Vink, W.; Stam, M.; Asselman, F.-L.; van Eijk, R.P.A.; van der Pol, W.L. Fatigability in spinal muscular atrophy: Validity and reliability of endurance shuttle tests. Orphanet J. Rare Dis. 2020, 15, 75. [Google Scholar] [CrossRef]
- Stam, M.; I Wadman, R.; A Wijngaarde, C.; Bartels, B.; Asselman, F.-L.; Otto, L.A.M.; Goedee, H.S.; E Habets, L.; de Groot, J.F.; Schoenmakers, M.A.G.C.; et al. Protocol for a phase II, monocentre, double-blind, placebo-controlled, cross-over trial to assess efficacy of pyridostigmine in patients with spinal muscular atrophy types 2–4 (SPACE trial). BMJ Open 2018, 8, e019932. [Google Scholar] [CrossRef]
- Jansen, M.; De Jong, M.; Coes, H.M.; Eggermont, F.; Van Alfen, N.; De Groot, I.J. The assisted 6-minute cycling test to assess endurance in children with a neuromuscular disorder. Muscle Nerve 2012, 46, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Morse, C.I.; Bostock, E.L.; Twiss, H.M.; Kapp, L.H.; Orme, P.; Jacques, M.F. The cardiorespiratory response and physiological determinants of the assisted 6-minute handbike cycle test in adult males with muscular dystrophy. Muscle Nerve 2018, 58, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Dirks, I.; Koene, S.; Verbruggen, R.; Smeitink, J.A.; Jansen, M.; De Groot, I.J. Assisted 6-minute cycling test: An exploratory study in children. Muscle Nerve 2016, 54, 232–238. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Henricson, E.K.; Abresch, R.T.; Florence, J.; Eagle, M.; Gappmaier, E.; Glanzman, A.M.; Spiegel, R.; Barth, J.; Elfring, G.; et al. The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: Reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve 2013, 48, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.; van Alfen, N.; Geurts, A.C.H.; de Groot, I.J.M. Assisted bicycle training delays functional deterioration in boys with Duchenne muscular dystrophy: The randomized controlled trial “no use is disuse”. Neurorehabil. Neural Repair 2013, 27, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Montes, J.; McDermott, M.P.; Martens, W.B.; Dunaway, S.; Glanzman, A.M.; Riley, S.; Quigley, J.; Montgomery, M.J.; Sproule, D.; Tawil, R.; et al. Six-Minute Walk Test demonstrates motor fatigue in spinal muscular atrophy. Neurology 2010, 74, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.; Tang, W.; Nelson, L.; Parker, D.; Pasternak, A.; Young, S.D.; Muni-Lofra, R.; Maczek, E.; Michell-Sodhi, J.; Moat, D.; et al. P.47 Adaptive test for neuromuscular disorders: Design of a wheelchair-based assessment. Neuromuscul. Disord. 2022, 32, S61. [Google Scholar] [CrossRef]
- Rodriguez-Torres, R.S.; Uher, D.; Gay, E.L.; Coratti, G.; Young, S.D.; Rohwer, A.; Lofra, R.M.; De Vivo, D.C.; Hirano, M.; Glynn, N.W.; et al. Measuring Fatigue and Fatigability in Spinal Muscular Atrophy (SMA): Challenges and Opportunities. J. Clin. Med. 2023, 12, 3458. [Google Scholar] [CrossRef]
- Binz, C.; Osmanovic, A.; Thomas, N.H.; Stolte, B.; Freigang, M.; Cordts, I.; Griep, R.; Uzelac, Z.; Wurster, C.D.; Kamm, C.; et al. Validity and reliability of the German multidimensional fatigue inventory in spinal muscular atrophy. Ann. Clin. Transl. Neurol. 2022, 9, 351–362. [Google Scholar] [CrossRef]
- Lerdal, A.; Kottorp, A.; Gay, C.; Aouizerat, B.E.; Portillo, C.J.; Lee, K.A. A 7-item version of the fatigue severity scale has better psychometric properties among HIV-infected adults: An application of a Rasch model. Qual. Life Res. 2011, 20, 1447–1456. [Google Scholar] [CrossRef]
- Werlauff, U.; Steffensen, B.F. The applicability of four clinical methods to evaluate arm and hand function in all stages of spinal muscular atrophy type II. Disabil. Rehabil. 2014, 36, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Werlauff, U.; Vissing, J.; Steffensen, B. Change in muscle strength over time in spinal muscular atrophy types II and III. A long-term follow-up study. Neuromuscul. Disord. NMD 2012, 22, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, B.; Hyde, S.; Lyager, S.; Mattsson, E. Validity of the EK scale: A functional assessment of non-ambulatory individuals with Duchenne muscular dystrophy or spinal muscular atrophy. Physiother. Res. Int. 2001, 6, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, B.F.; Lyager, S.; Werge, B.; Rahbek, J.; Mattsson, E. Physical capacity in non-ambulatory people with Duchenne muscular dystrophy or spinal muscular atrophy: A longitudinal study. Dev. Med. Child Neurol. 2007, 44, 623–632. [Google Scholar] [CrossRef]
- Mokkink, L.B.; Terwee, C.B.; Patrick, D.L.; Alonso, J.; Stratford, P.W.; Knol, D.L.; Bouter, L.M.; de Vet, H.C.W. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: An international Delphi study. Qual. Life Res. 2010, 19, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.; Wolford, C.; McDermott, M.P.; Macpherson, C.E.; Pasternak, A.; Glanzman, A.M.; Martens, W.B.; Kichula, E.; Darras, B.T.; De Vivo, D.C.; et al. Nusinersen Treatment in Adults With Spinal Muscular Atrophy. Neurol. Clin. Pract. 2021, 11, e317–e327. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Lucibello, S.; Perulli, M.; Coratti, G.; De Sanctis, R.; Pera, M.C.; Pane, M.; Montes, J.; De Vivo, D.C.; Darras, B.T.; et al. Longitudinal natural history of type I spinal muscular atrophy: A critical review. Orphanet J. Rare Dis. 2020, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Richardson, E.; Burnell, J.; Adams, H.R.; Bohannon, R.W.; Bush, E.N.; Campbell, M.; Chen, W.H.; Coons, S.J.; Papadopoulos, E.; Reeve, B.R.; et al. Developing and Implementing Performance Outcome Assessments: Evidentiary, Methodological, and Operational Considerations. Ther. Innov. Regul. Sci. 2019, 53, 146–153. [Google Scholar] [CrossRef]
- Zilio, E.; Piano, V.; Wirth, B. Mitochondrial Dysfunction in Spinal Muscular Atrophy. Int. J. Mol. Sci. 2022, 23, 10878. [Google Scholar] [CrossRef]
- Filler, K.; Lyon, D.; Bennett, J.; McCain, N.; Elswick, R.; Lukkahatai, N.; Saligan, L.N. Association of mitochondrial dysfunction and fatigue: A review of the literature. BBA Clin. 2014, 1, 12–23. [Google Scholar] [CrossRef]
- Burton, A.M.; Cowburn, I.; Thompson, F.; Eisenmann, J.C.; Nicholson, B.; Till, K. Associations Between Motor Competence and Physical Activity, Physical Fitness and Psychosocial Characteristics in Adolescents: A Systematic Review and Meta-analysis. Sports Med. 2023, 53, 2191–2256. [Google Scholar] [CrossRef] [PubMed]
- Bartels, B.; de Groot, J.F.; Habets, L.E.; Wadman, R.I.; Asselman, F.-L.; Nieuwenhuis, E.E.; van Eijk, R.P.; Goedee, H.S.; van der Pol, W.L. Correlates of Fatigability in Patients With Spinal Muscular Atrophy. Neurology 2021, 96, e845–e852. [Google Scholar] [CrossRef]
- Young, S.D.; Montes, J.; Kramer, S.S.; Podwika, B.; Rao, A.K.; De Vivo, D.C. Perceived Fatigue in Spinal Muscular Atrophy: A Pilot Study. J. Neuromuscul. Dis. 2019, 6, 109–117. [Google Scholar] [CrossRef]
- Maggi, L.; Bello, L.; Bonanno, S.; Govoni, A.; Caponnetto, C.; Passamano, L.; Grandis, M.; Trojsi, F.; Cerri, F.; Ferraro, M.; et al. Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1166–1174. [Google Scholar] [CrossRef]
- Kaufmann, P.; McDermott, M.P.; Darras, B.T.; Finkel, R.; Kang, P.; Oskoui, M.; Constantinescu, A.; Sproule, D.M.; Foley, A.R.; Yang, M.; et al. Observational Study of Spinal Muscular Atrophy Type 2 and 3: Functional Outcomes Over 1 Year. Arch. Neurol. 2011, 68, 779–786. [Google Scholar] [CrossRef]
- Mercuri, E.; Finkel, R.; Montes, J.; Mazzone, E.S.; Sormani, M.P.; Main, M.; Ramsey, D.; Mayhew, A.; Glanzman, A.M.; Dunaway, S.; et al. Patterns of disease progression in type 2 and 3 SMA: Implications for clinical trials. Neuromuscul. Disord. NMD 2016, 26, 126–131. [Google Scholar] [CrossRef] [PubMed]


| Clinical Characteristics | Motor Function Outcomes | Patient-Reported Outcomes |
|---|---|---|
| Functional Status Non-sitter, Sitter | A6MCT Total Revolutions, Percent Fatigue | OMNI RPE 3 Reported Minute 1 and Minute 6 |
| Age | RULM 1 | FSS 4 |
| SMN2 Copy Number | ATEND 2 | EK2 5 |
| Disease Duration | ||
| Treatment Duration |
| Overall (N = 38) | |
|---|---|
| Baseline Age (y) | |
| Mean (SD) | 30.3 (14.1) |
| Median [Min, Max] | 27.4 [5.90, 74.1] |
| Sex | |
| Female | 16 (42.1%) |
| Male | 22 (57.9%) |
| Baseline Disease Duration (y) | |
| Mean (SD) | 28.1 (14.6) |
| Median [Min, Max] | 26.7 [0.110, 69.8] |
| Baseline Treatment Duration (y) | |
| Mean (SD) | 3.17 (2.40) |
| Median [Min, Max] | 3.01 [−0.444, 10.3] |
| Baseline Treatment | |
| Nusinersen | 21 (55.3%) |
| Risdiplam | 17 (44.7%) |
| Function Group | |
| Non-sitter | 25 (65.8%) |
| Sitter | 13 (34.2%) |
| Type | |
| Type 2 | 19 (50.0%) |
| Type 3 | 19 (50.0%) |
| SMN2 Copy Number * | |
| Unknown † | 1 (2.6%) |
| 2 SMN2 | 1 (2.6%) |
| 3 SMN2 | 26 (68.4%) |
| ≥4 SMN2 | 10 (26.3%) |
| Gloves | |
| Required | 13 (34.2%) |
| Not Required | 25 (65.8%) |
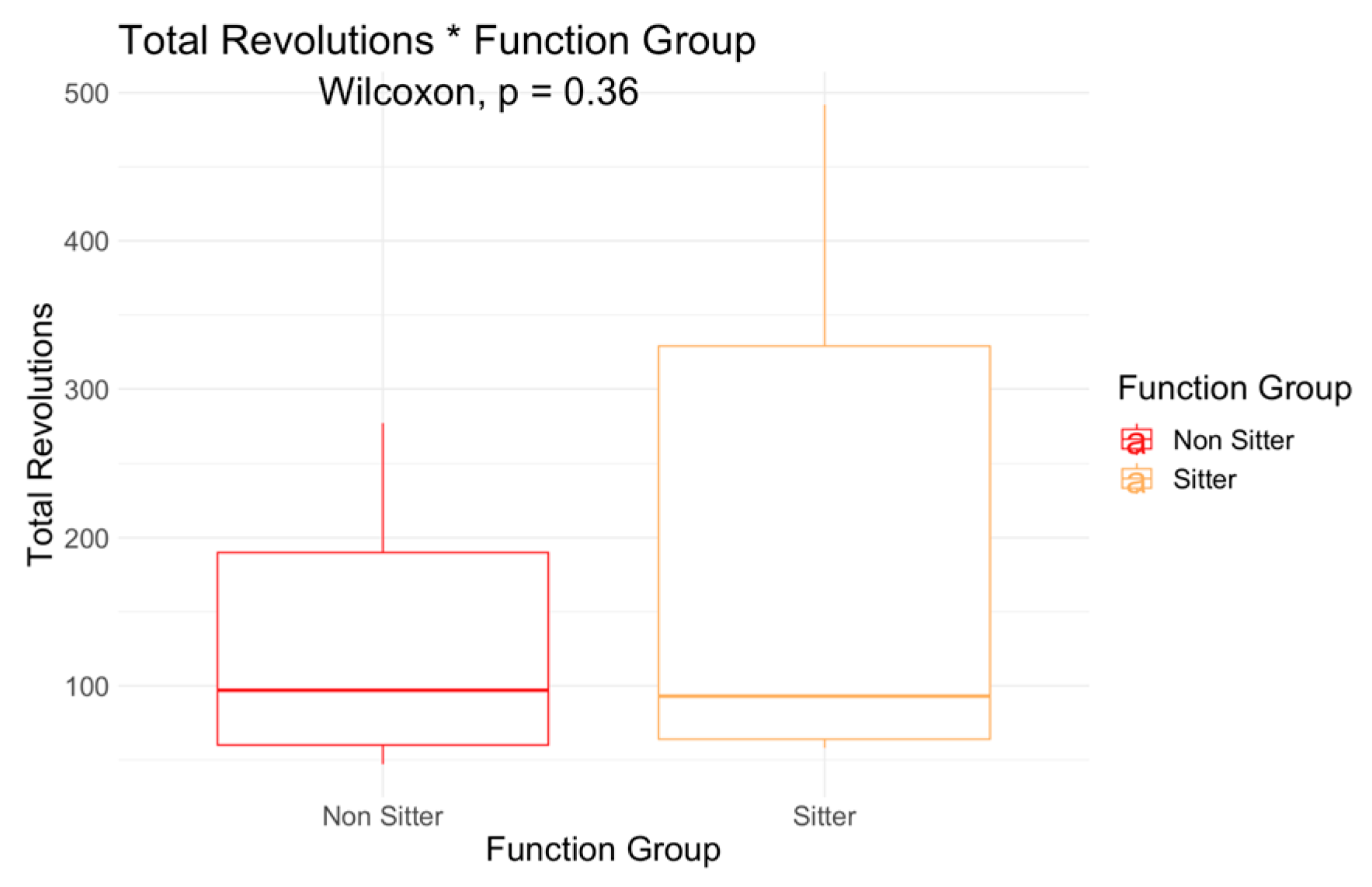
| Non-Sitter (N = 25) | Sitter (N = 13) | Overall (N = 38) | |
|---|---|---|---|
| A6MCT: Total Revolutions | |||
| Mean (SD) | 129 (77.0) | 204 (180) | 155 (125) |
| Median [Min, Max] | 97.0 [47.0, 277] | 93.0 [58.0, 492] | 93.5 [47.0, 492] |
| A6MCT: Percent Fatigue | |||
| Mean (SD) | 13.9 (19.3) | −10.9 (27.0) | 5.43 (24.9) |
| Median [Min, Max] | 11.1 [−14.3, 48.5] | 0 [−66.1, 33.8] | 2.78 [−66.1, 48.5] |
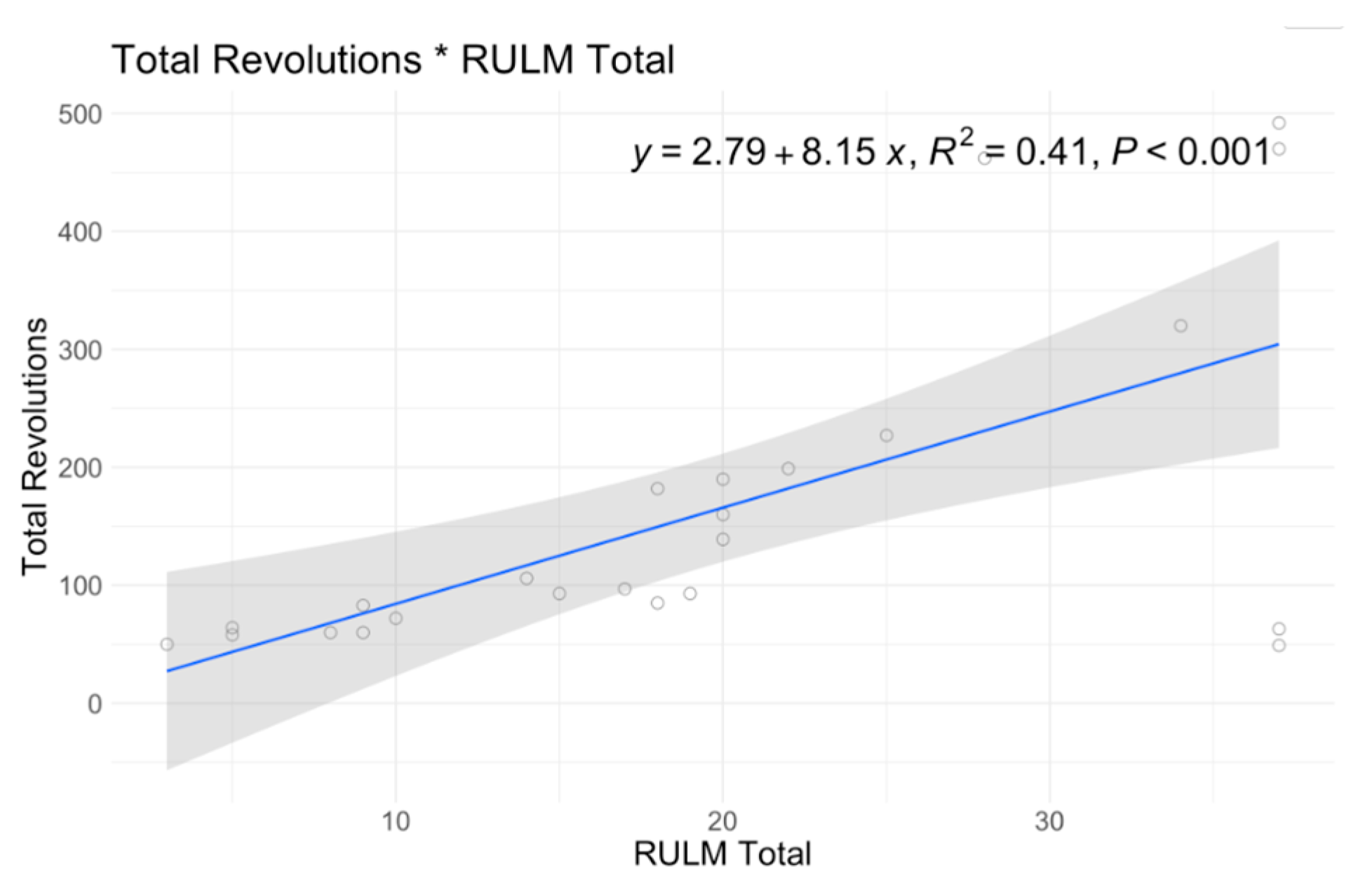
| RULM Best Side | |||
| Mean (SD) | 15.8 (8.87) | 25.6 (11.7) | 19.5 (10.9) |
| Median [Min, Max] | 17.0 [3.00, 37.0] | 28.0 [5.00, 37.0] | 18.5 [3.00, 37.0] |
| Missing | 10 (40.0%) | 4 (30.8%) | 14 (36.8%) |
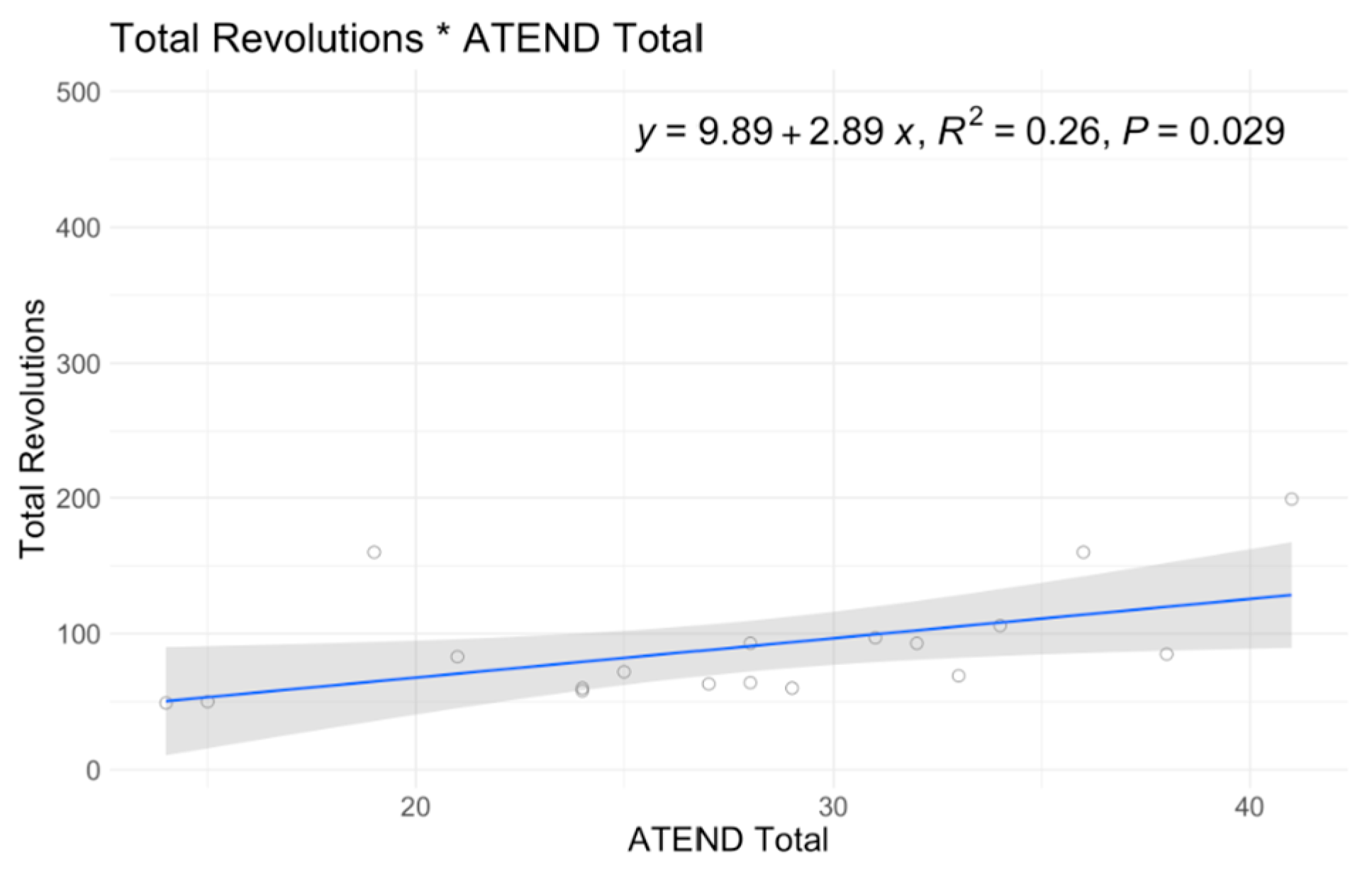
| ATEND Best Side | |||
| Mean (SD) | 26.4 (8.36) | 30.3 (5.01) | 27.7 (7.50) |
| Median [Min, Max] | 26.5 [14.0, 41.0] | 30.0 [24.0, 38.0] | 28.0 [14.0, 41.0] |
| Missing | 13 (52.0%) | 7 (53.8%) | 20 (52.6%) |
| ATEND Upper Extremity Score | |||
| 1 | 1 (4.3%) | 0 (0%) | 1 (2.6%) |
| 2 | 1 (4.3%) | 0 (0%) | 1 (2.6%) |
| 3 | 7 (30.4%) | 3 (20.0%) | 10 (26.3%) |
| 4 | 5 (17.4%) | 1 (6.7%) | 6 (15.8%) |
| 5 | 0 (0.0%) | 3 (20.0%) | 3 (7.9%) |
| 6 | 0 (0.0%) | 5 (33.3%) | 5 (13.2%) |
| Missing | 9 (39.1%) | 3 (20.0%) | 12 (31.6%) |
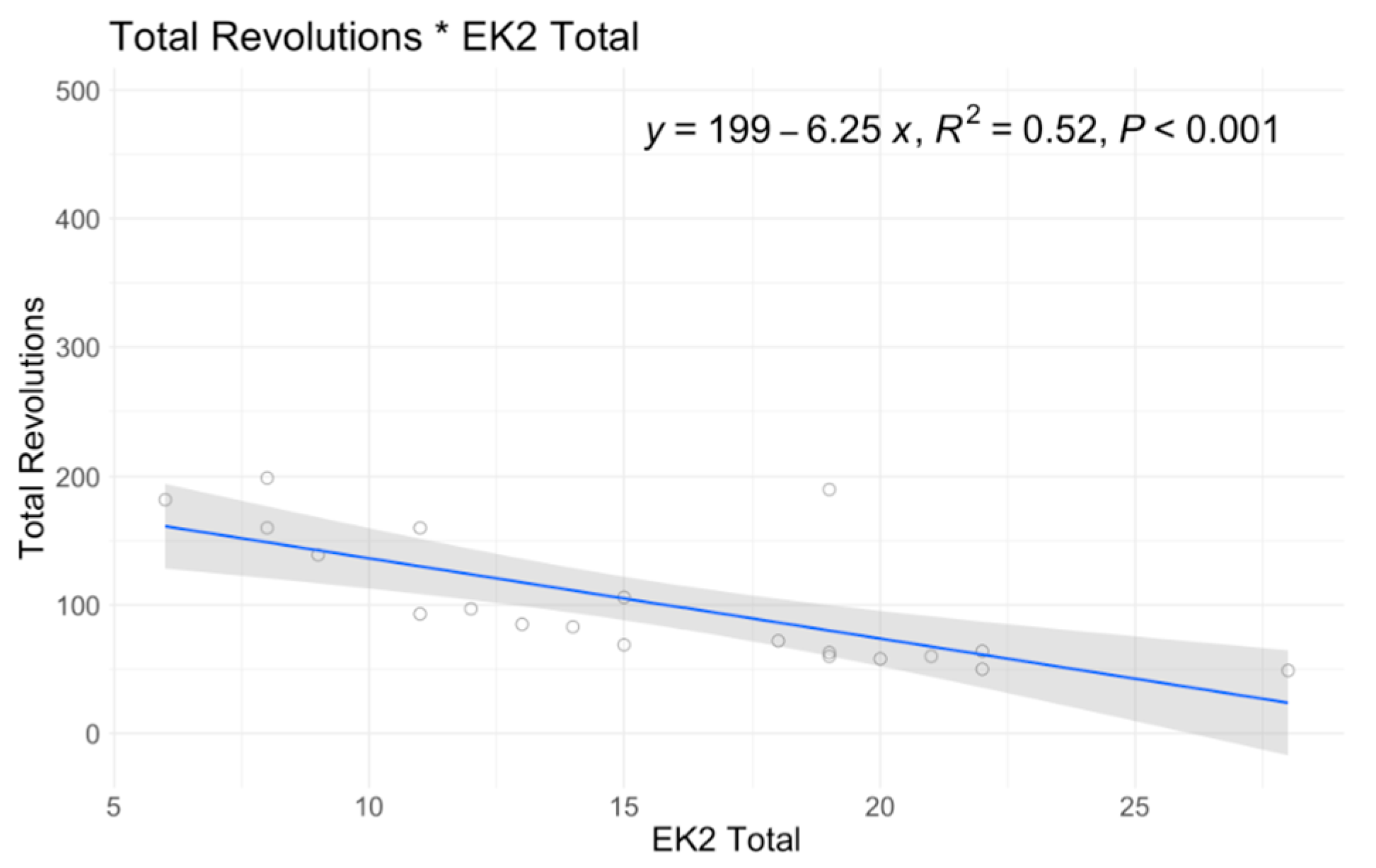
| EK2 Total | |||
| Mean (SD) | 15.5 (6.45) | 15.6 (3.85) | 15.5 (5.81) |
| Median [Min, Max] | 15.0 [6.00, 28.0] | 15.0 [11.0, 20.0] | 15.0 [6.00, 28.0] |
| Missing | 10 (40.0%) | 8 (61.5%) | 18 (47.4%) |
| FSS Average | |||
| Mean (SD) | 3.68 (1.38) | 3.27 (1.68) | 3.54 (1.46) |
| Median [Min, Max] | 3.50 [1.57, 6.86] | 2.86 [1.43, 5.71] | 3.43 [1.43, 6.86] |
| Missing | 11 (44.0%) | 6 (46.2%) | 17 (44.7%) |
| Minute 6 RPE | |||
| Mean (SD) | 6.67 (2.01) | 6.70 (2.36) | 6.68 (2.08) |
| Median [Min, Max] | 6.00 [3.00, 10.0] | 6.50 [2.00, 10.0] | 6.00 [2.00, 10.0] |
| Missing | 1 (4.0%) | 3 (23.1%) | 4 (10.5%) |
| Minute 6 RPE Above 7 | |||
| No | 16 (64.0%) | 10 (76.9%) | 26 (68.4%) |
| Yes | 9 (36.0%) | 3 (23.1%) | 12 (31.6%) |
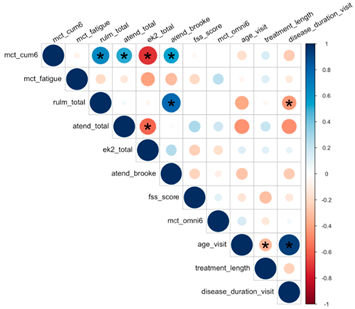 |
|---|
| A * sign indicates p-value < 0.05 |
| Gradient color of a circle indicates both magnitude and direction of correlation |
| The size of a circle indicates the magnitude of correlation |
| Magnitude of correlation ≠ significance of correlation |
| A6MCT | A6MCT (No Outlier) | Fatigue % | RULM Total | ATEND Total | EK2 Total | ATEND Brooke | FSS Mean | Minute 6 RPE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | |
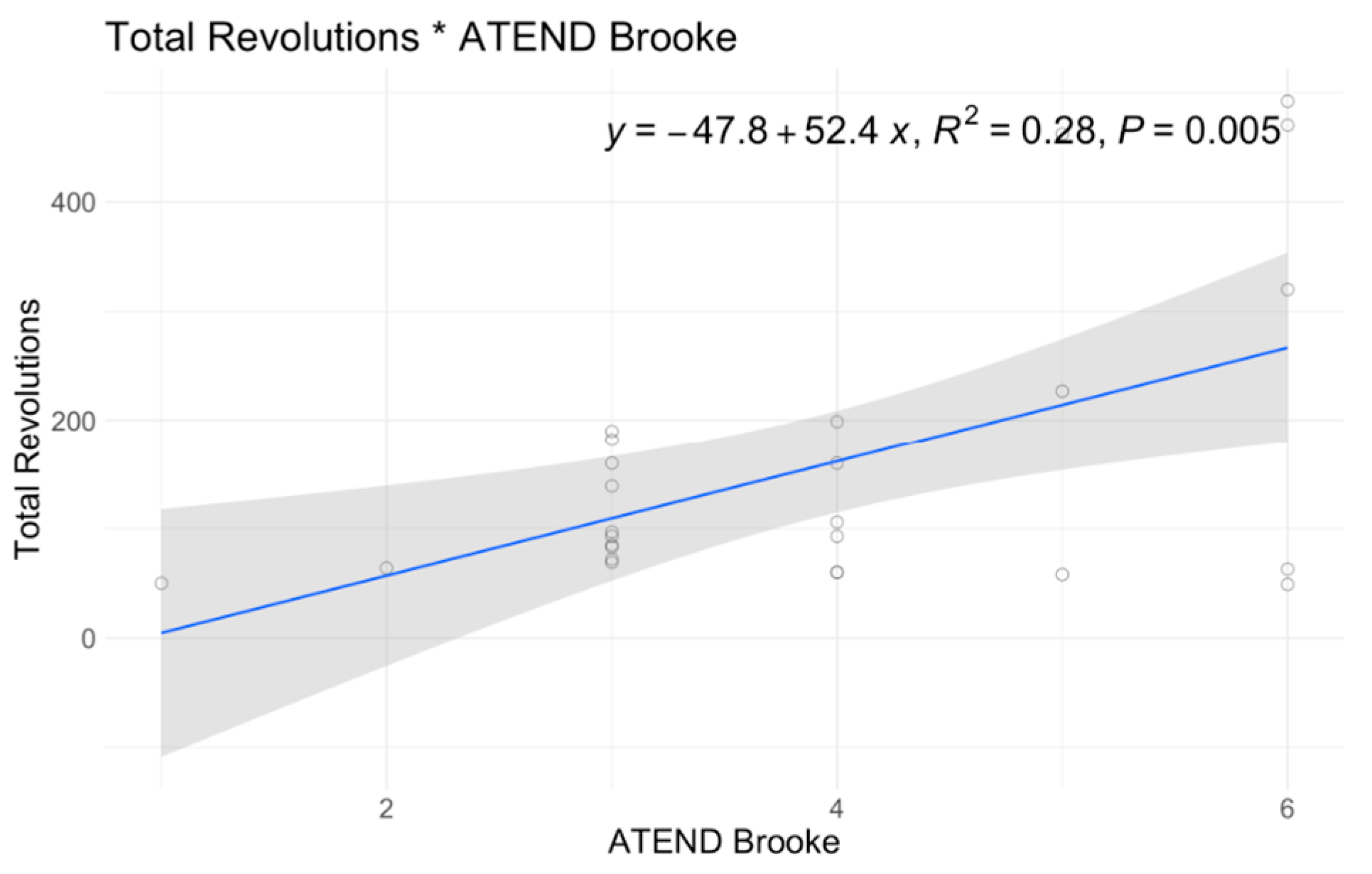
| Intercept | 80.96 | 0.56 | 57.83 | 0.655 | −4.36 | 0.853 | 29.94 | 0.012 | 19.2 | 0 | 23.82 | 0 | 3.88 | 0 | 3.4 | 0.038 | 4.9 | 0.029 |
| Time of Visit (yrs) | 29.79 | 0.062 | −5.1 | 0.614 | 4.71 | 0.1 | −1.16 | 0.372 | −0.03 | 0.937 | 0.88 | 0.309 | −0.2 | 0.277 | −0.33 | 0.091 | 0.06 | 0.833 |
| Age | 2.84 | 0.629 | 3.28 | 0.506 | 0.59 | 0.502 | 0.01 | 0.963 | 1.92 | 0.186 | 0.43 | 0.146 | 0 | 0.993 | −0.09 | 0.096 | 0.14 | 0.173 |
| Sitter | 94.02 | 0.079 | 72.13 | 0.121 | −18.72 | 0.023 | 7.26 | 0.063 | 5.32 | 0.09 | 0.93 | 0.841 | 1.16 | 0.008 | −1.15 | 0.084 | 0.51 | 0.554 |
| SMN2 = 2 | 304.19 | 0.115 | 279.61 | 0.116 | 40.99 | 0.159 | 1.41 | 0.649 | ||||||||||
| SMN2 = 3 | 70.1 | 0.585 | 82.55 | 0.507 | 21.69 | 0.262 | −1.34 | 0.815 | 13.97 | 0.022 | −17.28 | 0.008 | −0.21 | 0.859 | 0.93 | 0.483 | 0.31 | 0.893 |
| SMN2 = 4 | 138.08 | 0.325 | 134.79 | 0.359 | 17.6 | 0.388 | 1.31 | 0.895 | 13.5 | 0.046 | −20.19 | 0.005 | 0.09 | 0.903 | 2.46 | 0.111 | 0.65 | 0.771 |
| Treatment Duration | 5.04 | 0.596 | 8.88 | 0.315 | 0.26 | 0.872 | 0.09 | 0.843 | 0.26 | 0.771 | 0.77 | 0.245 | 0 | 0.987 | −0.11 | 0.37 | 0.15 | 0.421 |
| Disease Duration | −5.57 | 0.242 | −5.34 | 0.1999 | −0.82 | 0.298 | −0.33 | 0.322 | −2.23 | 0.125 | −0.29 | 0.273 | −0.02 | 0.569 | 0.08 | 0.108 | −0.11 | 0.203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.J.; Gu, B.; Montalvo, S.; Dunaway Young, S.; Parker, D.M.; de Monts, C.; Ataide, P.; Ni Ghiollagain, N.; Wheeler, M.T.; Tesi Rocha, C.; et al. Assessing the Assisted Six-Minute Cycling Test as a Measure of Endurance in Non-Ambulatory Patients with Spinal Muscular Atrophy (SMA). J. Clin. Med. 2023, 12, 7582. https://doi.org/10.3390/jcm12247582
Tang WJ, Gu B, Montalvo S, Dunaway Young S, Parker DM, de Monts C, Ataide P, Ni Ghiollagain N, Wheeler MT, Tesi Rocha C, et al. Assessing the Assisted Six-Minute Cycling Test as a Measure of Endurance in Non-Ambulatory Patients with Spinal Muscular Atrophy (SMA). Journal of Clinical Medicine. 2023; 12(24):7582. https://doi.org/10.3390/jcm12247582
Chicago/Turabian StyleTang, Whitney J., Bo Gu, Samuel Montalvo, Sally Dunaway Young, Dana M. Parker, Constance de Monts, Paxton Ataide, Noirin Ni Ghiollagain, Matthew T. Wheeler, Carolina Tesi Rocha, and et al. 2023. "Assessing the Assisted Six-Minute Cycling Test as a Measure of Endurance in Non-Ambulatory Patients with Spinal Muscular Atrophy (SMA)" Journal of Clinical Medicine 12, no. 24: 7582. https://doi.org/10.3390/jcm12247582
APA StyleTang, W. J., Gu, B., Montalvo, S., Dunaway Young, S., Parker, D. M., de Monts, C., Ataide, P., Ni Ghiollagain, N., Wheeler, M. T., Tesi Rocha, C., Christle, J. W., He, Z., Day, J. W., & Duong, T. (2023). Assessing the Assisted Six-Minute Cycling Test as a Measure of Endurance in Non-Ambulatory Patients with Spinal Muscular Atrophy (SMA). Journal of Clinical Medicine, 12(24), 7582. https://doi.org/10.3390/jcm12247582






