Multilevel Laminoplasty for CSM: Is C3 Laminectomy Better Than C3 Laminoplasty at the Superior Vertebra?
Abstract
:1. Introduction
2. Materials & Methods
2.1. Study Design, Setting, Participants
2.2. Variables
2.3. Primary Outcome
2.4. Quantitative Variables
2.5. Statistical Methods
3. Results
3.1. Descriptive Data
3.2. Outcome Data
3.3. Main Result
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirabayashi, K.; Watanabe, K. A Review of My Invention of Expansive Laminoplasty. Neurospine 2019, 16, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Kim, J.S.; Overley, S.C.; Merrill, R.K. Cervical Laminoplasty: Indications, Surgical Considerations, and Clinical Outcomes. J. Am. Acad. Orthop. Surg. 2018, 26, e142–e152. [Google Scholar] [CrossRef]
- Jung, J.-M.; Jahng, A.L.; Hyun, S.-J.; Kim, K.-J.; Jahng, T.-A. Comparison of Spinal Canal Expansion Following Cervical Laminoplasty Based on the Preoperative Lamina Angle: A Simulation Study. J. Korean Neurosurg. Soc. 2021, 64, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Ryken, T.C.; Heary, R.F.; Matz, P.G.; Anderson, P.A.; Groff, M.W.; Holly, L.T.; Kaiser, M.G.; Mummaneni, P.V.; Choudhri, T.F.; Vresilovic, E.J.; et al. Cervical laminectomy for the treatment of cervical degenerative myelopathy. J. Neurosurg. Spine 2009, 11, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Mummaneni, P.V.; Kaiser, M.G.; Matz, P.G.; Anderson, P.A.; Groff, M.W.; Heary, R.F.; Holly, L.T.; Ryken, T.C.; Choudhri, T.F.; Vresilovic, E.J.; et al. Cervical surgical techniques for the treatment of cervical spondylotic myelopathy. J. Neurosurg. Spine 2009, 11, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.A.; Wu, J.-C.; Mummaneni, P.V. Laminoplasty outcomes: Is there a difference between patients with degenerative stenosis and those with ossification of the posterior longitudinal ligament? Neurosurg. Focus. 2011, 30, E9. [Google Scholar] [CrossRef]
- Highsmith, J.M.; Dhall, S.S.; Haid, R.W.; Rodts, G.E.; Mummaneni, P.V. Treatment of cervical stenotic myelopathy: A cost and outcome comparison of laminoplasty versus laminectomy and lateral mass fusion. J. Neurosurg. Spine 2011, 14, 619–625. [Google Scholar] [CrossRef]
- Lau, D.; Winkler, E.A.; Than, K.D.; Chou, D.; Mummaneni, P.V. Laminoplasty versus laminectomy with posterior spinal fusion for multilevel cervical spondylotic myelopathy: Influence of cervical alignment on outcomes. J. Neurosurg. Spine 2017, 27, 508–517. [Google Scholar] [CrossRef]
- Abe, T.; Miyazaki, M.; Ishihara, T.; Kanezaki, S.; Notani, N.; Kataoka, M.; Tsumura, H. Analysis of the risk factors for increasing cervical sagittal vertical axis after cervical laminoplasty for cervical spondylotic myelopathy. Arch. Orthop. Trauma. Surg. 2022, 142, 553–560. [Google Scholar] [CrossRef]
- Alam, I.; Sharma, R.; Borkar, S.A.; Goda, R.; Katiyar, V.; Kale, S.S. Factors predicting loss of cervical lordosis following cervical laminoplasty: A critical review. J. Craniovertebral Junction Spine 2020, 11, 163–168. [Google Scholar] [CrossRef]
- Lee, J.S.; Son, D.W.; Lee, S.H.; Kim, D.H.; Lee, S.W.; Song, G.S. The Predictable Factors of the Postoperative Kyphotic Change of Sagittal Alignment of the Cervical Spine after the Laminoplasty. J. Korean Neurosurg. Soc. 2017, 60, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Borkar, S.A.; Goda, R.; Kale, S.S. Which factors predict the loss of cervical lordosis following cervical laminoplasty? A review of various indices and their clinical implications. Surg. Neurol. Int. 2019, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Cho, S.-M.; Hur, J.W.; Cha, J.; Kim, S.-H. Kinematics after cervical laminoplasty: Risk factors for cervical kyphotic deformity after laminoplasty. Spine J. 2021, 21, 1822–1829. [Google Scholar] [CrossRef]
- Kong, C.; Li, X.-Y.; Sun, X.-Y.; Guo, M.-C.; Ding, J.-Z.; Yang, Y.-M.; Lu, S.-B. The ratio of C2-C7 Cobb angle to T1 slope is an effective parameter for the selection of posterior surgical approach for patients with multisegmental cervical spondylotic myelopathy. J. Orthop. Sci. 2020, 25, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Son, D.W.; Shin, J.J.; Ha, Y.; Song, G.S.; Lee, J.S.; Lee, S.W. Preoperative Radiological Parameters to Predict Clinical and Radiological Outcomes after Laminoplasty. J. Korean Neurosurg. Soc. 2021, 64, 677–692. [Google Scholar] [CrossRef]
- Miyazaki, M.; Ishihara, T.; Abe, T.; Kanezaki, S.; Notani, N.; Sato, S.; Kataoka, M.; Tsumura, H. Analysis of the reciprocal changes in upper cervical profile and the risk factors for increasing cervical sagittal vertical axis after laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine. Clin. Neurol. Neurosurg. 2020, 194, 105788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Li, J.Q.; Niu, R.J.; Liu, Z.; Tong, T.; Shen, Y. Predictors of cervical lordosis loss after laminoplasty in patients with cervical spondylotic myelopathy. Eur. Spine J. 2017, 26, 1205–1210. [Google Scholar] [CrossRef]
- Lin, B.-J.; Hong, K.-T.; Lin, C.; Chung, T.-T.; Tang, C.-T.; Hueng, D.-Y.; Hsia, C.-C.; Ju, D.-T.; Ma, H.-I.; Liu, M.-Y.; et al. Impact of global spine balance and cervical regional alignment on determination of postoperative cervical alignment after laminoplasty. Medicine 2018, 97, e13111. [Google Scholar] [CrossRef]
- Kim, K.-R.; Lee, C.-K.; Park, J.-Y.; Kim, I.-S. Preoperative Parameters for Predicting the Loss of Lordosis After Cervical Laminoplasty. Spine 2020, 45, 1476–1484. [Google Scholar] [CrossRef]
- Liu, J.; Xie, R.; Ruan, H.; Rivera, J.; Li, B.; Mahmood, B.; Lee, J.; Guizar, R.; Mahmoudieh, Y.; Mummaneni, P.V.; et al. The Preoperative Cross-sectional Area of the Deep Cervical Extensor Muscles Does Not Predict Loss of Lordosis After Cervical Laminoplasty. Clin. Spine Surg. 2022, 35, E181–E186. [Google Scholar] [CrossRef]
- Lin, S.; Lin, T.; Wu, Z.; Chen, G.; Shangguan, Z.; Wang, Z.; Liu, W. Does the asymmetry and extension function of the preoperative cervical paraspinal extensor predict postoperative cervical sagittal deformity in patients who undergo modified laminoplasty? Spine J. 2022, 22, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Ishihara, T.; Notani, N.; Kanezaki, S.; Tsumura, H. Relationship of T1 slope with loss of lordosis and surgical outcomes after laminoplasty for cervical ossification of the posterior longitudinal ligament. Clin. Neurol. Neurosurg. 2018, 164, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Funaba, M.; Imajo, Y.; Suzuki, H.; Nishida, N.; Sakamoto, T.; Sakai, T. The Deterioration of Cervical Kyphosis During Neck Flexion after Laminoplasty Affects the Surgical Outcome of Cervical Spondylotic Myelopathy. Glob. Spine J. 2022, 13, 2497–2507. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Borkar, S.A.; Phalak, M.; Joseph, S.L.; Kale, S.S. Cervical alignment following laminoplasty for cervical spondylotic myelopathy. Surg. Neurol. Int. 2019, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, H.; Nakajima, T.; Iizuka, Y.; Sorimachi, Y.; Ara, T.; Nishinome, M.; Takagishi, K. Cervical malalignment after laminoplasty: Relationship to deep extensor musculature of the cervical spine and neurological outcome. J. Neurosurg. Spine 2007, 7, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Anastasio, A.T.; Rhee, J.M. Laminoplasty Achieves Improved Outcomes Despite Leading to a More Positive Sagittal Balance: Neither Preoperative nor Postoperative Sagittal Balance Correlated with Spine-specific Outcome Data. Clin. Spine Surg. 2022, 35, E150–E154. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-Y.; Ma, L.; Huo, L.-S.; Cao, Y.-X.; Yang, D.-L.; Wang, H.; Yang, S.-D.; Ding, W.-Y. Mini-plate fixation versus suture suspensory fixation in cervical laminoplasty: A meta-analysis. Medicine 2017, 96, e6026. [Google Scholar] [CrossRef]
- Tabaraee, E.; Mummaneni, P.; Abdul-Jabbar, A.; Shearer, D.; Roy, E.; Amin, B.; Ames, C.; Burch, S.; Deviren, V.; Berven, S.; et al. A Comparison of Implants Used in Open-Door Laminoplasty: Structural Rib Allografts Versus Metallic Miniplates. Clin. Spine Surg. 2017, 30, E523–E529. [Google Scholar] [CrossRef]
- Iizuka, H.; Shimizu, T.; Tateno, K.; Toda, N.; Edakuni, H.; Shimada, H.; Takagishi, K. Extensor musculature of the cervical spine after laminoplasty: Morphologic evaluation by coronal view of the magnetic resonance image. Spine 2001, 26, 2220–2226. [Google Scholar] [CrossRef]
- Sasai, K.; Saito, T.; Akagi, S.; Kato, I.; Ogawa, R. Cervical curvature after laminoplasty for spondylotic myelopathy—Involvement of yellow ligament, semispinalis cervicis muscle, and nuchal ligament. J. Spinal Disord. 2000, 13, 26–30. [Google Scholar] [CrossRef]
- Shimizu, K.; Mitsuhara, T.; Takeda, M.; Kurisu, K.; Yamaguchi, S. Effects of Preservation of the Semispinalis Cervicis Inserted into C2 on Craniocervical Alignment After Laminoplasty. World Neurosurg. 2021, 146, e1367–e1376. [Google Scholar] [CrossRef]
- Sakaura, H.; Hosono, N.; Mukai, Y.; Fujimori, T.; Iwasaki, M.; Yoshikawa, H. Preservation of muscles attached to the C2 and C7 spinous processes rather than subaxial deep extensors reduces adverse effects after cervical laminoplasty. Spine 2010, 35, E782–E786. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M.; Oxland, T.R.; Parks, E.H. Quantitative anatomy of cervical spine ligaments. Part II. Middle and lower cervical spine. J. Spinal Disord. 1991, 4, 277–285. [Google Scholar] [CrossRef]
- Michael, K.W.; Neustein, T.M.; Rhee, J.M. Where should a laminoplasty start? The effect of the proximal level on post-laminoplasty loss of lordosis. Spine J. 2016, 16, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, J.; Huang, Z.; Wu, X. The impact of plating level on the postoperative loss of cervical lordosis in alternative skipped-level plating laminoplasty. J. Orthop. Surg. 2020, 28, 2309499019896882. [Google Scholar] [CrossRef]
- Liu, G.; Fung, G.; Tan, J.; Ng, J.H.; Tan, J.-H. A Feasibility Study of a New Muscle Sparing “C3 Dome-Hybrid Open-Door Laminoplasty”: A Surgical Technique, Clinical Outcome, and Learning Curve Description. SPINE 2020, 45, E1256–E1263. [Google Scholar] [CrossRef] [PubMed]
- Umeda, M.; Sasai, K.; Kushida, T.; Wakabayashi, E.; Maruyama, T.; Ikeura, A.; Iida, H. A less-invasive cervical laminoplasty for spondylotic myelopathy that preserves the semispinalis cervicis muscles and nuchal ligament. J. Neurosurg. Spine 2013, 18, 545–552. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Liao, Z.; Gao, Y.; Shao, Z.; Yang, C. C3 laminectomy combined with modified unilateral laminoplasty and in situ reconstruction of the midline structures maintained cervical sagittal balance: A retrospective matched-pair case-control study. Spine J. 2020, 20, 1403–1412. [Google Scholar] [CrossRef]
- Mesfin, A.; Park, M.-S.; Piyaskulkaew, C.; Chuntarapas, T.; Song, K.S.; Kim, H.J.; Riew, K.D. Neck Pain following Laminoplasty. Glob. Spine J. 2015, 5, 17–22. [Google Scholar] [CrossRef]
- Riew, K.D.; Raich, A.L.; Dettori, J.R.; Heller, J.G. Neck Pain Following Cervical Laminoplasty: Does Preservation of the C2 Muscle Attachments and/or C7 Matter? Evid. Based Spine Care J. 2013, 4, 42–53. [Google Scholar] [CrossRef]
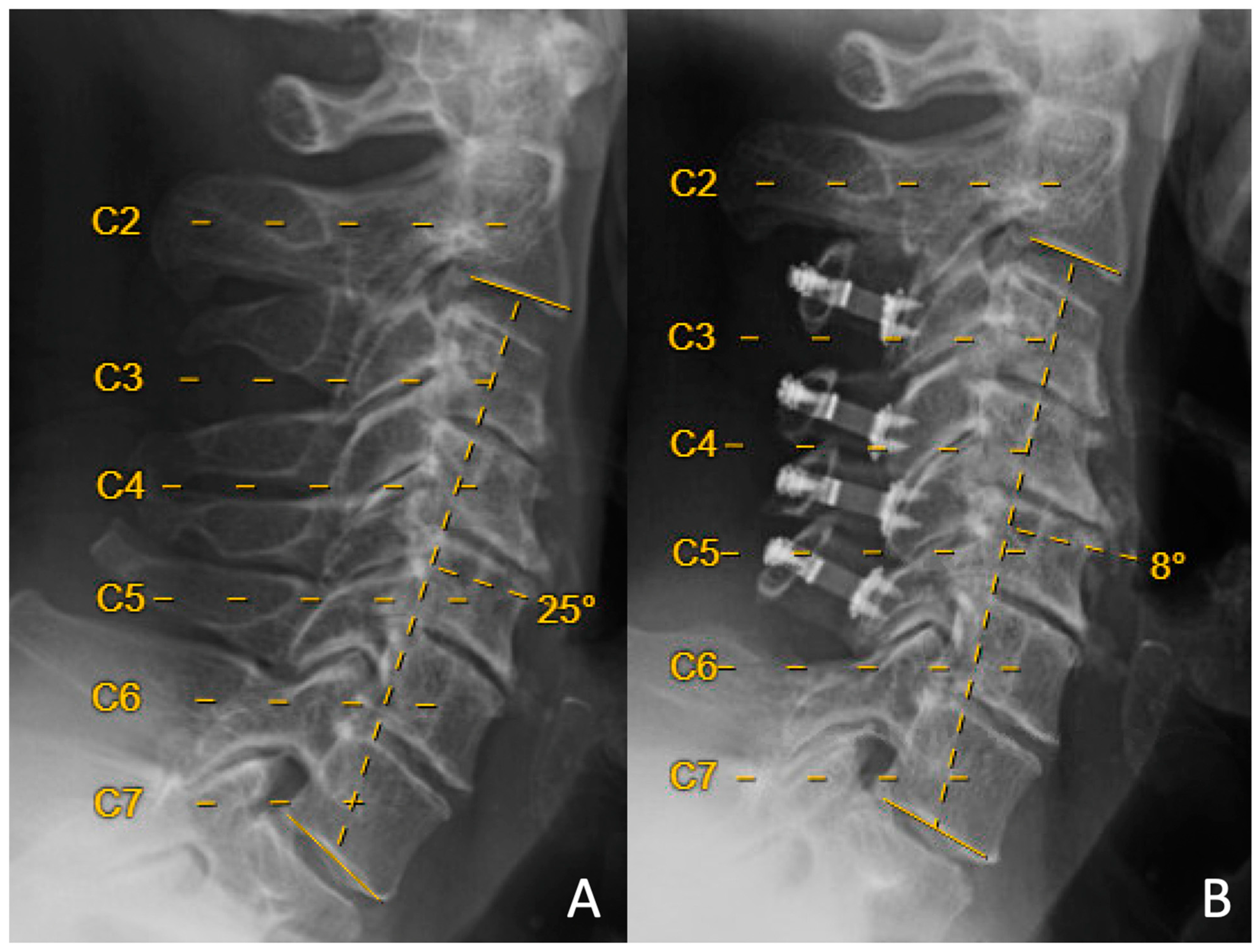
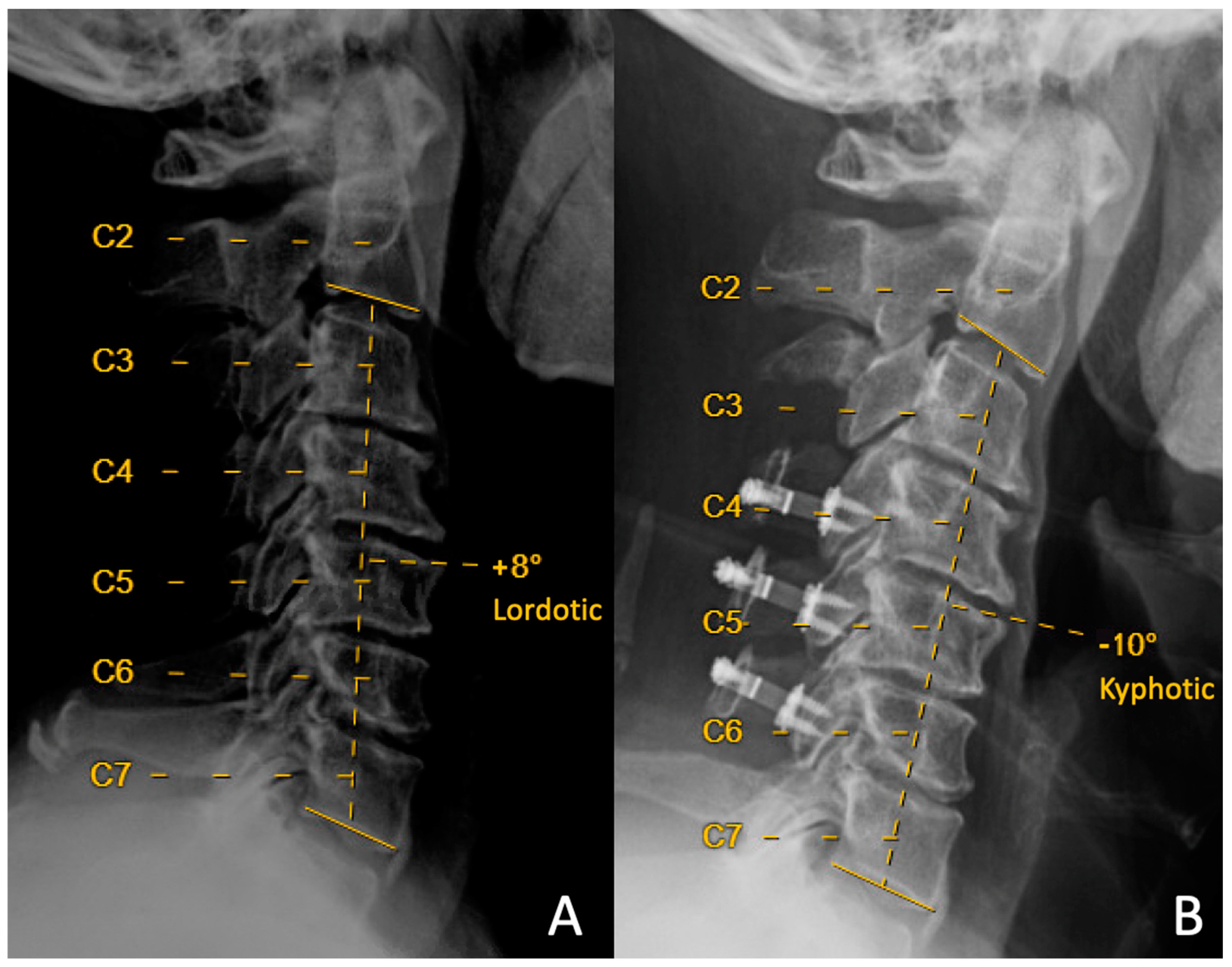
| Laminoplasty Only N = 61 | +Partial C3 Laminectomy N = 39 | p Value | |
|---|---|---|---|
| Male gender | 43 (70.5%) | 21 (53.9%) | 0.091 |
| Age | 66.4 ± 1.3 | 59.4 ± 2.3 | 0.012 |
| Height (cm) | 170.3 ± 1.4 | 169.5 ± 1.5 | 0.349 |
| Weight (kg) | 82.4 ± 2.4 | 81.8 ± 2.5 | 0.870 |
| Body Mass Index | 28.5 ± 0.8 | 28.5 ± 0.7 | 0.983 |
| Not current smoker | 55 (94.8%) | 34 (91.9%) | 0.566 |
| Diabetes | 12 (20.0%) | 4 (10.3%) | 0.198 |
| Coronary Artery Disease | 8 (13.3%) | 2 (5.1%) | 0.186 |
| Peripheral Vascular Disease | 1 (1.67%) | 0 (0.0%) | 0.418 |
| Chronic Kidney Disease | 4 (6.7%) | 1 (2.6%) | 0.362 |
| Osteoporosis | 5 (8.3%) | 2 (5.1%) | 0.543 |
| American Society of Anesthesiologists (ASA) Classification | |||
| 1 | 0 (0.0%) | 2 (5.3%) | 0.273 |
| 2 | 30 (63.8%) | 22 (57.9%) | |
| 3 | 17 (36.2%) | 14 (36.8%) | |
| Median Number of Intervertebral Levels Decompressed [Interquartile Range] | 4 [4, 4] | 3 [3, 3] | <0.001 * |
| Estimated Blood Loss (mL) | 179.0 ± 24.3 | 142.0 ± 12.2 | 0.178 |
| Operative Time (minutes) | 134.0 ± 8.2 | 138.8 ± 5.1 | 0.619 |
| Length of Stay (days) | 4.6 ± 0.9 | 3.5 ± 0.2 | 0.346 |
| Change in Cervical Lordosis | |||
| No change (<5°) | 40 (65.6%) | 29 (74.3%) | 0.644 |
| Mild change (5–10°) | 9 (14.8%) | 4 (10.3%) | |
| Moderate change (>10°) | 12 (19.7%) | 6 (15.4%) | |
| 90-day Readmission Rate | 1 (1.6%) | 2 (5.1%) | 0.318 |
| 1-year Reoperation Rate | 0 | 1 (2.5%) | 0.209 |
| Median Follow-up [Range], months | 26.5 months [12.2 months–9.0 years] | 15.3 months [12.1 months–6.4 years] | 0.002 |
| Laminoplasty Only | +Partial C3 Laminectomy | p Value | |
|---|---|---|---|
| Preoperative Measurements | |||
| Cervical Lordosis | 13.2° ± 1.2 | 11.1° ± 1.3 | 0.259 |
| T1 Slope | 32.9° ± 1.3 | 29.3° ± 1.6 | 0.073 |
| T1 Slope–Cervical Lordosis | 19.9° ± 1.2 | 18.6° ± 1.1 | 0.485 |
| Cervical–Sagittal Vertical Axis (mm) | 31.5 ± 2.1 | 27.4 ± 2.1 | 0.193 |
| Global Measurements | |||
| Pelvic Incidence | 54.4° ± 2.2 | 60.1° ± 2.3 | 0.085 |
| Pelvic Tilt | 21.1° ± 1.7 | 19.5° ± 1.7 | 0.503 |
| Lumbar Lordosis | 43.5° ± 2.9 | 52.4° ± 3.4 | 0.056 |
| Sagittal Vertical Axis (mm) | 28.9 ± 7.1 | 30.4 ± 7.0 | 0.891 |
| Postoperative Measurements | |||
| Cervical Lordosis | 9.4° ± 1.4 | 11.2° ± 1.2 | 0.331 |
| T1 Slope | 31.2° ± 1.2 | 27.8° ± 1.6 | 0.080 |
| T1 Slope–Cervical Lordosis | 21.7° ± 1.6 | 18.2° ± 1.5 | 0.126 |
| Cervical–Sagittal Vertical Axis (mm) | 34.0 ± 2.0 | 36.2 ± 2.4 | 0.480 |
| Adjusted Odds Ratio [95% Confidence Interval] | p Value | |
|---|---|---|
| Partial C3 “Dome” Laminectomy | 0.72 [0.29–1.8] | 0.479 |
| Age | 1.01 [0.97–1.05] | 0.593 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macki, M.; Chryssikos, T.; Meade, S.M.; Aabedi, A.A.; Letchuman, V.; Ambati, V.; Krishnan, N.; Tawil, M.E.; Tichelaar, S.; Rivera, J.; et al. Multilevel Laminoplasty for CSM: Is C3 Laminectomy Better Than C3 Laminoplasty at the Superior Vertebra? J. Clin. Med. 2023, 12, 7594. https://doi.org/10.3390/jcm12247594
Macki M, Chryssikos T, Meade SM, Aabedi AA, Letchuman V, Ambati V, Krishnan N, Tawil ME, Tichelaar S, Rivera J, et al. Multilevel Laminoplasty for CSM: Is C3 Laminectomy Better Than C3 Laminoplasty at the Superior Vertebra? Journal of Clinical Medicine. 2023; 12(24):7594. https://doi.org/10.3390/jcm12247594
Chicago/Turabian StyleMacki, Mohamed, Timothy Chryssikos, Seth M. Meade, Alexander A. Aabedi, Vijay Letchuman, Vardhaan Ambati, Nishanth Krishnan, Michael E. Tawil, Seth Tichelaar, Joshua Rivera, and et al. 2023. "Multilevel Laminoplasty for CSM: Is C3 Laminectomy Better Than C3 Laminoplasty at the Superior Vertebra?" Journal of Clinical Medicine 12, no. 24: 7594. https://doi.org/10.3390/jcm12247594
APA StyleMacki, M., Chryssikos, T., Meade, S. M., Aabedi, A. A., Letchuman, V., Ambati, V., Krishnan, N., Tawil, M. E., Tichelaar, S., Rivera, J., Chan, A. K., Tan, L. A., Chou, D., & Mummaneni, P. (2023). Multilevel Laminoplasty for CSM: Is C3 Laminectomy Better Than C3 Laminoplasty at the Superior Vertebra? Journal of Clinical Medicine, 12(24), 7594. https://doi.org/10.3390/jcm12247594






