Association between Gray and White Matter Lesions and Its Involvement in Clinical Symptoms of Alzheimer’s-Type Dementia
Abstract
:1. Introduction
2. Methods
Statistical Analysis
3. Results
3.1. Clinical Characteristics
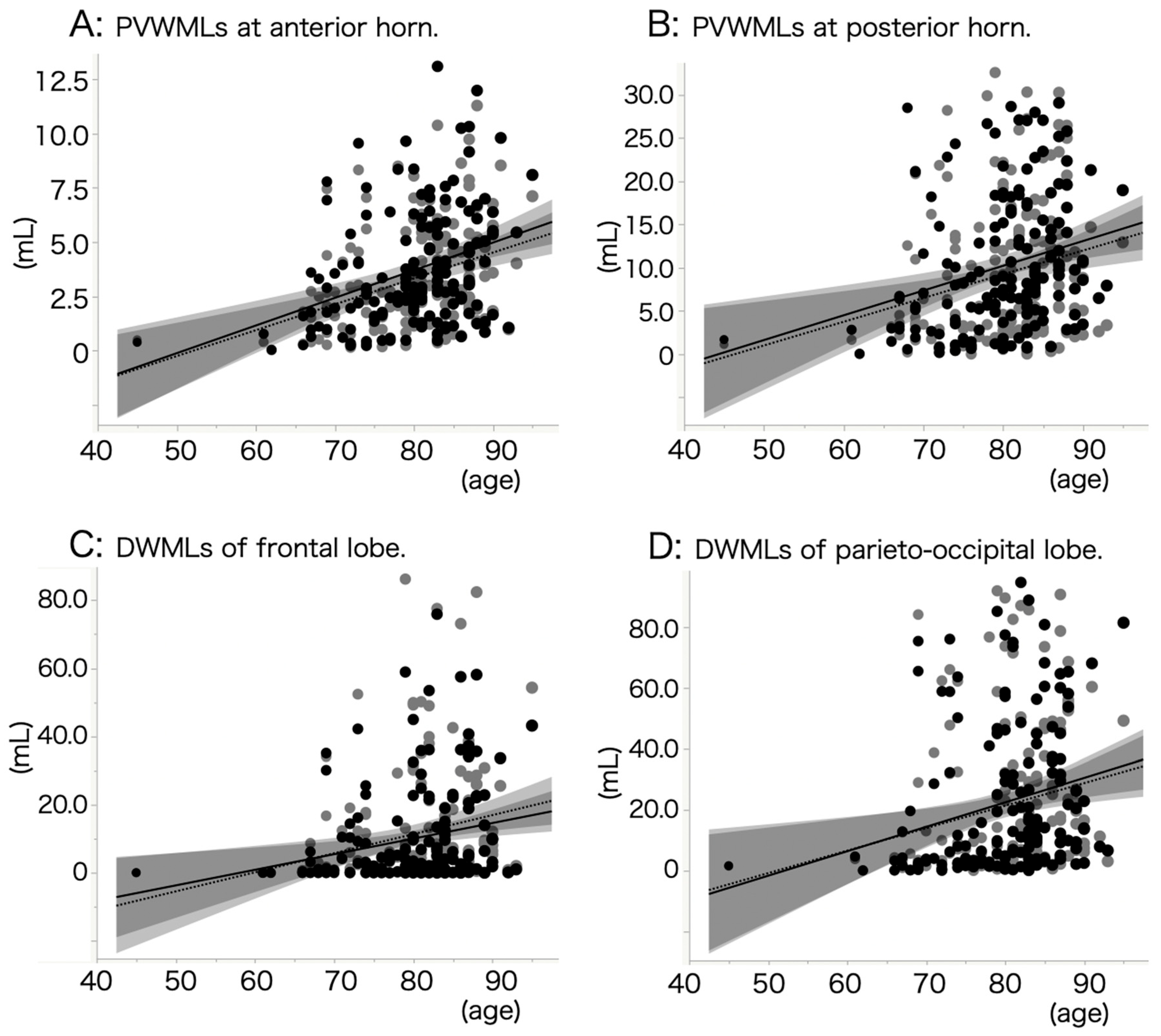
3.2. Backgrounds of White Matter Lesions
3.3. Connectivity between Symptoms and Brain Lesions
3.4. Correlations between Gray Matter Lesions and White Matter Lesions
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Sandrone, S. The brain as a crystal ball: The predictive potential of default mode network. Front. Hum. Neurosci. 2012, 6, 261. [Google Scholar] [CrossRef]
- Gili, T.; Cercignani, M.; Serra, L.; Perri, R.; Giove, F.; Maraviglia, B.; Caltagirone, C.; Bozzali, M. Regional brain atrophy and functional disconnection across Alzheimer’s disease evolution. J. Neurol. Neurosurg. Psychiatry 2011, 82, 58–66. [Google Scholar] [CrossRef]
- Filley, C.M.; Fields, R.D. White matter and cognition: Making the connection. J. Neurophysiol. 2016, 116, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Depp, C.; Sun, T.; Sasmita, A.O.; Spieth, L.; Berghoff, S.A.; Nazarenko, T.; Overhoff, K.; Steixner-Kumar, A.A.; Subramanian, S.; Arinrad, S.; et al. Myelin dysfunction drives amyloid-beta deposition in models of Alzheimer’s disease. Nature 2023, 618, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Viqar, F.; Zimmerman, M.E.; Narkhede, A.; Tosto, G.; Benzinger, T.L.; Marcus, D.S.; Fagan, A.M.; Goate, A.; Fox, N.C.; et al. White matter hyperintensities are a core feature of Alzheimer’s disease: Evidence from the dominantly inherited Alzheimer network. Ann. Neurol. 2016, 79, 929–939. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, F.E.; de Groot, J.C.; Achten, E.; Oudkerk, M.; Ramos, L.M.; Heijboer, R.; Hofman, A.; Jolles, J.; van Gijn, J.; Breteler, M.M. Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The Rotterdam Scan Study. J. Neurol. Neurosurg. Psychiatry 2001, 70, 9–14. [Google Scholar] [CrossRef]
- Schmidt, R.; Schmidt, H.; Haybaeck, J.; Loitfelder, M.; Weis, S.; Cavalieri, M.; Seiler, S.; Enzinger, C.; Ropele, S.; Erkinjuntti, T.; et al. Heterogeneity in age-related white matter changes. Acta Neuropathol. 2011, 122, 171–185. [Google Scholar] [CrossRef]
- Dadar, M.; Manera, A.L.; Ducharme, S.; Collins, D.L. White matter hyperintensities are associated with grey matter atrophy and cognitive decline in Alzheimer’s disease and frontotemporal dementia. Neurobiol. Aging 2022, 111, 54–63. [Google Scholar] [CrossRef]
- Puzo, C.; Labriola, C.; Sugarman, M.A.; Tripodis, Y.; Martin, B.; Palmisano, J.N.; Steinberg, E.G.; Stein, T.D.; Kowall, N.W.; McKee, A.C.; et al. Independent effects of white matter hyperintensities on cognitive, neuropsychiatric, and functional decline: A longitudinal investigation using the National Alzheimer’s Coordinating Center Uniform Data Set. Alzheimers Res. Ther. 2019, 11, 64. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J. Alzheimers Dis. 2001, 3, 75–80. [Google Scholar] [CrossRef]
- Beyreuther, K.; Bush, A.I.; Dyrks, T.; Hilbich, C.; Konig, G.; Monning, U.; Multhaup, G.; Prior, R.; Rumble, B.; Schubert, W.; et al. Mechanisms of amyloid deposition in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1991, 640, 129–139. [Google Scholar] [CrossRef]
- Khoury, M.A.; Bahsoun, M.A.; Fadhel, A.; Shunbuli, S.; Venkatesh, S.; Ghazvanchahi, A.; Mitha, S.; Chan, K.; Fornazzari, L.R.; Churchill, N.W.; et al. Delusional Severity Is Associated with Abnormal Texture in FLAIR MRI. Brain Sci. 2022, 12, 600. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Kaufer, D.I.; Cummings, J.L.; Ketchel, P.; Smith, V.; MacMillan, A.; Shelley, T.; Lopez, O.L.; DeKosky, S.T. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Pantoni, L.; Garcia, J.H. Pathogenesis of leukoaraiosis: A review. Stroke 1997, 28, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Pietroboni, A.M.; Colombi, A.; Carandini, T.; Scarpini, E.; Galimberti, D.; Bozzali, M. The Role of Amyloid-beta in White Matter Damage: Possible Common Pathogenetic Mechanisms in Neurodegenerative and Demyelinating Diseases. J. Alzheimers Dis. 2020, 78, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Chen, W.; Cai, W.J.; Hu, H.; Xu, W.; Wang, Z.T.; Cao, X.P.; Tan, L.; Yu, J.T.; Alzheimer’s Disease Neuroimaging Initiative. Associations of White Matter Hyperintensities with Cognitive Decline: A Longitudinal Study. J. Alzheimers Dis. 2020, 73, 759–768. [Google Scholar] [CrossRef]
- Martin, K.C.; Ketchabaw, W.T.; Turkeltaub, P.E. Plasticity of the language system in children and adults. Handb. Clin. Neurol. 2022, 184, 397–414. [Google Scholar] [CrossRef]
- Dieterich, M.; Brandt, T. Global orientation in space and the lateralization of brain functions. Curr. Opin. Neurol. 2018, 31, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Misquitta, K.; Dadar, M.; Louis Collins, D.; Tartaglia, M.C.; Alzheimer’s Disease Neuroimaging Initiative. White matter hyperintensities and neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s disease. Neuroimage Clin. 2020, 28, 102367. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O. Delusional misidentifications and duplications: Right brain lesions, left brain delusions. Neurology 2009, 72, 80–87. [Google Scholar] [CrossRef]
- Jenkins, L.M.; Wang, L.; Rosen, H.; Weintraub, S. A transdiagnostic review of neuroimaging studies of apathy and disinhibition in dementia. Brain 2022, 145, 1886–1905. [Google Scholar] [CrossRef] [PubMed]


| Total | AD Type | aMCI | p | |
|---|---|---|---|---|
| n | 156 | 102 | 54 | |
| Male/female (n) | 61/95 | 37/65 | 24/30 | 0.3198 |
| Age (mean ± SD years old) | 79.8 ± 7.4 | 80.7 ± 7.1 | 78.3 ± 7.7 | 0.0519 |
| Hypertension | 67.3% | 70.6% | 61.1% | 0.2300 |
| Hyperlipidemia | 37.8% | 36.3% | 40.7% | 0.5842 |
| Diabetes mellitus | 26.3% | 31.4% | 16.7% | 0.0471 |
| Heart diseases | 10.9% | 12.8% | 7.4% | 0.3088 |
| MMSE-J (mean ± SD) | 22.0 ± 4.1 | 20.4 ± 4.0 | 24.9 ± 2.4 | <0.0001 |
| Right-handedness | 94.2% | 90.2% | 96.2% | 0.1737 |
| Cognitive impairments | ||||
| Disorientation (mean ± SD) | 2.2 ± 2.1 | 2.9 ± 2.2 | 0.9 ± 1.1 | <0.0001 |
| Disturbed attention (mean ± SD) | 2.3 ± 1.5 | 2.5 ± 1.5 | 1.9 ± 1.4 | 0.0158 |
| Memory disturbance (mean ± SD) | 2.1 ± 1.0 | 2.4 ± 0.9 | 1.7 ± 1.1 | <0.0001 |
| Impairment of language skill (mean ± SD) | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.1 ± 0.3 | 0.2997 |
| Disorder of spatial perception (mean ± SD) | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.1 ± 0.3 | 0.0416 |
| BPSD | ||||
| Hypoactivity | 34.6% | 44.1% | 16.7% | 0.0006 |
| Hyperactivity | 18.6% | 20.6% | 14.8% | 0.3779 |
| Hallucination/delusion | 15.4% | 21.6% | 3.7% | 0.0033 |
| Abnormal behavior | 7.1% | 8.8% | 3.7% | 0.2347 |
| Disturbed circadian rhythm | 1.3% | 2.0% | 0% | 0.3004 |
| Total | HT | HL | DM | |||
|---|---|---|---|---|---|---|
| Correlation Coefficient | p | Correlation Coefficient | p | Correlation Coefficient | p | |
| L. anterior horn PVWML | 0.2444 | 0.0021 | 0.0215 | 0.7897 | 0.1201 | 0.1353 |
| R. anterior horn PVWML | 0.2273 | 0.0043 | 0.0233 | 0.773 | 0.0797 | 0.3225 |
| L. posterior horn PVWML | 0.1334 | 0.097 | 0.0118 | 0.8833 | 0.105 | 0.1919 |
| R. posterior horn PVWML | 0.1283 | 0.1106 | −0.091 | 0.2586 | 0.0718 | 0.3731 |
| L. frontal DWML | 0.0857 | 0.2876 | 0.0121 | 0.8807 | 0.0056 | 0.945 |
| R. frontal DWML | 0.1289 | 0.1089 | 0.0242 | 0.7642 | 0.0198 | 0.8058 |
| L. parieto-occipital DWML | 0.0567 | 0.4821 | −0.0197 | 0.8068 | 0.0118 | 0.8837 |
| R. parieto-occipital DWML | 0.1102 | 0.1707 | −0.0176 | 0.8276 | 0.0656 | 0.4161 |
| AD type | ||||||
| L. anterior horn PVWML | 0.1645 | 0.0986 | −0.0483 | 0.6296 | 0.1053 | 0.292 |
| R. anterior horn PVWML | 0.1543 | 0.1215 | −0.0304 | 0.7618 | 0.0916 | 0.3601 |
| L. posterior horn PVWML | 0.05 | 0.618 | −0.0464 | 0.6437 | 0.1047 | 0.2951 |
| R. posterior horn PVWML | 0.0286 | 0.7751 | −0.1233 | 0.217 | 0.0812 | 0.4174 |
| L. frontal DWML | −0.0143 | 0.8869 | −0.1105 | 0.269 | −0.0185 | 0.8538 |
| R. frontal DWML | 0.0664 | 0.5075 | −0.065 | 0.5164 | −0.0012 | 0.9906 |
| L. parieto-occipital DWML | −0.0549 | 0.5833 | −0.1177 | 0.2388 | −0.0224 | 0.8232 |
| R. parieto-occipital DWML | −0.012 | 0.9048 | −0.1226 | 0.2196 | 0.0225 | 0.8222 |
| aMCI | ||||||
| L. anterior horn PVWML | 0.4014 | 0.0029 | 0.1814 | 0.1937 | 0.1244 | 0.375 |
| R. anterior horn PVWML | 0.3788 | 0.0052 | 0.1768 | 0.2053 | −0.0226 | 0.8721 |
| L. posterior horn PVWML | 0.3018 | 0.0281 | 0.1754 | 0.2091 | 0.0363 | 0.7962 |
| R. posterior horn PVWML | 0.3323 | 0.015 | 0.0162 | 0.9083 | −0.0229 | 0.8705 |
| L. frontal DWML | 0.2632 | 0.0569 | 0.244 | 0.0783 | 0.0352 | 0.8023 |
| R. frontal DWML | 0.2631 | 0.057 | 0.2588 | 0.0613 | 0.0028 | 0.984 |
| L. parieto-occipital DWML | 0.2724 | 0.0485 | 0.2085 | 0.1341 | 0.0306 | 0.8279 |
| R. parieto-occipital DWML | 0.3619 | 0.0077 | 0.2455 | 0.0764 | 0.0987 | 0.4819 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakase, T.; Thyreau, B.; Tatewaki, Y.; Tomita, N.; Takano, Y.; Muranaka, M.; Taki, Y. Association between Gray and White Matter Lesions and Its Involvement in Clinical Symptoms of Alzheimer’s-Type Dementia. J. Clin. Med. 2023, 12, 7642. https://doi.org/10.3390/jcm12247642
Nakase T, Thyreau B, Tatewaki Y, Tomita N, Takano Y, Muranaka M, Taki Y. Association between Gray and White Matter Lesions and Its Involvement in Clinical Symptoms of Alzheimer’s-Type Dementia. Journal of Clinical Medicine. 2023; 12(24):7642. https://doi.org/10.3390/jcm12247642
Chicago/Turabian StyleNakase, Taizen, Benjamin Thyreau, Yasuko Tatewaki, Naoki Tomita, Yumi Takano, Michiho Muranaka, and Yasuyuki Taki. 2023. "Association between Gray and White Matter Lesions and Its Involvement in Clinical Symptoms of Alzheimer’s-Type Dementia" Journal of Clinical Medicine 12, no. 24: 7642. https://doi.org/10.3390/jcm12247642
APA StyleNakase, T., Thyreau, B., Tatewaki, Y., Tomita, N., Takano, Y., Muranaka, M., & Taki, Y. (2023). Association between Gray and White Matter Lesions and Its Involvement in Clinical Symptoms of Alzheimer’s-Type Dementia. Journal of Clinical Medicine, 12(24), 7642. https://doi.org/10.3390/jcm12247642











