The Evaluation and Management of Coronary Artery Disease in the Lung Transplant Patient
Abstract
:1. Introduction
1.1. Prevalence of Coronary Artery Disease among Lung Transplant Candidates
1.2. Impact of Coronary Artery Disease on Lung Transplant Candidates
1.3. Medical Therapy for the Prevention or Treatment of CAD in Lung Transplant Candidates
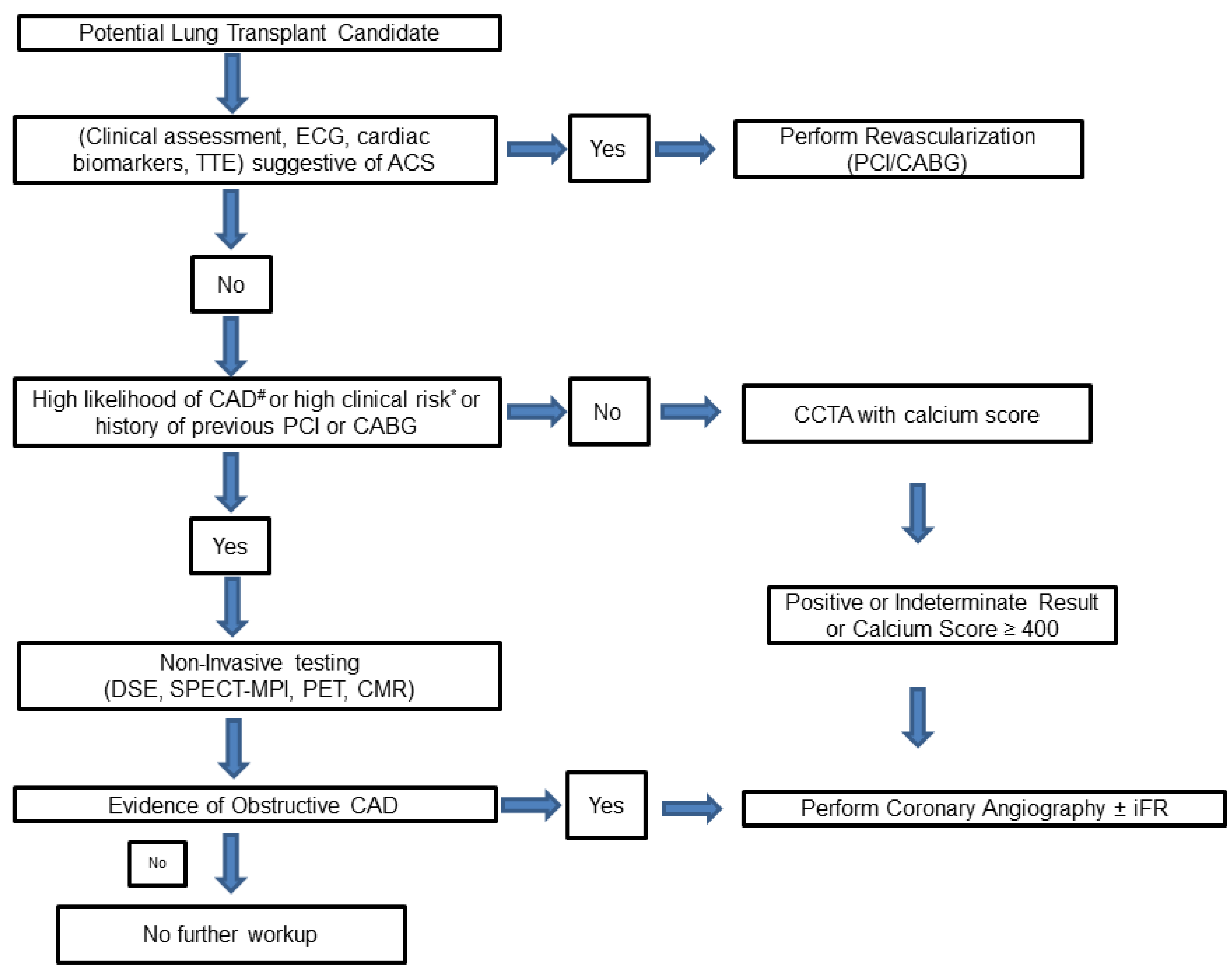
1.4. CAD Screening in Lung Transplantation Candidates
1.4.1. Exercise Testing
1.4.2. Dobutamine Stress Echocardiogram (DSE)
1.4.3. Myocardial Perfusion Imaging (MPI)
1.4.4. Stress Cardiac Magnetic Resonance (CMR)
1.4.5. Coronary Computed Tomography Angiography (CCTA)
1.4.6. Coronary Angiography
1.5. Indications for Revascularization
1.6. Percutaneous Coronary Intervention (PCI)
1.6.1. Stent Choice
1.6.2. DAPT Choice
1.6.3. Stent Optimization
1.7. Surgical Revascularization Coronary Artery Bypass Grafting (CABG)
1.7.1. Approach to Surgical Revascularization
1.7.2. Arterial Versus Venous Grafts
1.8. Immunosuppression and CAD in Lung Transplant Recipients
1.8.1. Calcineurin Inhibitors (Cyclosporine and Tacrolimus)
1.8.2. Anti-Proliferative Agents (Mycophenolate Mofetil or Sodium and Azathioprine)
1.8.3. mTOR Inhibitors (Sirolimus and Everolimus)
1.8.4. Corticosteroids
1.8.5. Statins
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koprivanac, M.; Budev, M.M.; Yun, J.J.; Kelava, M.; Pettersson, G.B.; McCurry, K.R.; Johnston, D.R.; Mangi, A.A.; Houghtaling, P.L.; Blackstone, E.H.; et al. How important is coronary artery disease when considering lung transplant candidates? J. Heart Lung Transplant. 2016, 35, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Makey, I.A.; Sui, J.W.; Huynh, C.; Das, N.A.; Thomas, M.; Johnson, S. Lung transplant patients with coronary artery disease rarely die of cardiac causes. Clin. Transplant. 2018, 32, e13354. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.W. Cardiovascular Toxicities of Immunosuppressive Agents. Am. J. Transplant. 2002, 2, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Wild, J.; Arrigo, M.; Isenring, B.D.; Buergi, U.; Kurowski, T.; Schuurmans, M.M.; Huber, L.C.; Benden, C. Coronary artery disease in lung transplant candidates: Role of routine invasive assessment. Respiration 2015, 89, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Khandhar, S.J.; Althouse, A.D.; Mulukutla, S.; Kormos, R.; Toma, C.; Marroquin, O.; Volz, E.; Tefera, L.; Bermudez, C. Postoperative outcomes and management strategies for coronary artery disease in patients in need of a lung transplantation. Clin. Transplant. 2017, 31, e13026. [Google Scholar] [CrossRef]
- Ben-Dor, I.; Shitrit, D.; Kramer, M.R.; Iakobishvili, Z.; Sahar, G.; Hasdai, D. Is routine coronary angiography and revascularization indicated among patients undergoing evaluation for lung transplantation? Chest 2005, 128, 2557–2562. [Google Scholar] [CrossRef]
- Kaza, A.K.; Dietz, J.F.; Kern, J.A.; Jones, D.R.; Robbins, M.K.; Fiser, S.M.; Long, S.M.; Bergin, J.D.; Kron, I.L.; Tribble, C.G. Coronary risk stratification in patients with end-stage lung disease. J. Heart Lung Transplant. 2002, 21, 334–339. [Google Scholar] [CrossRef]
- Seoane, L.; Arcement, L.M.; Valentine, V.G.; McFadden, P.M. Long-term survival in lung transplant recipients after successful preoperative coronary revascularization. J. Thorac. Cardiovasc. Surg. 2005, 130, 538–541. [Google Scholar] [CrossRef]
- Jones, R.M.; Enfield, K.B.; Mehrad, B.; Keeley, E.C. Prevalence of obstructive coronary artery disease in patients undergoing lung transplantation: Case series and review of the literature. Catheter. Cardiovasc. Interv. 2014, 84, 1–6. [Google Scholar] [CrossRef]
- Kizer, J.R.; Zisman, D.A.; Blumenthal, N.P.; Kotloff, R.M.; Kimmel, S.E.; Strieter, R.M.; Arcasoy, S.M.; Ferrari, V.A.; Hansen-Flaschen, J. Association between pulmonary fibrosis and coronary artery disease. Arch. Intern. Med. 2004, 164, 551–556. [Google Scholar] [CrossRef]
- Snell, G.I.; Richardson, M.; Griffiths, A.P.; Williams, T.J.; Esmore, D.S. Coronary artery disease in potential lung transplant recipients > 50 years old: The role of coronary intervention. Chest 1999, 116, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Leard, L.E.; Holm, A.M.; Valapour, M.; Glanville, A.R.; Attawar, S.; Aversa, M.; Campos, S.V.; Christon, L.M.; Cypel, M.; Dellgren, G.; et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2021, 40, 1349–1379. [Google Scholar] [CrossRef] [PubMed]
- Chassot, P.G.; Delabays, A.; Spahn, D. Preoperative evaluation of patients with, or at risk of, coronary artery disease undergoing non-cardiac surgery. Br. J. Anaesth. 2002, 89, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Chandiramani, R.; Capodanno, D.; Berger, J.S.; Levin, M.A.; Hawn, M.T.; Angiolillo, D.J.; Mehran, R. Non-cardiac surgery in patients with coronary artery disease: Risk evaluation and periprocedural management. Nat. Rev. Cardiol. 2021, 18, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F. Immunosuppression minimization: Current and future trends in transplant immunosuppression. J. Am. Soc. Nephrol. 2003, 14, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Chaikriangkrai, K.; Jyothula, S.; Jhun, H.Y.; Estep, J.; Loebe, M.; Scheinin, S.; Torre-Amione, G. Impact of pre-operative coronary artery disease on cardiovascular events following lung transplantation. J. Heart Lung Transplant. 2016, 35, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Halloran, K.; Hirji, A.; Li, D.; Jackson, K.; Kapasi, A.; Meyer, S.; Mullen, J.; Lien, D.; Weinkauf, J. Coronary Artery Disease and Coronary Artery Bypass Grafting at the Time of Lung Transplantation Do Not Impact Overall Survival. Transplantation 2019, 103, 2190–2195. [Google Scholar] [CrossRef] [PubMed]
- Choong, C.K.; Meyers, B.F.; Guthrie, T.J.; Trulock, E.P.; Patterson, G.A.; Moazami, N. Does the presence of preoperative mild or moderate coronary artery disease affect the outcomes of lung transplantation? Ann. Thorac. Surg. 2006, 82, 1038–1042. [Google Scholar] [CrossRef]
- Zanotti, G.; Hartwig, M.G.; Castleberry, A.W.; Martin, J.T.; Shaw, L.K.; Williams, J.B.; Lin, S.S.; Davis, R.D. Preoperative mild-to-moderate coronary artery disease does not affect long-term outcomes of lung transplantation. Transplantation 2014, 97, 1079–1085. [Google Scholar] [CrossRef]
- Jones, M.; Patel, S.; Tefera, L.; Borrebach, J.; Bermudez, C.; Bhama, J.; Kormos, R.; Shigemura, N.; D’Cunha, J.; Mulukutla, S. The impact of coronary artery disease on lung transplantation outcomes. J. Heart Lung Transplant. 2014, 33, S80. [Google Scholar] [CrossRef]
- Sherman, W.; Rabkin, D.G.; Ross, D.; Saggar, R.; Lynch, J.P., 3rd; Belperio, J.; Saggar, R.; Hamilton, M.; Ardehali, A. Lung transplantation and coronary artery disease. Ann. Thorac. Surg. 2011, 92, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Castleberry, A.W.; Martin, J.T.; Osho, A.A.; Hartwig, M.G.; Hashmi, Z.A.; Zanotti, G.; Shaw, L.K.; Williams, J.B.; Lin, S.S.; Davis, R.D. Coronary revascularization in lung transplant recipients with concomitant coronary artery disease. Am. J. Transplant. 2013, 13, 2978–2988. [Google Scholar] [CrossRef] [PubMed]
- Mateen, F.J.; Dierkhising, R.A.; Rabinstein, A.A.; Van De Beek, D.; Wijdicks, E.F.M. Neurological complications following adult lung transplantation. Am. J. Transplant. 2010, 10, 908–914. [Google Scholar] [CrossRef]
- Shigemura, N.; Sclabassi, R.J.; Bhama, J.K.; Gries, C.J.; Crespo, M.M.; Johnson, B.; Pilewski, J.M.; Bermudez, C.A. Early major neurologic complications after lung transplantation: Incidence, risk factors, and outcome. Transplantation 2013, 95, 866–871. [Google Scholar] [CrossRef]
- Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; Meade, T.; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar] [PubMed]
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996, 348, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [PubMed]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Braunwald, E. Ezetimibe plus a Statin after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 373, 1473–1477. [Google Scholar]
- Wright, J.T., Jr.; Williamson, J.D.; Whelton, P.K.; Snyder, J.K.; Sink, K.M.; Rocco, M.V.; Reboussin, D.M.; Rahman, M.; Oparil, S.; Lewis, C.E.; et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med. 2015, 373, 2103–2116. [Google Scholar] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [PubMed]
- Arnold, S.V.; Bhatt, D.L.; Barsness, G.W.; Beatty, A.L.; Deedwania, P.C.; Inzucchi, S.E.; Kosiborod, M.; Leiter, L.A.; Lipska, K.J.; Newman, J.D.; et al. Clinical Management of Stable Coronary Artery Disease in Patients With Type 2 Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e779–e806. [Google Scholar] [CrossRef] [PubMed]
- Sorbets, E.; Steg, P.G.; Young, R.; Danchin, N.; Greenlaw, N.; Ford, I.; Tendera, M.; Ferrari, R.; Merkely, B.; Parkhomenko, A.; et al. β-blockers, calcium antagonists, and mortality in stable coronary artery disease: An international cohort study. Eur. Heart J. 2019, 40, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Belsey, J.; Savelieva, I.; Mugelli, A.; Camm, A.J. Relative efficacy of antianginal drugs used as add-on therapy in patients with stable angina: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2015, 22, 837–848. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar]
- International guidelines for the selection of lung transplant candidates. The American Society for Transplant Physicians (ASTP)/American Thoracic Society(ATS)/European Respiratory Society(ERS)/International Society for Heart and Lung Transplantation(ISHLT). Am. J. Respir. Crit. Care Med. 1998, 158, 335–339.
- Weill, D.; Benden, C.; Corris, P.A.; Dark, J.H.; Davis, R.D.; Keshavjee, S.; Lederer, D.J.; Mulligan, M.J.; Patterson, G.A.; Singer, L.G.; et al. A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2015, 34, 1–15. [Google Scholar] [CrossRef]
- Bossone, E.; Martinez, F.J.; Whyte, R.I.; Iannettoni, M.D.; Armstrong, W.F.; Bach, D.S. Dobutamine stress echocardiography for the preoperative evaluation of patients undergoing lung volume reduction surgery. J. Thorac. Cardiovasc. Surg. 1999, 118, 542–546. [Google Scholar] [CrossRef]
- Manoushagian, S.; Meshkov, A. Evaluation of solid organ transplant candidates for coronary artery disease. Am. J. Transplant. 2014, 14, 2228–2234. [Google Scholar] [CrossRef] [PubMed]
- Henzlova, M.J.; Padilla, M.L.; Freilich, A.; Gass, A.L.; Courtney, M.C.; Diamond, J.A.; Machac, J. Dobutamine thallium 201 perfusion imaging in candidates for lung transplantation. J. Heart Lung Transplant. 1995, 14, 251–256. [Google Scholar]
- Schiopu, S.R.I.; Zacherl, M.; Todica, A.; Bartenstein, P.; Milger, K.; Leuschner, G.; Munker, D.; Bauer, M.; Massberg, S.; Behr, J.; et al. Feasibility and accuracy of SPECT myocardial perfusion imaging in end-stage lung disease. J. Nucl. Cardiol. 2020, 27, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.H.; Bateman, T.M.; Berman, D.S.; Chareonthaitawee, P.; De Blanche, L.E.; Dilsizian, V.; Dorbala, S.; Gropler, R.J.; Shaw, L.; Soman, P.; et al. Appropriate Use Criteria for PET Myocardial Perfusion Imaging. J. Nucl. Med. 2020, 61, 1221–1265. [Google Scholar] [CrossRef] [PubMed]
- Jaarsma, C.; Leiner, T.; Bekkers, S.C.; Crijns, H.J.; Wildberger, J.E.; Nagel, E.; Nelemans, P.J.; Schalla, S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: A meta-analysis. J. Am. Coll. Cardiol. 2012, 59, 1719–1728. [Google Scholar] [PubMed]
- Koshy, A.N.; Ha, F.J.; Gow, P.J.; Han, H.C.; Amirul-Islam, F.M.; Lim, H.S.; Teh, A.W.; Farouque, O. Computed tomographic coronary angiography in risk stratification prior to non-cardiac surgery: A systematic review and meta-analysis. Heart 2019, 105, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Balayla, G.; Braghiroli, J. Coronary artery disease in lung transplant patients. Clin. Transplant. 2020, 34, e14078. [Google Scholar] [CrossRef] [PubMed]
- Gotberg, M.; Christiansen, E.H.; Gudmundsdottir, I.J.; Sandhall, L.; Danielewicz, M.; Jakobsen, L.; Olsson, S.E.; Ohagen, P.; Olsson, H.; Omerovic, E.; et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N. Engl. J. Med. 2017, 376, 1813–1823. [Google Scholar] [CrossRef]
- Halvorsen, S.; Mehilli, J.; Cassese, S.; Hall, T.S.; Abdelhamid, M.; Barbato, E.; De Hert, S.; de Laval, I.; Geisler, T.; Hinterbuchner, L.; et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur. Heart J. 2022, 43, 3826–3924. [Google Scholar] [CrossRef]
- McFalls, E.O.; Ward, H.B.; Moritz, T.E.; Goldman, S.; Krupski, W.C.; Littooy, F.; Pierpont, G.; Santilli, S.; Rapp, J.; Hattler, B.; et al. Coronary-artery revascularization before elective major vascular surgery. N. Engl. J. Med. 2004, 351, 2795–2804. [Google Scholar] [CrossRef]
- Fleisher, L.A.; Fleischmann, K.E.; Auerbach, A.D.; Barnason, S.A.; Beckman, J.A.; Bozkurt, B.; Davila-Roman, V.G.; Gerhard-Herman, M.D.; Holly, T.A.; Kane, G.C.; et al. 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2014, 130, e278–e333. [Google Scholar] [PubMed]
- Kanaparthi, J.; Kashem, M.A.; Suryapalam, M.; Zhao, H.; Brann, S.; Leotta, E.; Minakata, K.; Keshavamurthy, S.; Shigemura, N.; Toyoda, Y. Prior and Perioperative Revascularization Does Not Affect Survival in Lung Transplant Patients. Ann. Thorac. Surg. 2020, 109, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Dalal, A. Organ transplantation and drug eluting stents: Perioperative challenges. World J. Transplant. 2016, 6, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef]
- Vranckx, P.; Valgimigli, M.; Jüni, P.; Hamm, C.; Steg, P.G.; Heg, D.; van Es, G.A.; McFadden, E.P.; Onuma, Y.; van Meijeren, C.; et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: A multicentre, open-label, randomised superiority trial. Lancet 2018, 392, 940–949. [Google Scholar] [PubMed]
- Urban, P.; Meredith, I.T.; Abizaid, A.; Pocock, S.J.; Carrié, D.; Naber, C.; Lipiecki, J.; Richardt, G.; Iñiguez, A.; Brunel, P.; et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N. Engl. J. Med. 2015, 373, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.-P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2017, 39, 213–260. [Google Scholar]
- Teng, R. Ticagrelor: Pharmacokinetic, Pharmacodynamic and Pharmacogenetic Profile: An Update. Clin. Pharmacokinet. 2015, 54, 1125–1138. [Google Scholar] [CrossRef]
- Butler, K.; Maya, J.; Teng, R. Effect of ticagrelor on pulmonary function in healthy elderly volunteers and asthma or chronic obstructive pulmonary disease patients. Curr. Med. Res. Opin. 2013, 29, 569–577. [Google Scholar] [CrossRef]
- Caldeira, D.; Pinto, F.J.; Ferreira, J.J. Dyspnea and reversibility profile of P2Y12 antagonists: Systematic review of new antiplatelet drugs. Am. J. Cardiovasc. Drugs 2014, 14, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xu, W.; Li, O.; Zhang, B. The risk of dyspnea in patients treated with third-generation P2Y12 inhibitors compared with clopidogrel: A meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2020, 20, 140. [Google Scholar] [CrossRef] [PubMed]
- Nanau, R.M.; Delzor, F.; Neuman, M.G. Efficacy and safety of prasugrel in acute coronary syndrome patients. Clin. Biochem. 2014, 47, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Abro, S.; Bikeyeva, V.; Naqvi, W.A.; Anigbo, C.L.; Tariq, F.; Hussain Rafay, R.; Umar, M.F.; Singh, R. Clopidogrel-Associated Interstitial Lung Disease: A Case Report and Literature Review. Cureus 2022, 14, e28394. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, Y.; Miyajima, T.; Nagasawa, C.; Awaya, Y. Clopidogrel-induced pneumonia. BMJ Case Rep. 2021, 14, e244564. [Google Scholar] [CrossRef] [PubMed]
- Mintz, G.S.; Bourantas, C.V.; Chamié, D. Intravascular Imaging for Percutaneous Coronary Intervention Guidance and Optimization: The Evidence for Improved Patient Outcomes. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100413. [Google Scholar] [CrossRef]
- Niu, Y.; Bai, N.; Ma, Y.; Zhong, P.Y.; Shang, Y.S.; Wang, Z.L. Efficacy of intravascular imaging-guided drug-eluting stent implantation: A systematic review and meta-analysis of randomized clinical trials. BMC Cardiovasc. Disord. 2022, 22, 327. [Google Scholar] [CrossRef]
- Buccheri, S.; Franchina, G.; Romano, S.; Puglisi, S.; Venuti, G.; D’Arrigo, P.; Francaviglia, B.; Scalia, M.; Condorelli, A.; Barbanti, M.; et al. Clinical Outcomes Following Intravascular Imaging-Guided Versus Coronary Angiography–Guided Percutaneous Coronary Intervention With Stent Implantation. JACC Cardiovasc. Interv. 2017, 10, 2488–2498. [Google Scholar] [CrossRef]
- Darmoch, F.; Alraies, M.C.; Al-Khadra, Y.; Moussa Pacha, H.; Pinto, D.S.; Osborn, E.A. Intravascular Ultrasound Imaging-Guided Versus Coronary Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e013678. [Google Scholar] [CrossRef]
- Ali, Z.A.; Landmesser, U.; Maehara, A.; Matsumura, M.; Shlofmitz, R.A.; Guagliumi, G.; Price, M.J.; Hill, J.M.; Akasaka, T.; Prati, F.; et al. Optical Coherence Tomography–Guided versus Angiography-Guided PCI. N. Engl. J. Med. 2023, 389, 1466–1476. [Google Scholar] [CrossRef]
- Holm, N.R.; Andreasen, L.N.; Neghabat, O.; Laanmets, P.; Kumsars, I.; Bennett, J.; Olsen, N.T.; Odenstedt, J.; Hoffmann, P.; Dens, J.; et al. OCT or Angiography Guidance for PCI in Complex Bifurcation Lesions. N. Engl. J. Med. 2023, 389, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Maehara, A. Intravascular Imaging to Guide Percutaneous Coronary Intervention Will Be Mandatory Soon. Circ. Cardiovasc. Interv. 2022, 15, e012120. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Gao, X.F.; Kan, J.; Kong, X.Q.; Zuo, G.F.; Ye, F.; Tian, N.L.; Lin, S.; Liu, Z.Z.; Shao, Y.B.; et al. Comparison of one-month versus twelve-month dual antiplatelet therapy after implantation of drug-eluting stents guided by either intravascular ultrasound or angiography in patients with acute coronary syndrome: Rationale and design of prospective, multicenter, randomized, controlled IVUS-ACS and ULTIMATE-DAPT trial. Am. Heart J. 2021, 236, 49–58. [Google Scholar] [PubMed]
- Lee, R.; Meyers, B.F.; Sundt, T.M.; Trulock, E.P.; Patterson, G.A. Concomitant coronary artery revascularization to allow successful lung transplantation in selected patients with coronary artery disease. J. Thorac. Cardiovasc. Surg. 2002, 124, 1250–1251. [Google Scholar] [CrossRef]
- Parekh, K.; Meyers, B.F.; Patterson, G.A.; Guthrie, T.J.; Trulock, E.P.; Damiano, R.J.; Moazami, N. Outcome of lung transplantation for patients requiring concomitant cardiac surgery. J. Thorac. Cardiovasc. Surg. 2005, 130, 859–863. [Google Scholar] [CrossRef]
- Patel, V.S.; Palmer, S.M.; Messier, R.H.; Davis, R.D. Clinical outcome after coronary artery revascularization and lung transplantation. Ann. Thorac. Surg. 2003, 75, 372–377. [Google Scholar] [CrossRef]
- Mohite, P.N.; Sabashnikov, A.; Patil, N.P.; Garcia-Saez, D.; Zych, B.; Zeriouh, M.; Romano, R.; Soresi, S.; Reed, A.; Carby, M.; et al. The role of cardiopulmonary bypass in lung transplantation. Clin. Transplant. 2016, 30, 202–209. [Google Scholar] [CrossRef]
- Khatchatourian, G.; Chevalley, C.; Spiliopoulos, A.; Licker, M. Myocardial revascularization and bilateral lung transplantation without cardiopulmonary bypass. Eur. J. Cardiothorac. Surg. 2001, 20, 1042–1044. [Google Scholar] [CrossRef]
- Chikwe, J.; Lee, T.; Itagaki, S.; Adams, D.H.; Egorova, N.N. Long-Term Outcomes After Off-Pump Versus On-Pump Coronary Artery Bypass Grafting by Experienced Surgeons. J. Am. Coll. Cardiol. 2018, 72, 1478–1486. [Google Scholar] [CrossRef]
- Quin, J.A.; Wagner, T.H.; Hattler, B.; Carr, B.M.; Collins, J.; Almassi, G.H.; Grover, F.L.; Shroyer, A.L. Ten-Year Outcomes of Off-Pump vs On-Pump Coronary Artery Bypass Grafting in the Department of Veterans Affairs: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Hoetzenecker, K.; Schwarz, S.; Muckenhuber, M.; Benazzo, A.; Frommlet, F.; Schweiger, T.; Bata, O.; Jaksch, P.; Ahmadi, N.; Muraközy, G.; et al. Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J. Thorac. Cardiovasc. Surg. 2018, 155, 2193–2206.e2193. [Google Scholar] [CrossRef] [PubMed]
- Machuca, T.N.; Collaud, S.; Mercier, O.; Cheung, M.; Cunningham, V.; Kim, S.J.; Azad, S.; Singer, L.; Yasufuku, K.; de Perrot, M.; et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J. Thorac. Cardiovasc. Surg. 2015, 149, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, C.A.; Shiose, A.; Esper, S.A.; Shigemura, N.; D’Cunha, J.; Bhama, J.K.; Richards, T.J.; Arlia, P.; Crespo, M.M.; Pilewski, J.M. Outcomes of intraoperative venoarterial extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation. Ann. Thorac. Surg. 2014, 98, 1936–1942; discussion 1942–1933. [Google Scholar] [CrossRef] [PubMed]
- Biscotti, M.; Yang, J.; Sonett, J.; Bacchetta, M. Comparison of extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J. Thorac. Cardiovasc. Surg. 2014, 148, 2410–2415. [Google Scholar] [CrossRef]
- Ius, F.; Kuehn, C.; Tudorache, I.; Sommer, W.; Avsar, M.; Boethig, D.; Fuehner, T.; Gottlieb, J.; Hoeper, M.; Haverich, A.; et al. Lung transplantation on cardiopulmonary support: Venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 2012, 144, 1510–1516. [Google Scholar] [CrossRef]
- Collins, P.; Webb, C.M.; Chong, C.F.; Moat, N.E. Radial Artery Versus Saphenous Vein Patency Randomized Trial. Circulation 2008, 117, 2859–2864. [Google Scholar] [CrossRef]
- Bos, S.; Vos, R.; Van Raemdonck, D.E.; Verleden, G.M. Survival in adult lung transplantation: Where are we in 2020? Curr. Opin. Organ Transplant. 2020, 25, 268–273. [Google Scholar] [CrossRef]
- Thabut, G.; Mal, H. Outcomes after lung transplantation. J. Thorac. Dis. 2017, 9, 2684–2691. [Google Scholar] [CrossRef]
- Caliskan, E.; de Souza, D.R.; Böning, A.; Liakopoulos, O.J.; Choi, Y.H.; Pepper, J.; Gibson, C.M.; Perrault, L.P.; Wolf, R.K.; Kim, K.B.; et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat. Rev. Cardiol. 2020, 17, 155–169. [Google Scholar] [CrossRef]
- Aldea, G.S.; Bakaeen, F.G.; Pal, J.; Fremes, S.; Head, S.J.; Sabik, J.; Rosengart, T.; Kappetein, A.P.; Thourani, V.H.; Firestone, S.; et al. The Society of Thoracic Surgeons Clinical Practice Guidelines on Arterial Conduits for Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2016, 101, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.M.; Landee, F.A.; Reslock, M.A. Chemically Oriented Storage and Retrieval System. 1. Storage and Verification of Structural Information. J. Chem. Doc. 1967, 7, 43–47. [Google Scholar] [CrossRef]
- Silverborn, M.; Jeppsson, A.; Mårtensson, G.; Nilsson, F. New-onset cardiovascular risk factors in lung transplant recipients. J. Heart Lung Transplant. 2005, 24, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Nso, N.; Lakhdar, S.; Kondaveeti, R.; Buttar, C.; Bhangoo, H.; Awad, M.; Sheikh, N.S.; Soliman, K.M.; Munira, M.S.; et al. New onset hypertension after transplantation. World J. Transplant. 2022, 12, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.S.; Rein, J.L. The Many Faces of Calcineurin Inhibitor Toxicity-What the FK? Adv. Chronic Kidney Dis. 2020, 27, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Nash, E.F.; Stephenson, A.; Helm, E.J.; Durie, P.R.; Tullis, E.; Singer, L.G.; Chaparro, C. Impact of lung transplantation on serum lipids in adults with cystic fibrosis. J. Heart Lung Transplant. 2011, 30, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Akman, B.; Uyar, M.; Afsar, B.; Sezer, S.; Ozdemir, F.N.; Haberal, M. Lipid profile during azathioprine or mycophenolate mofetil combinations with cyclosporine and steroids. Transplant. Proc. 2007, 39, 135–137. [Google Scholar] [CrossRef]
- Tory, R.; Sachs-Barrable, K.; Hill, J.S.; Wasan, K.M. Cyclosporine A and Rapamycin induce in vitro cholesteryl ester transfer protein activity, and suppress lipoprotein lipase activity in human plasma. Int. J. Pharm. 2008, 358, 219–223. [Google Scholar] [CrossRef]
- Jenssen, T.; Hartmann, A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat. Rev. Endocrinol. 2019, 15, 172–188. [Google Scholar] [CrossRef]
- Chakkera, H.A.; Mandarino, L.J. Calcineurin inhibition and new-onset diabetes mellitus after transplantation. Transplantation 2013, 95, 647–652. [Google Scholar] [CrossRef]
- Ollech, J.E.; Kramer, M.R.; Peled, N.; Ollech, A.; Amital, A.; Medalion, B.; Saute, M.; Shitrit, D. Post-transplant diabetes mellitus in lung transplant recipients: Incidence and risk factors. Eur. J. Cardiothorac. Surg. 2008, 33, 844–848. [Google Scholar] [CrossRef]
- Kobashigawa, J.; Miller, L.; Renlund, D.; Mentzer, R.; Alderman, E.; Bourge, R.; Costanzo, M.; Eisen, H.; Dureau, G.; Ratkovec, R.; et al. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenolate Mofetil Investigators. Transplantation 1998, 66, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Ducloux, D.; Courivaud, C. Prevention of Post-Transplant Diabetes Mellitus: Towards a Personalized Approach. J. Pers. Med. 2022, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Diekmann, F.; Campistol, J.M. Practical considerations for the use of mTOR inhibitors. Transplant. Res. 2015, 4, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Geissler, E.K.; Schlitt, H.J.; Thomas, G. mTOR, cancer and transplantation. Am. J. Transplant. 2008, 8, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Elezaby, A.; Dexheimer, R.; Sallam, K. Cardiovascular effects of immunosuppression agents. Front. Cardiovasc. Med. 2022, 9, 981838. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, R.J.; Lee, M.S. Transplant Coronary Artery Disease. JACC Cardiovasc. Interv. 2010, 3, 367–377. [Google Scholar] [CrossRef]
- Ternstrom, L.; Jeppsson, A.; Ricksten, A.; Nilsson, F. Tumor necrosis factor gene polymorphism and cardiac allograft vasculopathy. J. Heart Lung Transplant. 2005, 24, 433–438. [Google Scholar] [CrossRef]
- Keogh, A.; Richardson, M.; Ruygrok, P.; Spratt, P.; Galbraith, A.; O’Driscoll, G.; Macdonald, P.; Esmore, D.; Muller, D.; Faddy, S. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: A randomized clinical trial. Circulation 2004, 110, 2694–2700. [Google Scholar] [CrossRef]
- Simon, M.; Gomberg-Maitland, M.; Oudiz, R.J.; Machado, R.; Rischard, F.; Elinoff, J.M.; Grigorian, B.; Schmid, A.N.; Hou, S.; Desai, N.; et al. Severe Pulmonary Arterial Hypertension Treated with ABI-009, nab-Sirolimus, an mTOR Inhibitor. J. Heart Lung Transplant. 2019, 38, S487. [Google Scholar] [CrossRef]
- Temiz-Resitoglu, M.; Guden, D.S.; Senol, S.P.; Vezir, O.; Sucu, N.; Kibar, D.; Yılmaz, S.N.; Tunctan, B.; Malik, K.U.; Sahan-Firat, S. Pharmacological Inhibition of Mammalian Target of Rapamycin Attenuates Deoxycorticosterone Acetate Salt-Induced Hypertension and Related Pathophysiology: Regulation of Oxidative Stress, Inflammation, and Cardiovascular Hypertrophy in Male Rats. J. Cardiovasc. Pharmacol. 2022, 79, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Basdevant, A. Steroids and lipid metabolism: Mechanism of action. Int. J. Fertil. 1992, 37 (Suppl. S2), 93–97. [Google Scholar] [PubMed]
- Sever, M.S. Transplantation–steroid–impaired glucose metabolism: A hope for improvement? Nephrol. Dial. Transplant. 2013, 29, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.; Biddle, K.; Augustine, T.; Azmi, S. Post-Transplantation Diabetes Mellitus. Diabetes Ther. 2020, 11, 779–801. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.M.; Forrest, I.A.; Corris, P.A.; Johnson, G.E.; Small, T.; Jones, D.; Fisher, A.J.; Egan, J.J.; Cawston, T.E.; Ward, C.; et al. Simvastatin attenuates release of neutrophilic and remodeling factors from primary bronchial epithelial cells derived from stable lung transplant recipients. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 294, L592–L599. [Google Scholar] [CrossRef] [PubMed]
- Raphael, J.; Collins, S.R.; Wang, X.-Q.; Scalzo, D.C.; Singla, P.; Lau, C.L.; Kozower, B.D.; Durieux, M.E.; Blank, R.S. Perioperative statin use is associated with decreased incidence of primary graft dysfunction after lung transplantation. J. Heart Lung Transplant. 2017, 36, 948–956. [Google Scholar] [CrossRef]
- Johnson, B.A.; Iacono, A.T.; Zeevi, A.; McCurry, K.R.; Duncan, S.R. Statin use is associated with improved function and survival of lung allografts. Am. J. Respir. Crit. Care Med. 2003, 167, 1271–1278. [Google Scholar] [CrossRef]
- Bello, I.; Sandiumenge, A.; Coll, E.; de la Torre, M.; Mosteiro, F.; Álvarez, C.; Mora, V.; Miñambres, E.; Crowley, S.; Ussetti, P.; et al. Value of Preoperative Use of Statins as a Protective Factor for Severe Graft Dysfunction After Lung Transplantation: A Multicenter Propensity Score Analysis. Arch. Bronconeumol. 2021, 57, 720–722. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Corsini, A.; Davidson, M.H.; Holdaas, H.; Jacobson, T.A.; Leitersdorf, E.; März, W.; Reckless, J.P.D.; Stein, E.A. Risk for Myopathy With Statin Therapy in High-Risk Patients. Arch. Intern. Med. 2003, 163, 553–564. [Google Scholar] [CrossRef]
- Lemahieu, W.P.; Hermann, M.; Asberg, A.; Verbeke, K.; Holdaas, H.; Vanrenterghem, Y.; Maes, B.D. Combined therapy with atorvastatin and calcineurin inhibitors: No interactions with tacrolimus. Am. J. Transplant. 2005, 5, 2236–2243. [Google Scholar] [CrossRef]
- Elsby, R.; Hilgendorf, C.; Fenner, K. Understanding the critical disposition pathways of statins to assess drug-drug interaction risk during drug development: It’s not just about OATP1B1. Clin. Pharmacol. Ther. 2012, 92, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Migliozzi, D.R.; Asal, N.J. Clinical Controversy in Transplantation: Tacrolimus Versus Cyclosporine in Statin Drug Interactions. Ann. Pharmacother. 2020, 54, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Amundsen, R.; Christensen, H.; Zabihyan, B.; Asberg, A. Cyclosporine A, but not tacrolimus, shows relevant inhibition of organic anion-transporting protein 1B1-mediated transport of atorvastatin. Drug Metab. Dispos. 2010, 38, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, B.S.; Saseen, J.J.; Page, R.L.; Reed, B.N.; Sneed, K.; Kostis, J.B.; Lanfear, D.; Virani, S.; Morris, P.B. Recommendations for Management of Clinically Significant Drug-Drug Interactions With Statins and Select Agents Used in Patients With Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e468–e495. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrao, G.; Vinayak, M.; Nicolas, J.; Subramaniam, V.; Lai, A.C.; Laskey, D.; Kini, A.; Seethamraju, H.; Scheinin, S. The Evaluation and Management of Coronary Artery Disease in the Lung Transplant Patient. J. Clin. Med. 2023, 12, 7644. https://doi.org/10.3390/jcm12247644
Serrao G, Vinayak M, Nicolas J, Subramaniam V, Lai AC, Laskey D, Kini A, Seethamraju H, Scheinin S. The Evaluation and Management of Coronary Artery Disease in the Lung Transplant Patient. Journal of Clinical Medicine. 2023; 12(24):7644. https://doi.org/10.3390/jcm12247644
Chicago/Turabian StyleSerrao, Gregory, Manish Vinayak, Johny Nicolas, Varsha Subramaniam, Ashton C. Lai, Daniel Laskey, Annapoorna Kini, Harish Seethamraju, and Scott Scheinin. 2023. "The Evaluation and Management of Coronary Artery Disease in the Lung Transplant Patient" Journal of Clinical Medicine 12, no. 24: 7644. https://doi.org/10.3390/jcm12247644




