Sex Differences in Red Blood Cell Transfusions and 30-Day Mortality in Cardiac Surgery: A Single Center Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Informed Consent
2.2. Patient Selection
2.3. Anesthetic Management
2.4. Outcomes and Measurements
2.5. Statistical Methods
3. Results
3.1. Baseline Characteristics
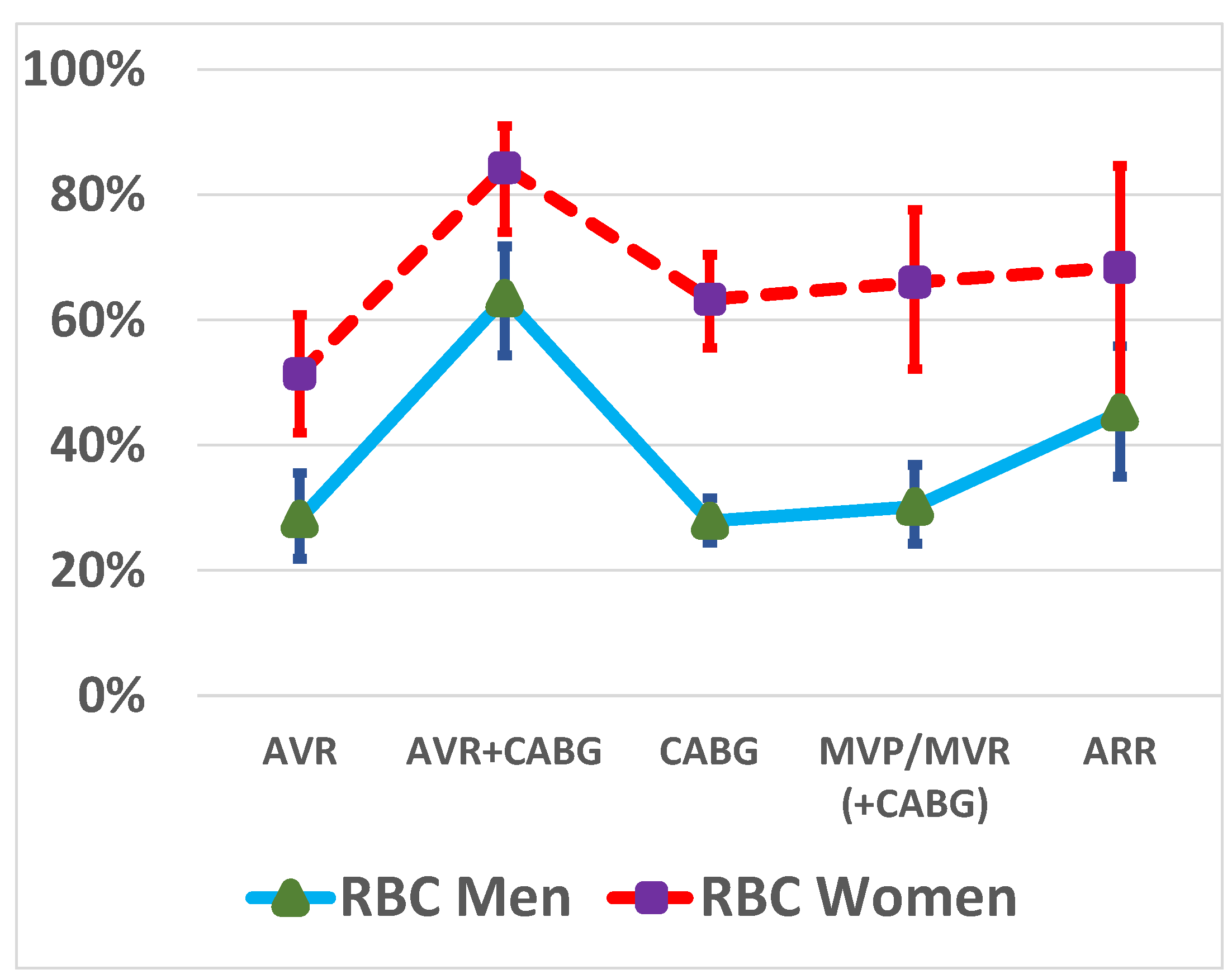
3.2. RBC Transfusions by Sex and Type of Operation
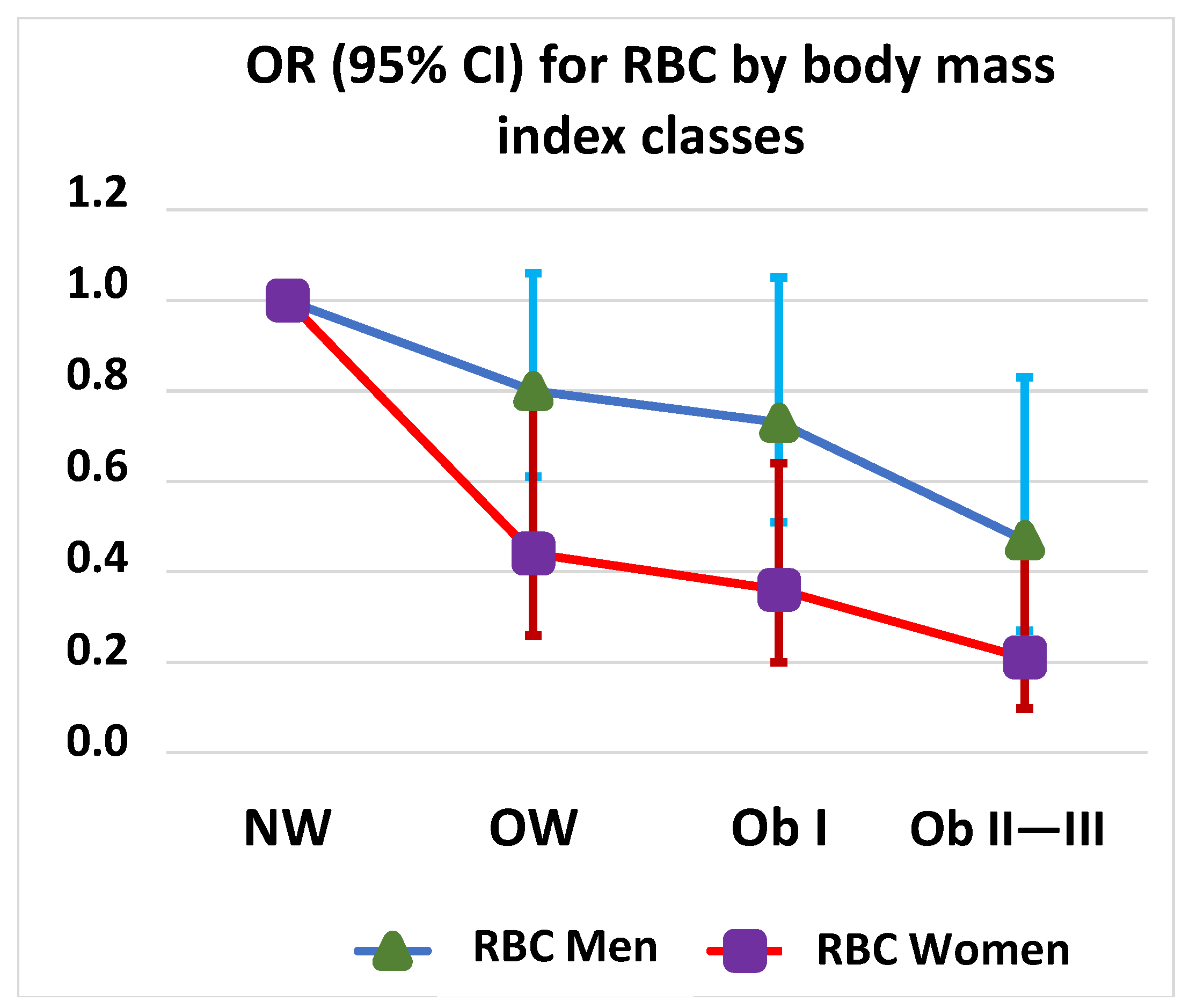
3.3. Linear Regression between BMI and RBC Transfusions by Sex
3.4. Univariable Logistic Regression Analysis
3.5. Multivariable Logistic Regression Model
3.6. Sensitivity Analysis
3.7. 30-Day Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, G.J.; Reeves, B.C.; Rogers, C.A.; Rizvi, S.I.; Culliford, L.; Angelini, G.D. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007, 116, 2544–2552. [Google Scholar] [CrossRef] [PubMed]
- Ad, N.; Massimiano, P.S.; Rongione, A.J.; Taylor, B.; Schena, S.; Alejo, D.; Fonner, C.E.; Salenger, R.; Whitman, G.; Metkus, T.S.; et al. Number and type of blood products are negatively associated with outcomes after cardiac surgery. Ann. Thorac. Surg. 2022, 113, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Sultan, I.; Bianco, V.; Brown, J.A.; Kilic, A.; Habertheuer, A.; Aranda-Michel, E.; Navid, F.; Humar, R.; Wang, Y.; Gleason, T.G. Long-term impact of perioperative red blood cell transfusion on patients undergoing cardiac surgery. Ann. Thorac. Surg. 2021, 112, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Kuduvalli, M.; Oo, A.Y.; Newall, N.; Grayson, A.D.; Jackson, M.; Desmond, M.J.; Fabri, B.M.; Rashid, A. Effect of peri-operative red blood cell transfusion on 30-day and 1-year mortality following coronary artery bypass surgery. Eur. J. Cardio-Thorac. Surg. 2005, 27, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Woldendorp, K.; Manuel, L.; Srivastava, A.; Doane, M.; Bassin, L.; Marshman, D. Perioperative transfusion and long-term mortality after cardiac surgery: A meta-analysis. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Ravn, H.B.; Andreasen, J.J.; Greisen, J.; Thomassen, S.; Fabrin, A.; Jakobsen, C.J. Fewer transfusions are still more-red blood cell transfusions affect long-term mortality in cardiac surgery. Eur. J. Cardio-Thorac. Surg. 2023, 63, ezad101. [Google Scholar] [CrossRef] [PubMed]
- Schwann, T.A.; Vekstein, A.M.; Engoren, M.; Grau-Sepulveda, M.; O’Brien, S.; Engelman, D.; Lobdell, K.W.; Gaudino, M.F.; Salenger, R.; Habib, R.H. Perioperative anemia and transfusions and late mortality in coronary artery bypass patients. Ann. Thorac. Surg. 2023, 115, 759–769. [Google Scholar] [CrossRef]
- Jakobsen, C.J.; Ryhammer, P.K.; Tang, M.; Andreasen, J.J.; Mortensen, P.E. Transfusion of blood during cardiac surgery is associated with higher long-term mortality in low-risk patients. Eur. J. Cardio-Thorac. Surg. 2012, 42, 114–120. [Google Scholar] [CrossRef]
- Rao, S.V.; Jollis, J.G.; Harrington, R.A.; Granger, C.B.; Newby, L.K.; Armstrong, P.W.; Moliterno, D.J.; Lindblad, L.; Pieper, K.; Topol, E.J.; et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004, 292, 1555–1562. [Google Scholar] [CrossRef]
- Allonen, J.; Nieminen, M.S.; Hiippala, S.; Sinisalo, J. Relation of use of red blood cell transfusion after ccute coronary syndrome to long-term mortality. Am. J. Cardiol. 2018, 121, 1496–1504. [Google Scholar] [CrossRef]
- O’Shaughnessy, S.; Tangel, V.; Dzotsi, S.; Jiang, S.; White, R.; Hoyler, M. Non-white race/ethnicity and female sex are associated with increased allogeneic red blood cell transfusion in cardiac surgery patients: 2007–2018. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.C.; Légaré, J.F.; Buth, K.J.; Sullivan, J.A.; Hirsch, G.M. Identifying patients at risk of intraoperative and postoperative transfusion in isolated CABG: Toward selective conservation strategies. Ann. Thorac. Surg. 2004, 78, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.C.; Simpson, A.N.; Baker, R.A.; Wu, X.; Zhang, M.; Thompson, M.P.; Paone, G.; Delucia, A., 3rd; Likosky, D.S.; Registry, P.E.; et al. Determinants of hospital variability in perioperative red blood cell transfusions during coronary artery bypass graft surgery. J. Thorac. Cardiovasc. Surg. 2022, 163, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Eranki, A.; Wilson-Smith, A.; Ali, U.; Merry, C. Preoperative patient factors associated with blood product use in cardiac surgery, a retrospective cohort study. J. Cardiothorac. Surg. 2022, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, D.M.; Klein, J.J.; Shander, A.; Cousineau, K.M.; Goldweit, R.S.; Bodian, C.; Perelman, S.I.; Kang, H.; Fink, D.A.; Rothman, H.C.; et al. Predictors of transfusion requirements for cardiac surgical procedures at a blood conservation center. Ann. Thorac. Surg. 2004, 77, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Ter Woorst, J.; Sjatskig, J.; Soliman-Hamad, M.; Akca, F.; Haanschoten, M.; van Straten, A. Evolution of perioperative blood transfusion practice after coronary artery bypass grafting in the past two decades. J. Cardiothorac. Surg. 2020, 35, 1220–1227. [Google Scholar] [CrossRef]
- Blasberg, J.D.; Schwartz, G.S.; Balaram, S.K. The role of gender in coronary surgery. Eur. J. Cardio-Thorac. Surg. 2011, 40, 715–721. [Google Scholar] [CrossRef]
- Robinson, N.B.; Naik, A.; Rahouma, M.; Morsi, M.; Wright, D.; Hameed, I.; Di Franco, A.; Girardi, L.N.; Gaudino, M. Sex differences in outcomes following coronary artery bypass grafting: A meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 841–847. [Google Scholar] [CrossRef]
- Blankstein, R.; Ward, R.P.; Arnsdorf, M.; Jones, B.; Lou, Y.B.; Pine, M. Female gender is an independent predictor of operative mortality after coronary artery bypass graft surgery: Contemporary analysis of 31 Midwestern hospitals. Circulation 2005, 112, I323–I327. [Google Scholar] [CrossRef]
- Gaudino, M.; Chadow, D.; Rahouma, M.; Soletti, G.J.; Sandner, S.; Perezgrovas-Olaria, R.; Audisio, K.; Cancelli, G.; Bratton, B.A.; Fremes, S.; et al. Operative outcomes of women undergoing coronary artery bypass surgery in the US, 2011 to 2020. JAMA Surg. 2023, 158, 494–502. [Google Scholar] [CrossRef]
- Bradley, S.; White, R.S.; Jiang, S.Y.; Ma, X.; Hoyler, M.M.; Muehlschlegel, J.D.; Karamnov, S.; Tangel, V.E.; Lanahan, J.; Rong, L.Q. Sex differences in in-hospital mortality after open cardiac valve surgery. Anesth. Analg. 2022, 135, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Mokhles, M.M.; Soloukey Tbalvandany, S.; Siregar, S.; Versteegh, M.I.M.; Noyez, L.; van Putte, B.; Vonk, A.B.A.; Roos-Hesselink, J.W.; Bogers, A.; Takkenberg, J.J.M. Male-female differences in aortic valve and combined aortic valve/coronary surgery: A national cohort study in the Netherlands. Open Heart 2018, 5, e000868. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Mesana, T.G.; Lee, D.S.; Eddeen, A.B.; Sun, L.Y. Sex differences in long-term survival after major cardiac surgery: A population-based cohort study. J. Am. Heart Assoc. 2019, 8, e013260. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.M.; Feng, L.; He, X.; Xian, Y.; Jacobs, J.P.; Badhwar, V.; Kurlansky, P.A.; Furnary, A.P.; Cleveland, J.C., Jr.; Lobdell, K.W.; et al. The Society of Thoracic Surgeons 2018 adult cardiac surgery risk models: Part 2-statistical methods and results. Ann. Thorac. Surg. 2018, 105, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardio-Thorac. Surg. 2012, 41, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Bartoszko, J.; Karkouti, K. Can predicting transfusion in cardiac surgery help patients? Br. J. Anaesth. 2017, 119, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Shehata, N.; Mistry, N.; da Costa, B.R.; Pereira, T.V.; Whitlock, R.; Curley, G.F.; Scott, D.A.; Hare, G.M.T.; Jüni, P.; Mazer, C.D. Restrictive compared with liberal red cell transfusion strategies in cardiac surgery: A meta-analysis. Eur. Heart J. 2019, 40, 1081–1088. [Google Scholar] [CrossRef]
- Gombotz, H.; Schreier, G.; Neubauer, S.; Kastner, P.; Hofmann, A. Gender disparities in red blood cell transfusion in elective surgery: A post hoc multicentre cohort study. BMJ Open 2016, 6, e012210. [Google Scholar] [CrossRef]
- Shevde, K.; Pagala, M.; Tyagaraj, C.; Udeh, C.; Punjala, M.; Arora, S.; Elfaham, A. Preoperative blood volume deficit influences blood transfusion requirements in females and males undergoing coronary bypass graft surgery. J. Clin. Anesth. 2002, 14, 512–517. [Google Scholar] [CrossRef]
- Stammers, A.H.; Tesdahl, E.A.; Mongero, L.B.; Stasko, A. Gender and intraoperative blood transfusion: Analysis of 54,122 non-reoperative coronary revascularization procedures. Perfusion 2019, 34, 236–245. [Google Scholar] [CrossRef]
- Tran, L.; Greiff, G.; Pleym, H.; Wahba, A.; Stenseth, R.; Videm, V. Transfusion of red blood cells in coronary surgery: Is there an effect on long-term mortality when adjusting for risk factors and postoperative complications? Eur. J. Cardio-Thorac. Surg. 2018, 53, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Loor, G.; Koch, C.G.; Sabik, J.F., 3rd; Li, L.; Blackstone, E.H. Implications and management of anemia in cardiac surgery: Current state of knowledge. J. Thorac. Cardiovasc. Surg. 2012, 144, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, J.G.; Smith, M.M.; Hanson, A.C.; Schulte, P.J.; Portner, E.R.; Kor, D.J.; Warner, M.A. Sex-specific associations between preoperative anemia and postoperative clinical outcomes in patients undergoing cardiac surgery. Anesth. Analg. 2021, 132, 1101–1111. [Google Scholar] [CrossRef]
- Tanaka, K.A.; Alejo, D.; Ghoreishi, M.; Salenger, R.; Fonner, C.; Ad, N.; Whitman, G.; Taylor, B.S.; Mazzeffi, M.A. Impact of preoperative hematocrit, body mass index, and red cell mass on allogeneic blood product usage in adult cardiac surgical patients: Report from a Statewide Quality Initiative. J. Cardiothorac. Vasc. Anesth. 2023, 37, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Mariscalco, G.; Wozniak, M.J.; Dawson, A.G.; Serraino, G.F.; Porter, R.; Nath, M.; Klersy, C.; Kumar, T.; Murphy, G.J. Body mass index and mortality among adults undergoing cardiac surgery: A nationwide study with a systematic review and meta-analysis. Circulation 2017, 135, 850–863. [Google Scholar] [CrossRef]
- Stamou, S.C.; Nussbaum, M.; Stiegel, R.M.; Reames, M.K.; Skipper, E.R.; Robicsek, F.; Lobdell, K.W. Effect of body mass index on outcomes after cardiac surgery: Is there an obesity paradox? Ann. Thorac. Surg. 2011, 91, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Reeves, B.C.; Ascione, R.; Chamberlain, M.H.; Angelini, G.D. Effect of body mass index on early outcomes in patients undergoing coronary artery bypass surgery. J. Am. Coll. Cardiol. 2003, 42, 668–676. [Google Scholar] [CrossRef]
- Bhavsar, R.; Tang, M.; Greisen, J.; Jakobsen, C.J. Increasing obesity is associated with lower postoperative bleeding in coronary bypass patients. J. Cardiothorac. Vasc. Anesth. 2023, 37, 1129–1137. [Google Scholar] [CrossRef]
- Alam, M.; Bandeali, S.J.; Kayani, W.T.; Ahmad, W.; Shahzad, S.A.; Jneid, H.; Birnbaum, Y.; Kleiman, N.S.; Coselli, J.S.; Ballantyne, C.M.; et al. Comparison by meta-analysis of mortality after isolated coronary artery bypass grafting in women versus men. Am. J. Cardiol. 2013, 112, 309–317. [Google Scholar] [CrossRef]
- Chang, F.C.; Chen, S.W.; Chan, Y.H.; Lin, C.P.; Wu, V.C.; Cheng, Y.T.; Chen, D.Y.; Hung, K.C.; Chu, P.H.; Chou, A.H. Sex differences in risks of in-hospital and late outcomes after cardiac surgery: A nationwide population-based cohort study. BMJ Open 2022, 12, e058538. [Google Scholar] [CrossRef]
- Gupta, S.; Lui, B.; Ma, X.; Walline, M.; Ivascu, N.S.; White, R.S. Sex differences in outcomes after coronary artery bypass grafting. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Movahed, M.R.; Etemad, S.; Hashemzadeh, M.; Hashemzadeh, M. Persistent reduction in the age adjusted mortality rate from aortic valve surgery in the United State with elimination of gender gap in recent years. Am. J. Cardiovasc. Dis. 2020, 10, 522–527. Available online: https://www.ncbi.nlm.nih.gov/pubmed/33224604 (accessed on 1 August 2023). [PubMed]
- Bairey Merz, C.N.; Shaw, L.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Pepine, C.J.; Mankad, S.; Sharaf, B.L.; et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: Gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J. Am. Coll. Cardiol. 2006, 47, S21–S29. [Google Scholar] [CrossRef] [PubMed]
- Bairey Merz, C.N.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L. Ischemia and no obstructive coronary artery disease (INOCA): Developing evidence-based therapies and research agenda for the next decade. Circulation 2017, 135, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- Taqueti, V.R.; Shaw, L.J.; Cook, N.R.; Murthy, V.L.; Shah, N.R.; Foster, C.R.; Hainer, J.; Blankstein, R.; Dorbala, S.; Di Carli, M.F. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation 2017, 135, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, L.E.; Bootsma, M.; Jukema, J.W.; Schalij, M.J.; Vliegen, H.W.; Bruschke, A.V.G. Chest pain in the absence of obstructive coronary artery disease: A critical review of current concepts focusing on sex specificity, microcirculatory function, and clinical implications. Int. J. Cardiol. 2019, 280, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Lee, K.E.; Lee, J.M.; Her, A.Y.; Kim, C.H.; Choi, K.H.; Song, Y.B.; Hahn, J.Y.; Kim, H.Y.; Choi, J.H.; et al. Effect of sex difference of coronary microvascular dysfunction on long-term outcomes in deferred lesions. JACC Cardiovasc. Interv. 2020, 13, 1669–1679. [Google Scholar] [CrossRef]
- Khan, N.A.; Daskalopoulou, S.S.; Karp, I.; Eisenberg, M.J.; Pelletier, R.; Tsadok, M.A.; Dasgupta, K.; Norris, C.M.; Pilote, L.; Team, G.P. Sex differences in acute coronary syndrome symptom presentation in young patients. JAMA Intern. Med. 2013, 173, 1863–1871. [Google Scholar] [CrossRef]
- Canto, J.G.; Rogers, W.J.; Goldberg, R.J.; Peterson, E.D.; Wenger, N.K.; Vaccarino, V.; Kiefe, C.I.; Frederick, P.D.; Sopko, G.; Zheng, Z.J.; et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA 2012, 307, 813–822. [Google Scholar] [CrossRef]
- Jawitz, O.K.; Lawton, J.S.; Thibault, D.; O’Brien, S.; Higgins, R.S.D.; Schena, S.; Vemulapalli, S.; Thomas, K.L.; Zwischenberger, B.A. Sex differences in coronary artery bypass grafting techniques: A Society of Thoracic Surgeons database analysis. Ann. Thorac. Surg. 2022, 113, 1979–1988. [Google Scholar] [CrossRef]


| Women, n = 402 | Men, n = 1181 | p-Value | |
|---|---|---|---|
| Age | 73.0 (65.0–78.0) | 66.0 (59.0–74.0) | <0.001 |
| Body mass index | 27.6 (24.3–31.6) | 27.2 (24.7–30.1) | 0.271 |
| Body mass index category 1 | <0.001 | ||
| Normal weight | 123 (30.6%) | 331 (28.0%) | |
| Overweight | 138 (34.3%) | 548 (46.4%) | |
| Obesity Class I | 105 (26.1%) | 221 (18.7%) | |
| Obesity Class II–III | 36 (9.0%) | 81 (6.9%) | |
| Operation | <0.001 | ||
| CABG | 158 (39.3%) | 617 (52.2%) | |
| AVR | 105 (26.1%) | 163 (13.8%) | |
| AVR + CABG | 70 (17.4%) | 115 (9.7%) | |
| MVP/MVR (+CABG) | 50 (12.4%) | 202 (17.1%) | |
| ARR | 19 (4.7%) | 84 (7.1%) | |
| Chronic obstructive pulmonary disease | 7 (1.7%) | 34 (2.9%) | 0.215 |
| Current smoking | 32 (8.0%) | 171 (14.5%) | < 0.001 |
| Estimated glomerular filtration rate | 74.0 (60.3–86.5) | 82.6 (70.6–92.7) | <0.001 |
| LVEF < 40% | 19 (4.8%) | 86 (7.3%) | 0.077 |
| NYHA class | <0.001 | ||
| No symptoms | 2 (0.5%) | 48 (4.1%) | |
| 1 | 10 (2.5%) | 76 (6.4%) | |
| 2 | 113 (28.1%) | 347 (29.4%) | |
| 3 | 208 (51.7%) | 461 (39.0%) | |
| 4 | 69 (17.2%) | 249 (21.1%) | |
| Preoperative atrial fibrillation | 33 (8.2%) | 112 (9.5%) | 0.444 |
| Peripheral vascular disease | 33 (8.2%) | 104 (8.8%) | 0.713 |
| Preoperative hemoglobin | 130.0 (121.0–139.0) | 143.0 (133.0–151.0) | <0.001 |
| Preoperative anemia | 88 (21.9%) | 229 (19.5%) | 0.293 |
| Preoperative LMWH | 56 (13.9%) | 206 (17.4%) | 0.102 |
| Preoperative warfarin | 30 (7.5%) | 94 (8.0%) | 0.749 |
| Cardiopulmonary bypass time, min | 105 (82–137) | 104 (81–136) | 0.726 |
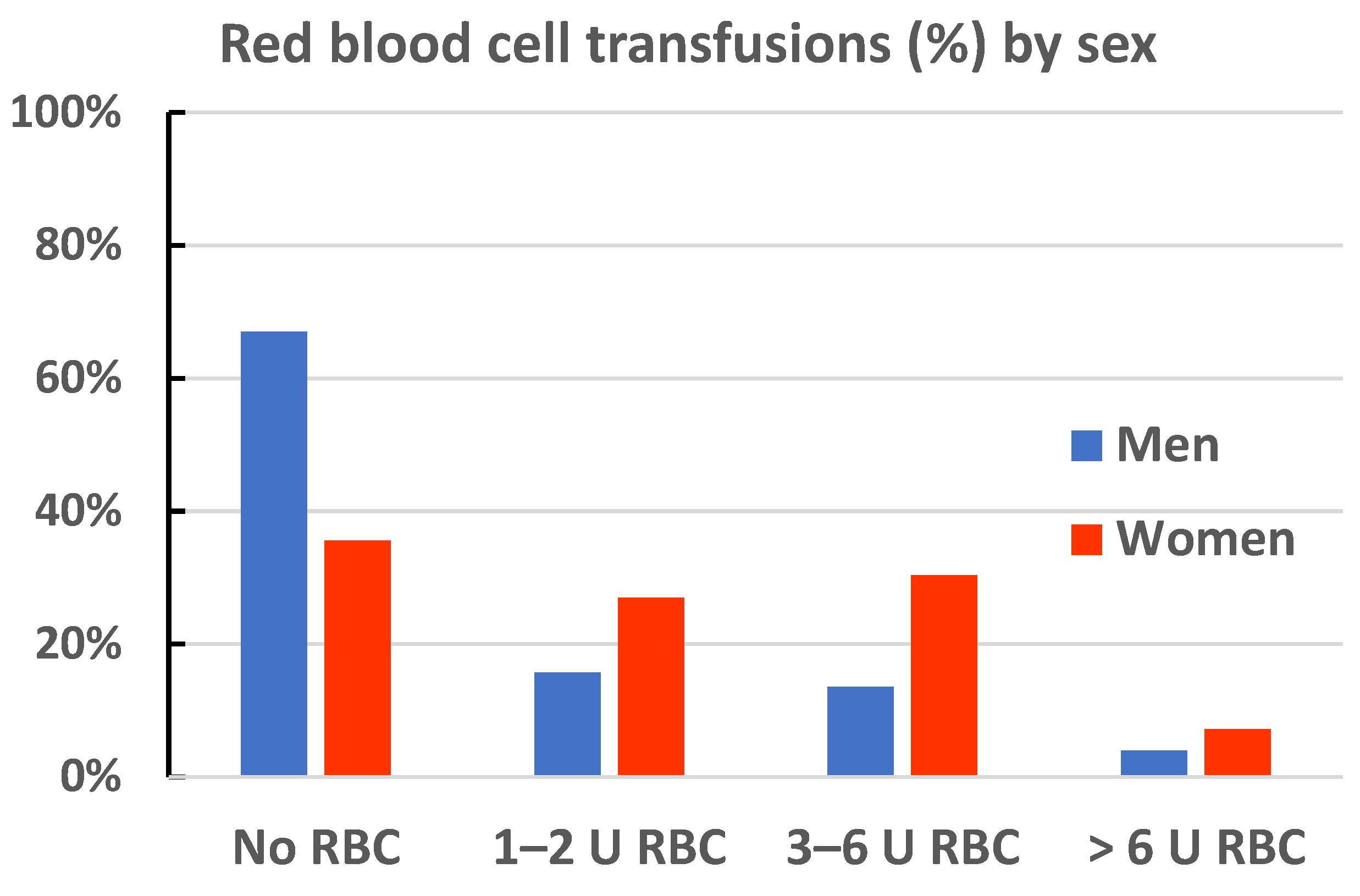
| Red blood cell transfusion | <0.001 | ||
| 0 U | 143 (35.6%) | 791 (67.0%) | |
| 1–2 U | 108 (26.9%) | 185 (15.7%) | |
| 3–6 U | 122 (30.3%) | 159 (13.5%) | |
| >6 U | 29 (7.2%) | 46 (3.9%) |
| Women | Men | All | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Female sex | 3.67 (2.90–4.66) | < 0.001 | ||||
| Age (10 y) | 1.79 (1.42–2.26) | <0.001 | 1.51 (1.33–1.72) | <0.001 | 1.74 (1.56–1.94) | <0.001 |
| Body surface area (+0.1) | 0.75 (0.67–0.85) | <0.001 | 0.87 (0.81–0.93) | <0.001 | 0.76 (0.73–0.80) | <0.001 |
| Body mass index class 1 | ||||||
| Normal weight | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Overweight | 0.44 (0.26–0.77) | 0.004 | 0.80 (0.61–1.06) | 0.124 | 0.66 (0.52–0.84) | <0.001 |
| Obesity Cl I | 0.36 (0.20–0.64) | <0.001 | 0.73 (0.51–1.05) | 0.092 | 0.68 (0.51–0.90) | 0.008 |
| Obesity Cl II–III | 0.21 (0.098–0.47) | <0.001 | 0.47 (0.27–0.83) | 0.009 | 0.43 (0.28–0.66) | <0.001 |
| Preoperative hemoglobin (10 g/L) | 0.61 (0.51–0.73) | <0.001 | 0.62 (0.56–0.68) | <0.001 | 0.57 (0.53–0.62) | <0.001 |
| Preoperative anemia | 4.08 (2.17–7.65) | <0.001 | 4.63 (3.42–6.28) | <0.001 | 4.18 (3.21–5.45) | <0.001 |
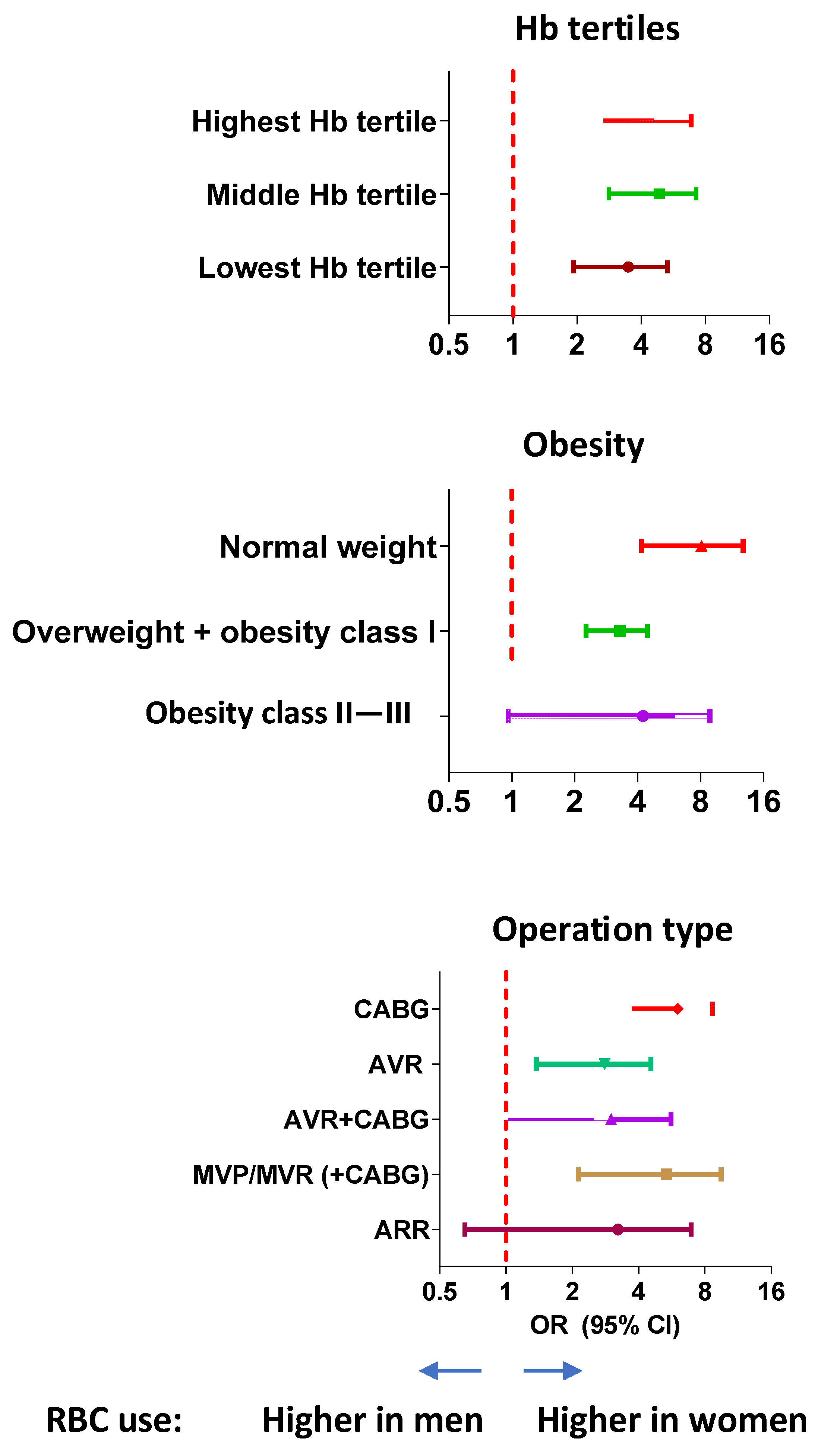
| Preoperative hemoglobin tertiles 2 | ||||||
| Highest | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Middle | 2.04 (1.25–3.32) | 0.004 | 1.78 (1.28–2.48) | <0.001 | 1.76 (1.36–2.28) | <0.001 |
| Lowest | 4.35 (2.52–7.51) | <0.001 | 4.50 (3.27–6.21) | <0.001 | 3.98 (3.07–5.17) | <0.001 |
| eGFR (10 mL/min/1.73 m2) | 0.85 (0.75–0.95) | 0.006 | 0.82 (0.77–0.88) | <0.001 | 0.80 (0.75–0.84) | <0.001 |
| LVEF < 40% | 1.23 (0.46–3.30) | 0.686 | 1.09 (0.69–1.73) | 0.712 | 1.00 (0.67–1.50) | 0.996 |
| NYHA class 3–4 | 1.39 (0.90–2.14) | 0.142 | 1.54 (1.20–1.99) | <0.001 | 1.60 (1.29–1.97) | <0.001 |
| Preoperative atrial fibrillation | 0.84 (0.40–1.74) | 0.633 | 1.73 (1.17–2.56) | 0.006 | 1.38 (0.98–1.95) | 0.062 |
| Peripheral vascular disease | 1.11 (0.52–2.37) | 0.779 | 1.84 (1.23–2.77) | 0.003 | 1.56 (1.10–2.22) | 0.012 |
| Cardiopulmonary bypass time (+10 min) | 1.16 (1.09–1.23) | <0.001 | 1.13 (1.09–1.16) | <0.001 | 1.12 (1.10–1.15) | <0.001 |
| Preoperative LMWH | 1.78 (0.94–3.39) | 0.078 | 1.33 (0.97–1.81) | 0.074 | 1.29 (0.99–1.68) | 0.062 |
| Preoperative warfarin | 0.95 (0.44–2.06) | 0.896 | 2.17 (1.42–3.31) | <0.001 | 1.71 (1.18–2.47) | 0.004 |
| Women | Men | All | ||||
|---|---|---|---|---|---|---|
| OR | p-Value | OR | p-Value | OR | p-Value | |
| Female sex | 3.88 (2.95–5.11) | <0.001 | ||||
| Age (+10 y) | 1.49 (1.12–1.98) | 0.006 | 1.19 (1.01–1.39) | 0.034 | 1.24 (1.08–1.43) | 0.002 |
| Body mass index class | ||||||
| Normal weight | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Overweight | 0.43 (0.23–0.79) | 0.006 | 0.84 (0.61–1.15) | 0.282 | 0.72 (0.55–0.95) | 0.020 |
| Obesity class I | 0.38 (0.20–0.72) | 0.003 | 0.75 (0.50–1.12) | 0.165 | 0.62 (0.44–0.86) | 0.005 |
| Obesity class II–III | 0.24 (0.10–0.57) | 0.001 | 0.53 (0.28–0.98) | 0.044 | 0.42 (0.25–0.70) | <0.001 |
| Preoperative Hb tertiles 1 | ||||||
| Highest tertile | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| Middle tertile | 1.85 (1.07–3.18) | 0.027 | 1.72 (1.22–2.44) | 0.002 | 1.71 (1.29–2.29) | <0.001 |
| Lowest tertile | 3.29 (1.79–6.05) | <0.001 | 4.12 (2.92–5.82) | <0.001 | 3.85 (2.86–5.19) | <0.001 |
| eGFR (+10 mL/min/1.73 m2) | 0.92 (0.80–1.07) | 0.299 | 0.92 (0.85–1.01) | 0.067 | 0.93 (0.86–0.99) | 0.041 |
| NYHA 3–4 vs. 1–2 | 0.94 (0.57–1.57) | 0.822 | 1.41 (1.05–1.90) | 0.023 | 1.25 (0.97–1.61) | 0.084 |
| Operation | ||||||
| CABG | 1 (ref.) | 1 (ref.) | 1 (ref.) | |||
| AVR | 0.42 (0.23–0.75) | 0.003 | 0.94 (0.61–1.43) | 0.789 | 0.74 (0.53–1.03) | 0.073 |
| AVR + CABG | 2.03 (0.93–4.45) | 0.077 | 4.40 (2.79–6.93) | <0.001 | 3.67 (2.48–5.45) | <0.001 |
| MVP/MVR + (CABG) | 0.95 (0.46–1.99) | 0.901 | 1.38 (0.93–2.05) | 0.110 | 1.25 (0.89–1.75) | 0.208 |
| ARR | 1.53 (0.51–4.59) | 0.450 | 3.67 (2.18–6.17) | <0.001 | 3.05 (1.90–4.88) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Räsänen, J.; Ellam, S.; Hartikainen, J.; Juutilainen, A.; Halonen, J. Sex Differences in Red Blood Cell Transfusions and 30-Day Mortality in Cardiac Surgery: A Single Center Observational Study. J. Clin. Med. 2023, 12, 7674. https://doi.org/10.3390/jcm12247674
Räsänen J, Ellam S, Hartikainen J, Juutilainen A, Halonen J. Sex Differences in Red Blood Cell Transfusions and 30-Day Mortality in Cardiac Surgery: A Single Center Observational Study. Journal of Clinical Medicine. 2023; 12(24):7674. https://doi.org/10.3390/jcm12247674
Chicago/Turabian StyleRäsänen, Jenni, Sten Ellam, Juha Hartikainen, Auni Juutilainen, and Jari Halonen. 2023. "Sex Differences in Red Blood Cell Transfusions and 30-Day Mortality in Cardiac Surgery: A Single Center Observational Study" Journal of Clinical Medicine 12, no. 24: 7674. https://doi.org/10.3390/jcm12247674
APA StyleRäsänen, J., Ellam, S., Hartikainen, J., Juutilainen, A., & Halonen, J. (2023). Sex Differences in Red Blood Cell Transfusions and 30-Day Mortality in Cardiac Surgery: A Single Center Observational Study. Journal of Clinical Medicine, 12(24), 7674. https://doi.org/10.3390/jcm12247674







