New Antimicrobials and New Therapy Strategies for Endocarditis: Weapons That Should Be Defended
Abstract
:1. Introduction
2. Materials and Methods
3. New Antimicrobials
3.1. Ceftobiprole
3.1.1. Mechanism of Action and Indication
3.1.2. Clinical Evidence in Infective Endocarditis
| Authors | Study Design | Endpoint | N° Patients/ IE Type | Pathogens | Dosage and Duration | Combination | Outcomes | Safety |
|---|---|---|---|---|---|---|---|---|
| Holland, T.L. et al., 2022 * [39] | Randomised double-blind trial (ERADICATE study) BPR vs. DAP ±Aztreonam | Clinical success Success required survival, symptom improvement, SAB clearance, no new SAB complications, and no use of other potentially effective antibiotics | 390 SAB 192 BPR vs. 198 DAP IE 33 BPR: 20, 15 right-sided, 5 left-sided DAP: 13, 10 right-sided, 3 left-sided | MSSA 287 MRSA 94 | 500 mg/6 h up to 42 d | ±Aztreonam | Overall clinical success: 69.8% in BPR vs. 68.7% for DAP There were no significant differences in mortality or microbiological eradication between treatment groups | ≥1 AE: 63% BPR vs. 59% DAP |
| Gentile, I. et al., 2023 [7] | Multicentre observational and ambispective study Mono vs. combination therapy | Clinical success: As a composite of the clinical cure, improvement or de-scalation feasibility in 30 d FU | 195, 34% mono vs. 66% combination (pneumonia 74%; BSI 19%; SSTI 5%; bone infection 4%) IE 7 (4%), all combination | Polymicrobial infection (25%) MSSA (11%) MRSA (38%) In IE subgroup: 2/7 MRSA; 5/7 MRCoNS | No data reported | MER 31% In IE subgroup: DAP 6/7 and LNZ 1/7 | Overall, clinical success 79%, microbiological cure 87%, 8 infection recurrences In IE subgroup: Clinical success 29% Microbiological cure 29% (presumed eradication) | 7 AE (2 rash, 2 myoclonus, 1 allergic reaction, 1 seizure, 1 CDI) 4 AE (rash or myoclonus) were BRP + DAP |
| Mahmoud, E. et al., 2020 [36] | Case series | N/A | 6 BSI (2 osteomyelitis,1 IE, 1 CLABSI, 1 SSTI, 1 pneumonia) IE 1 NVE | MRSA | No data reported on the dosage 31 d | All VAN | All demonstrated microbiological and clinical cure at 14 d | No data reported |
| Tascini, C. et al., 2020 [37] | Case series BPR + DAP or BPR | N/A | IE 12 8 PVE, 3 NVE, 1 CIED-IE 5 surgeries for vegetation size (n.3) or severe valve disfunction with heart failure (n. 2) 9/12 previous therapy BPR + DAP 11 BPR 1 | 25% polymicrobial 33.3% MSSA 33.3% MRSA | No data reported on dosage Up to 84 d | 91.7% DAP | Clinical success: 10/12 (83%) Microbiological cure: In 9/12 (75%) cases, patients were switched to BPR following failure of the previous antimicrobial regimen. In 3/3 patients in which BPR was administered because of persistently positive blood culture, bacteraemia clearance was rapidly achieved. | No data reported |
| Zhanel, G.G. et al., 2021 [38] | Case series Mono and combination therapy | N/A | 38 infections 42.1% IE 23.7% BJIs 15.8% HABP 5.3% SSTI 2.6% CNS 2.6% DRI 2.6% BSI 9 mono and 29 combination | MRSA | 500 mg/8 h No data on duration | Combination therapy 76.3%: - DAP 21/29 - VAN 7/29 - FLUORO 1/29 | Overall, clinical success 84.8%, microbiological cure 97.0% In IE subgroup: - Microbiological cure: 14/16, 2/16 unknown - Clinical success: 11/16, 4/16 unknown; 1/16 death | 2.6% AE (gastrointestinal symptoms) |
| Giuliano, S. et al., 2023 [40] | Case series | N/A | 21 BSI 13 left-sided IE 8 PVE, 5 NVE, 1 PVE + NVE | E. faecalis AMP S | 15/21 500 mg/8 h 3/21 500 mg/12 h 3/21 350 mg/8 h Among patients with IE, the mean duration of the ABPR regimen was 27.8 ± 14.5 days. In patients with E. faecalis bacteraemia, the mean duration of ABPR treatment was 20.4 ± 11.1 days. | All ampicillin | Overall clinical success 81%, microbiological cure 86% In IE subgroup: - Clinical success: 9 (6 PVE, 3 NVE) - Microbiological cure: 10 (5 PVE, 5 NVE) 1 relapse in NVE (pt did not adhere to the partial oral treatment) | 9% experienced ABPR-related side effects (seizure and skin rash) |
| Oltolini, C. et al., 2016 [35] | Case report | N/A | 1 PVE | MRSA | 250 mg/2 h then 500 mg/8 h according to GRF 11 weeks | DAP | Clearance of bacteraemia Complete disappearance of the vegetation at echocardiography IE recurrence (it was not attributable to antibiotic failure but to EVS with the implantation of a new prosthesis during an uncontrolled infection status and also the recurrence of PVE and the need for chronic antibiotic therapy) | No data reported |
3.2. Ceftaroline
3.2.1. Mechanism of Action and Indication
3.2.2. Clinical Evidence in Infective Endocarditis
| Authors | Study Design | Endpoint | N° Patients/ IE Type | Pathogens | Dosage and Duration | Combination | Outcomes | Safety | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Geriak, M. et al., 2019 [8] | Randomised clinical trial DAP + CPT vs. VAN/DAP | Primary endpoints: duration of bacteraemia and in-hospital mortality Secondary endpoints: 60 d and 90 d mortality, hospital stay | 40 BSI, 17 DAP + CPT vs. 23 VAN/DAP (VAN 21, DAP 2) 7 IE, 3 DAP + CPT vs. 4 VAN/DAP (1 bilateral, 1 right-sided, 1 aortic PVE, 1 mitral NVE, 1 aortic NVE, 2 CIED) | MRSA | CPT 600 mg 8 h (or adjusted for GFR) Mean 11 d | DAP 8 mg/kg/24 h | Overall, 30 d, 90 d, and in-hospital mortality: DAP + CPT 0 vs. VAN/DAP 6, 0 vs. 7, 0 vs. 6 Treatment failure *: 1 vs. 3 IE subgroup: in-hospital mortality, 0 vs. 2 | No AE reported | |||
| Casapao, A.M. et al., 2014 [66] | Multicentre observational retrospective study CPT in various infections | Clinical and microbiological success/failure, hospital length of stay, AEs, 30 d readmission, in-hospital mortality, and 30 d mortality. | 527 infections 148 (28.1%) BSI 35 IE | 138 SAB with 92% MRSA in IE group 6 hVISA | Overall, 85.6% 600 mg/12 h, 14.4% 600 mg/8 h Median 9 (4–15) in BSI group | 29.2% combination therapy, 42% of which was with metronidazole | In IE subgroup: Clinical failure 30.3% Mortality 22.9% | In the BSI group: 12.8% AE | |||
| Arshad, S. et al., 2017 [76] | Retrospective case-control study CPT vs. VAN vs. DAP | Composite failure: 30 d mortality from infection onset, 42 d BSI recurrence, or 30 d readmission after the end of treatment | 132 BSI, monotherapy 30 CPT vs. 46 VAN vs. 56 DAP 39 IE 7 vs. 13 vs. 19 | MRSA | No data reported | No data reported | Overall, 30 d mortality: CPT group 13% vs. DAP group 24% and VAN group 11% (p = 0.188) Overall and in the IE subgroup, no statistically significant difference in 30 d mortality, 42 d recurrence, and 30 d readmission | No data reported | |||
| Britt, R.S. et al., 2017 [67] | Multicentre observational retrospective study CPT in various infections | AEs within 30 d of therapy initiation All-cause in-hospital mortality | 764 infections 46 IE | No data reported | No data reported | No data reported | Overall, in hospital mortality 5%, 30 d readmission 33% IE subgroup mortality 11%, 30 d readmission 28% | AE < 1% (eosinophilia, leukopenia, fibromyalgia, myalgia and myositis, and polymyalgia) | |||
| Zasowski, E.J. et al., 2017 [93] | Multicentre observational-retrospective study CPT mono vs. combination therapy in BSI | Safety and efficacy outcomes | 211 BSI, 126 included in the efficacy population 31 IE 20 CPT mono vs. 11 combination therapy | MRSA 1% VAN resistant strain | In efficacy population, most common dose 600 mg (60.3%) and frequency every 8 h (52.4%) In efficacy population, median 13 d (IQR 5–21) | DAP combination in 75.7% | In efficacy population no statistical differences between monotherapy and combination. Clinical success § 86/126 (68.3%) monotherapy 69.7% vs. combination 64.9%, BSI clearance 115/126 §§ (91.3), 88.8% vs. 97.3%, Mortality 28/126 (22.2%), 19.1% vs. 29.7% | Overall, 16 AE (6 CDI, 7 rash, 3 neutropenia) | |||
| Cortes-Penfield, N. et al., 2018 [71] | Observational retrospective study DAP + CPT vs. DAP in BSI | Duration of bacteraemia, mortality, BSI recurrence | 17 BSI, 5 IE 12 DAP + CPT and 5 DAP | MRSA | No data on dosage Mean 32.5 d | DAP median dose 7.6 mg/kg/24 h (5.7–13.8) | Overall, shorter duration of bacteraemia in DAP + CPT group IE subgroup mortality 3/5 | No data reported | |||
| Destache, C.J. et al., 2019 [65] | Multicentre observational retrospective study CPT mono or combination therapy in IE | Clinical outcomes | 55 IE, 26 right-sided, 25 left sided, 4 bilateral | MRSA 44/55 MSSA 4 CoNS 4 E. faecalis 1 Streptococcus 1 | Mainly 600 mg/12 h Mean (SD) 13.4 d (9.7) | 32, most common drugs (>5% of pt) DAP (n. 19), VAN (n. 9), RIF (n. 7). Other drugs: CFZ, LVX, LNZ, GEN, AMP. | Overall, clinical successes 39 (70.9%): monotherapy 19/23 (82.6%), combination 20/32 (62.5%) High success rate with CPT as first or second line therapy | 2 AE (AKI and rash) with CPT withdrawal | |||
| McCreary, E.K. et al., 2019 [69] | Multicentre observational retrospective study DAP + CPT vs. SoC (case-control) | All-cause mortality, duration of bacteraemia, and BSI recurrence | 171 BSI, 58 DAP + CPT vs. 113 SoC (VAN or DAP), 70 EI, 23 vs. 47 | MRSA | No data reported | No data reported | No statistically significant difference in all-cause 30 d mortality and 90 d BSI recurrence | No data reported | |||
| Ahmad, O. et al., 2020 [70] | Retrospective case-control study VAN or DAP vs. VAN/DAP +CPT | Treatment outcomes: in-hospital mortality, BSI recurrence, 30 d readmission, AKI, leukopenia | 30 BSI, 15 VAN/DAP vs. 15 VAN/DAP + CPT 21 IE, all NVE (14 vs. 7) | MRSA | 600 mg/8–12 h Median 6 weeks | VAN 15–20 mg/kg/8–12 h DAP 8–10 mg/kg/24 h | No difference in AKI, leukopenia, BSI recurrence, 30 d readmission, or mortality | No AE reported | |||
| Morrisette, T. et al., 2020 [75] | Observational retrospective study DAP vs. DAP + CPT | Composite success: 30 d mortality, 60 d recurrence, worsening of respiratory status, change in therapy due to failure | 29 BSI with septic pulmonary emboli, 14 DAP vs. 15 DAP + CPT 24 IE, all NVE (11 vs. 13) | MRSA | 600 mg/8 h Median 11 d (9–12) | DAP median 9.9 mg/kg (8.8–9.8) duration median 36 d (22–42) | No difference in the primary outcome of compositive success | 1 AE (thrombocytopenia) with CPT withdrawal | |||
| Johnson, T.M. et al., 2021 [73] | Observational retrospective study DAP + CPT vs. SoC | Clinical failure: MRSA-related mortality and 60 d recurrent infection | 60 BSI, 30 DAP + CPT vs. 30 SoC, 22 IE, 15 vs. 7 (14 left-sided, 6 right-sided, 2 bilateral) | MRSA | 1800 mg/24 h (or adjusted for GFR) DAP + CPT median 7 d (3–11) | DAP 10 mg/kg/24 h | Overall, clinical failure DAP + CPT 20% vs. SoC 43%, 60 d BSI recurrence 0% vs. 30%, 90 d mortality 27% vs. 23%, DAP + CPT inversely associated with clinical failure 90 d (p = 0.03) | No statistically significant AE reported | |||
| Nichols, C.N. et al., 2021 [94] | Observational retrospective study DAP + CPT vs. de-escalation with DAP/CPT/or VAN | Composite endpoint: inpatient infection-related mortality, 60 d readmission, and 60 d BSI recurrence | 140 BSI, 66 DAP + CPT vs. 74 de-escalation in monotherapy DAP/CPT/VAN 63 IE, 37 vs. 26 | MRSA | No data on dosage Median 56 d in combination group | DAP | No differences between combo and monotherapy for inpatient infection-related mortality, 60 d readmission, or 60 d BSI recurrence | In the combination group, 2 AE (bone marrow suppression, oedema) | |||
| Zasowski, E.J. et al., 2022 [68] | Multicentre observational retrospective study CPT vs. DAP monotherapy | Composite treatment failure: 30 d mortality, BSI duration ≥ 7 d on study drug, and 60 d MRSA BSI recurrence. | 270 BSI, 83 CPT and 187 DAP 82 IE 27 vs. 55 | MRSA | Most common dose 600 mg (68.7%) and frequency every 12 h (56.6%) Median 10 d (IQR 5–18) | No Monotherapy DAP median 8.5 mg/kg 24 h | In all populations and the IE subgroup, CPT not inferior to DAP No differences in any endpoints | Overall, 17 AE (9 rash, 4 CDI, 5 others) No data on CPT discontinuation was reported | |||
| Brandariz-Nunez, D. et al., 2022 [64] | Observational retrospective study CPT combination in IE | Treatment failure: presence of fever or positive BC at 7 d, positive BC recurrence, early antibiotic withdrawal due to lack of clinical response, AE or death | 70 IE, 30 NVE, 36 PVE, 10 ICED-IE | MRSA 6/26; MR CoNS 15/26; E. faecalis AMP-S 5; Streptococcus 5 | 600 mg/8–12 h (or adjusted on GFR) Mean 21.26 d (DS 16.17) | 70/70 combination DAP (n.52), GEN (n.18), RIF (n.6) | Overall, 42 d in-hospital mortality 30%; 42 d treatment failure 38.6% | 6 AE with 4 CPT discontinuation | |||
| Kufel, W.D. et al., 2023 [74] | Observational retrospective study CPT + VAN in BSI | Effectiveness and safety Bacteraemia clearance post-CPT initiation | 30 BSI, 20 IE, 7 tricuspid, 7 mitral, 4 aortic and 2 multiple valves | MRSA | 600 mg/8 h Median 16 d (IQR 13.2) | All combination, VAN median 1250 mg/24 h | Overall, microbiological cure 96.7%; 90 d readmission for MRSA BSI 6.7%, all-cause 90 d mortality 26.7%, MRSAB-related mortality+ 13.3% | 2 AE (rash) with CPT discontinuation | |||
| Lin, J.C. et al., 2013 [92] | Case series | N/A | 10 infections 5 IE, 4 probable and 1 possible. 1 right-sided, 1 CIED, 1 NV+ CIED-IE, 2 no vegetation | MRSA | 600 mg/8 h (or adjusted por GFR) Between 3 d to 7 weeks | No data reported | IE subgroup Clinical cure 3/5 Microbiological cure 4/5 | 2 AE, 1 CDI, 1 fever + rash + eosinophilia with CPT discontinuation | |||
| Ho, T.T. et al., 2012 [91] | Case series CPT monotherapy | N/A | 6 BSI, 3 IE Cases 1 and 2: middle-aged men with mitral NVE Case 3: middle-age woman with mitral NVE | MRSA | 600 mg/8 h Case 1: 42 d Case 2–3: 3 weeks | No | IE subgroup Case 1–3: microbiological cure and clinical cure | No data reported | |||
| Polenakovik, H.M. and Pleiman, C.M. 2013 [78] | Case series | N/A | 31 BSI, 10 IE, 3 left-sided, 6 right-sided, and 1 CIED-IE | MRSA | CPT 1200–1800 mg/24 h (1 case GFR dose-adjusted) Overall median 30.4 d (IQR 7–60) | 4 IE combinations with DAP, RIF, GEN, LNZ | Overall, microbiological cure 64.5% (IE 9 pt); Clinical success 74.2% (IE 9 pt); Treatment failure ° 25.8% (IE 1 pt) Recurrence 9.7% (IE 1 pt); Death 6.5% | Overall, 2 AE (eosinophilia) without CPT discontinuation (1 IE) 3 AE (eosinophilic pneumoniae, rash, diarrhoea) with CPT discontinuation | |||
| Fabre, V. et al., 2014 [72] | Case series | N/A | 29 BSI 18 IE 4 right-sided, 11 left-sided, 1 CIED, 2 LVAD | MRSA | 600 mg/8 h (or adjusted on GFR) No data on duration | 24 combination therapies: 22 with TMP-SMZ 10–15 mg/kg/24 h 2 with DAP | Overall, microbiological success: 26/29 (90%); Treatment success # with 6 months FU: 9 (31%); Treatment failure ##: 4 (13%) (1 death, 3 recurrence) | 1 AE (rash) with CPT discontinuation | |||
| Tattevin, P. et al., 2014 [79] | Multicentre case series CPT in IE | N/A | 8 IE 3 aortic PVE, 1 aortic PV plus pulmonary valve, 1 CIED, 1 mitral and aortic NVE, 1 aortic NVE, 1 CIED plus aortic NVE | 5 MRSA 3 MR CoNS | From 400 mg/12 h to 800 mg/8 h Median 13 d (5–42) | 3 combination DAP (n 2) RIF (n 1) | Clinical success: 5/8 Clinical failure: 3/8 | No AE reported | |||
| Gritsenko, D. et al., 2017 [90] | Case series CPT + VAN | N/A | 5 BSI, 2 IE, Case 2: 42 y man with tricuspid NVE Case 5: 50 y mitral NVE | MRSA | Case 2: 400 mg/12 h (adjusted for GFR) 6 weeks Case 5: 600 mg/12 h (then adjusted for GFR) 7 d | Case 2 and 5: combo with VAN Case 5: 7 d | IE subgroup Case 2: microbiological cure and clinical success Case 5: death | No data reported | |||
| Hornak, J.P. et al., 2019 [77] | Case series CPT + DAP in BSI | N/A | 10 BSI 6 IE, 1 mitral NVE, 3 aortic NVE, 1 CIED, 1 LVAD | MRSA | 4600 mg/12 h, 1600 mg/8 h, 1 400 mg/h 8. Overall, median time 9 d (IQR 6–24) | All IE combination with DAP | IE subgroup microbiological cure 6/6; no recurrence; 30 d mortality and in-hospital mortality 1/6 | 3 AE (rash, eosinophilia, thrombocytopenia) without CPT discontinuation 1 eosinophilia in IE group | |||
| Rose, W.E. el al., 2012 [89] | Case report Failure with DAP | N/A | 1 right atrial vegetation | MRSA and DNS | 200 mg/12 h (haemodialysis dose-adjusted) 54 d | DAP 10 mg/kg/24 h | Microbiological cure and clinical success after failure with 11 d of monotherapy with DAP 6 mg/kg 48 h | No data reported | |||
| Jongsma, K. et al., 2013 [88] | Case report | N/A | 1 tricuspid and aortic NVE | MRSA and DNS | 600 mg/12 h 44 d | No | No resolution after 23 d of DAP and VAN, debridement on 19 d, microbiological cure at 7 d after CPT start, clinical success | No data reported | |||
| Sakoulas, G. et al., 2013 [87] | Case report Failure with AMP-based regimens | N/A | 1 aortic NVE | HLGR E. faecalis | 600 mg/8 h 6 weeks | DAP 8 mg/kg/24 h | Microbiological cure and clinical success achieved after failure with CRO + AMP (6 weeks) and then DAP + AMP (7 d). 2 weeks after CPT + DAP start, aortic valve replacement was performed | No data reported | |||
| Baxi, S.M. et al., 2015 [86] | Case-report CPT + DAP | N/A | 1 mitral NVE | MRSA VISA and DNS | 400 mg/12 h 6 weeks of CPT + DAP | DAP 10 mg/kg after dialysis | Negative BC from day 11 of DAP + CPT, remain negative at 28 d after discontinuation | No AE reported | |||
| Cunha, B.A. et al., 2015 [85] | Case report Persistent bacteraemia with DAP | N/A | 1 aortic PVE | MRSA | 600 mg/12 h 6 weeks | DAP 10–12 mg/kg/24 h | Persistent bacteraemia for 14 d under DAP 10 mg/kg 24 h BC negative after 4 d of DAP+ CPT, no recurrence | No data reported | |||
| Sundaragiri, P.R. et al., 2015 [84] | Case report | N/A | 1 tricuspid NVE | MRSA | No data reported | No data reported | 9 d valve replacement Death | No data reported | |||
| Duss, F.R. et al., 2019 [83] | Case report Persistent bacteraemia with VAN | N/A | 1 left NVE | MRSA (MIC: VAN 1.5 mg/L, DAP 2 mg/L) | 600 mg/12 h 6 weeks | DAP 10 mg/kg/24 h | BC positive under VAN 5 d; switch DAP + FOS; day 10 surgery and culture valve negative. After surgery CPT + DAP for 6 weeks. Negative BC and persistent negative at 6 months FU | No data reported | |||
| Jilani, T.N. and Masood, S.O. 2018 [82] | Case report Failure with DAP and VAN | N/A | 1 pulmonic NVE | MRSA | 600 mg/8 h 4 weeks after 2 weeks of VAN and DAP | No | Microbiological cure after 2 d of CPT and clinical success | No data reported | |||
| Lin, S.Y. et al., 2021 [81] | Case report Failure with DAP and VAN | N/A | 1 mitral NVE | hVISA | 600 mg/12 h 5 weeks | DAP 9 mg/kg/24 h | Microbiological cure and clinical success achieved after failure with monotherapy VAN (14 d) and then DAP (7 d) | No data reported | |||
| Warren, E.F. et al., 2022 [80] | Case report CPT+ nafcillin | N/A | Case 1, tricuspid NVE Case 2, CIED-IE | MSSA | Case 1 600 mg/8 h Case 2 600 mg/12 h (GFR dose-adjusted) Case 1: 11 d Case 2: 7 d | Case 1 and 2: nafcillin 12 g 24 h | Microbiological cure and clinical success | No data reported | |||
3.3. Dalbavancin
3.3.1. Mechanism of Action and Indication
3.3.2. Clinical Evidence in Infective Endocarditis
| Authors | Study Design | Endpoint | N° Patients/ IE Type | Pathogens | Dosage and Duration | Combination, Dosage | Outcomes | Safety |
|---|---|---|---|---|---|---|---|---|
| Bouza, E. et al., 2018 [128] | Multicentre retrospective study | Efficacy, tolerability, and cost reductions in people receiving DAL for various indications | 69, mainly prosthetic joint infections (29%) and ABSSSI (21.7%) Previous therapy 97% 7 IE, type unspecified. | IE subgroup: CoNS (2), Enterococcus spp. (2), MRSA (1), Streptococcus spp. (1), negative culture (1) | Most common regimen: 1000 mg Day 1, then weekly 500 mg | Overall, 36.2% | Overall clinical success 84.1% and significant cost reduction IE subgroup Clinical success: 85.7%. Failure in 1 IE patient attributed to inadequate source control | Overall, AE in 13%. Most common AE: rash and tachycardia. |
| Tobudic, S. et al., 2018 [14] | Observational retrospective study DAL in IE mainly administered as OPAT | Clinical cure and safety | 27 IE Previous therapy 88.9% 16 NVE, 6 PVE and 5 CIED-IE | S. aureus (33.3%), CoNS (22%), and E. faecalis (14.8%) main pathogens | Administered as twice-weekly regimen in 63.0% Median duration of 6 weeks (range, 1–30 weeks). | No | Clinical and microbiological success: 92.6%. Failure in 1 patient with MRSA CIED-IE and incomplete surgical control | 2 AE: 1 nausea and vomiting after the second dose, therapy continued. 1 creatinine increase, resolved with dose reduction. |
| Bryson-Cahn, C. et al., 2019 [115] | Observational retrospective study on vulnerable patients S. aureus serious infection | Clinical response: any patient who had an FU visit within 1 year without evidence of ongoing/relapsed infection | 32 infections (BSI 40.6%, osteoarticular 28%) Previous therapy 100%. 9 IE tricuspid NVE | 2 IE MSSA 7 IE MRSA | 22 received a single 1000 mg dose, 7 received 2 weekly doses | No | IE subgroup: Clinical response 5/9 Lost to FU 4/9 | No AE reported |
| Bork, J.T. et al., 2019 [116] | Multicentre retrospective study on vulnerable patients Invasive Gram-positive infections | Clinical cure | 45 infections (osteomyelitis 45%, endovascular 25%) Previous therapy 100%. 6 IE, type unspecified | MRSA (29%) and MSSA (21%) main pathogens | Median of 3 doses prescribed | 6 patients with concomitant oral fluoroquinolone. | Overall, 30 day cure was achieved by 50% of patients with endovascular infection; >25% loss to FU. IE subgroup unspecified. | AEs documented in 6.7% (2 acute kidney injuries and 1 rash) |
| Dinh, A. et al., 2019 [108] | Multicentre retrospective study French national cohort | Clinical cure | 75 infections (most frequent bone and joint 64%, endocarditis 25%). Previous therapy 98.7% 19 IE: 9 NVE and 10 PVE | S. aureus (51.4%) and CoNS (44.4%) main pathogens | In IE most frequent regimen was 1500 mg single or double dose | Overall, 45.3%, mainly rifampicin, cotrimoxazole, quinolones and tetracyclines | Overall, clinical cure 79%. IE subgroup Clinical cure: 72.2% | Five AE in the cohort (6.7%) with no treatment discontinuation |
| Hidalgo-Tenorio, C. et al., 2023 [9] | Multicentre retrospective study DAL as consolidation treatment | Effectiveness of DAL as consolidation therapy | 124 IE (46.8% native valve, 43.6% prosthetic valve and 9.6% pacemaker lead IE). Previous therapy 100%. | CoNS (38.7%), MSSA (22.6%) E. faecalis (19.4%) and Streptococcus species (9.7%) the most isolated pathogens | Single 1500 mg dose the most prescribed DAL regimen (33.3%) | No data reported | Clinical success in subjects that completed the 1 year follow-up: 95.9% Mean reduction in hospital stay: 14 days. | AE in 3.2% |
| Morrisette, T. et al., 2019 [107] | Multicentre retrospective study DAL or ORI in various infections | Clinical success | 56 infections (ABSSSI 36%, osteomyelitis 27%), 40 DAL, 14 ORI and 2 both. Previous therapy 91% 5 IE, type unspecified. | MSSA (25%), MRSA (19%) and E. faecalis (11%) main pathogens | No data reported | 30% of the whole cohort (drugs unspecified) | IE subgroup Clinical success: 100% among the 3 evaluable IE | Mild AE in 11%. |
| Wunsch, S. et al., 2019 [109] | Multicentre retrospective study DAL as sequential treatment | Clinical success | 101 infections (prosthetic joint 31%, osteomyelitis 30%, IE 25%) Previous therapy 100% 25 IE: 15 NVE, 6 PVE, 4 CIED-IE | CoNS (33%), MSSA (16%), MRSA (9%) main pathogens | In IE, 9 single 1500 mg dose and 1000 mg dose followed by 500 mg 1 week apart. | Overall, 64% of the cohort, mainly rifampicin (64%) and fluoroquinolones (15%) | Overall, clinical success 89%. IE subgroup Clinical success: 92% | Three AE in the cohort (3%), requiring treatment discontinuation |
| Ajaka, L. et al., 2020 [117] | Observational retrospective study in people with barriers to SoC | Cure: lack of clinical or microbiological persistent/recurrent infection within 90 days or negative BCs within 90 days after completion of DAL | 28 infections (24 BSI and 4 IE) Previous therapy 100%. PWID 67% 4 IE, type unspecified | MRSA (39%) and MSSA (17%) main pathogens | LD of 1500 mg followed by 1 maintenance dose | No | Overall, 44% clinical cure, 33% failed treatment, and 22% lost to FU. | No data reported |
| Bai, F. et al., 2020 [106] | Multicentre retrospective study DAL in various infections | Clinical cure | 206 infections (124 ABSSSI, 82 other site infection) Previous therapy 77.8% 6 IE, type unspecified. | MRSA (29%), CoNS (35%) and MSSA (17%) in the non-ABSSSI group. | Overall, single 1500 mg dose in 60.2% | In 37.2% of non-ABSSSI patients, mainly fluoroquinolones, rifampicin, and tetracycline | Overall clinical cure in non-ABSSSI 75%. IE subgroup Clinical cure: 83.3% | 5.4% had an AE, mainly dermatologic. One serious AE (Stevens–Johnson). |
| Núñez-Núñez, M. et al., 2020 [110] | Observational prospective study. DAL as sequential treatment | Clinical success | 22 infections (osteoarticular 46%, BSI 23%). Previous therapy 100%. 3 IE, type unspecified. | S. aureus (55%), CoNS (27%) | 63% of the whole cohort received 1000 mg followed by 500 mg | No data reported | Overall, clinical success 95% | AE 1 (4.5%), infusion site reaction |
| Veve, M.P. et al., 2020 [119] | Observational retrospective study DAL vs. SOC | Incidence of infection-related readmission within 90 d of hospital discharge or outpatient DAL administration | 215 infections (most common BSI, osteoarticular and IE) 70 DAL vs. 145 SoC Previous therapy 100%. IE 54: 9 DAL vs. 45 SOC | MRSA 82% | Most frequent regimen 2: 1500 mg doses 1 week apart | in 13% of DAL treated. | Overall, DAL was associated with lower 90-day infection-related readmissions and shorter length of stay. | AE 2.9% in the DAL group, 1 required discontinuation. |
| Durante-Mangoni, E. et al., 2021 [111] | Observational single-centre retrospective study DAL in IE | Clinical and microbiological cure | 10 IE: 3 NVE, 5 PVE, 2 CIED-IE At least 2 weeks previous therapy 100% | Mainly caused by staphylococci and enterococci. | Median of 2.5 DAL doses per patient | No data reported | Clinical and microbiological cure 70% | 1 AE (rash after the third dose) with treatment withdrawal |
| Arrieta-Loitegui, M. et al., 2022 [112] | Observational retrospective study DAL as sequential treatment | Clinical and microbiological cure | 102 infections (SSTI 30%, BSI 15.7%, IE 13.7%) Previous therapy 100%. 14 IE, type unspecified | S. aureus in 70.6% | IE patients, 1500 mg as LD followed by a range of 1–6: 1500 mg doses | 16.7%, mainly moxifloxacin and linezolid | Overall, clinical and microbiological success: 93.7%. Median reduction in hospitalization 14 days (range 7–84). | AE in 3.9%, 1 patient discontinued. |
| Taylor, K. et al., 2022 [114] | Observational retrospective study DAL as sequential treatment | Clinical success | 48 infections (osteomyelitis 54%, IE 23%, BSI 15%). 11 IE, type unspecified. Previous therapy 100% | MRSA (42%) and MSSA (19%) main pathogens | Most patients received 1500 mg doses 44% 1 dose, 52% 2 doses. | 27%, mainly rifampin and quinolones | Overall clinical success 85%. IE subgroup: Clinical success at 90 days 82%. | No AE reported |
| Lueking, R. et al., 2023 [120] | Observational retrospective study Vulnerable people receiving DAL | Clinical failure (not defined) | 40 infections (BSI 67.5%, ABSSSI 45%) Previous therapy 100%. 4 IE, type unspecified | MRSA (57.5%) and MSSA (30%) main pathogens | Most frequent regimen 1500 mg single dose | In 15% of the whole cohort. | IE subgroup: Clinical success in all patients | AE in 5% |
| Vazquez Deida, A.A. et al., 2020 [118] | Case series Vulnerable people receiving DAL | N/A | 27 infections (BSI 26%, IE 26%). Previous therapy 100% PWID 67% 9 right side IE | S. aureus 100% (48% MRSA). | Single DAL dose 7–10 days before the planned end of therapy | No | IE subgroup: Clinical success in 6/9 Estimated cost avoidance of USD 9600 per patient in the whole cohort | AE in 7.4% (mild events) |
| Guleri, A. et al., 2021 [113] | Case series DAL in IE | N/A | 11 IE, 4 aortic NVE, 3 aortic PVE, 1 mitro-aortic NVE, 1 mitral NVE, 1 ICD-IE, 1 tricuspid NVE) Previous therapy 100%. | MSSA and E. faecalis, main pathogens | 1 or 2: 1500 mg doses | 9, mostly oral amoxicillin. | Clinical cure in all but one patient | No AE reported |
| Hitzenbichler, F. et al., 2021 [127] | Case series DAL after clearance of bacteraemia | N/A | 4 IE 2 PVE 2 LVAD | MRSA E. faecalis E. faecium | Long-term suppressive DAL, various regimens | No | Clinical success with prolonged infection suppression in all IE cases | No AE reported |
| Steele, J.M. et al. 2018 [121] | Case report DNS strain | N/A | 1 Tricuspid NVE | DNS MRSA | 1000 mg LD, then 3 weekly 500 mg doses | No | Clinical and microbiological failure, bacteraemia relapse, isolation of a VISA and telavancin-non susceptible MRSA | No AE reported |
| Kussmann, M. et al., 2018 [125] | Case report | N/A | 1 CIED-IE with incomplete PMK explantation | MRSA | Unspecified dosing 30 weekly administrations | No | Clinical and microbiological failure, bacteraemia relapse, isolation of a SCV strain teicoplanin-resistant and DAL non-susceptible | No AE reported |
| Howard-Anderson, J. et al., 2019 [122] | Case report Suppressive therapy | N/A | 1 LVDA | MRSA | Weekly 1500 mg for 10 weeks, then 1500 mg biweekly. Total DAL exposure: 235 days | No | Clinical success with prolonged infection suppression | No AE reported |
| Spaziante, M. et al., 2019 [126] | Case report | N/A | 1 Aortic PVE in a man with unacceptable perioperative risk | MRSE | 1500 mg whenever serum bactericidal activity titers detected ≤ 1:8 | No | Clinical and radiological improvement with no recurrence | No AE reported |
| Hakim, A. et al., 2020 [123] | Case report DAL as primary regimen | N/A | 1 Tricuspid NVE | MSSA | 1500 mg LD, followed by 5 weekly 500 mg doses | No | Clinical success | No AE reported |
| Teigell-Muñoz, F.J. et al., 2023 [124] | Case report DAL as consolidation therapy | N/A | 1 Aortic NVE | E. faecalis | 1000 mg single dose, after 4 weeks of therapy and valve replacement | No | Clinical success | No AE reported |
3.4. Oritavancin
3.4.1. Mechanism of Action and Indication
3.4.2. Clinical Evidence in Infective Endocarditis
| Authors | Study Design | Endpoint | N° Patients/ IE Type | Pathogens | Dosage and Duration | Combination, Dosage | Outcomes | Safety |
|---|---|---|---|---|---|---|---|---|
| Stewart, C.L. et al., 2017 [145] | Observational retrospective study ORI as an off-label indication | Clinical cure | 10 infections (BSI 50%) 1 tricuspid NVE in a PWID with previous therapy: VAN (3 days), then CRO (4 days) | Streptococcus agalactiae | IE patient 1200 mg 1 dose and then discharged | No | Clinical failure with need for valve replacement 3 months after ORI administration | No AE reported |
| Ahiskali, A. et al., 2020 [143] | Observational retrospective study on a vulnerable population of PWID receiving ORI | Clinical cure | 23 infections (BSI 50%) Previous therapy 100%. 2 IE, type unspecified | 1 MSSA 1 MRSA | MSSA IE: single 1200 mg dose, MRSA IE: two 1200 mg doses | No | IE subgroup: Clinical cure 1 (MSSA), Clinical failure 1 (MRSA) | AE in 8.7%, mild |
| Brownell, L.E. et al., 2020 [11] | Multicentre observational retrospective study ORI as primary treatment | Clinical cure | 75 infections (ABSSSI 49%) No previous treatment 4 IE, type unspecified | MSSA (31.5%) and MRSA (17.8%) | All patients included received initial 1200 mg dose followed by 1200 or 800 mg weekly | No data reported | IE subgroup: Clinical cure 75% Average hospital days avoided in IE: 18 d | AE in 12%, most commonly back pain with infusion. All resolved upon discontinuation |
| Salcedo, D.A.T. et al., 2018 [146] | Case series of Gram-positive IE in PWID | N/A | 5 IE Previous therapy 100%. | MRSA (20%), MSSA (20%), Streptococcus (10%) | 2 received 4 ORI doses, 3 received only 1 dose | No | Clinical cure: 3/5 Lost to FU: 2/5 | AE in 1 patient (allergic reaction treated with oral prednisone) |
| Johnson, J.A. et al., 2015 [144] | Case report Limited treatment options | N/A | 1 Aortic PVE | VR E. faecium. | 1200 mg every other day for 3 doses, then weekly for 6 weeks, then 1200 mg biweekly for 10 weeks after recurrence and valve exchange | GEN for the first 4 days, discontinued due to renal toxicity | Recurrence after the first treatment course attributed to lack in source control. Clinical cure after valve exchange and a second prolonged course of ORI | Mild increase in transaminases |
3.5. Old Antibiotics with a Renewed Interest: Fosfomycin
3.5.1. Mechanism of Action and Indication
3.5.2. Clinical Evidence in Infective Endocarditis
| Authors | Study Design | Endpoint | N° Patients/ IE Type | Pathogens | Dosage and Duration | Combination, Dosage | Outcomes | Safety |
|---|---|---|---|---|---|---|---|---|
| Del Rio, A. et al., 2014 [180] | Multicentre prospective clinical trial IMI + FOS as rescue therapy for MRSA BSI | Primary endpoints: negative BC at 72 h, clinical success § rate assessed at the test-of-cure visit in the ITT population | 16 BSI 12 IE | MRSA | 2 g/6 h * Median 28 d (SD 4–75) | IMI 1 g/6 h * | Overall, negative BC 72 h after the first dose in all the patients, No MRSAB breakthrough episodes, Clinical success: 91.6%, Mortality: 5 (31%), only 1 related to the infection or to the antibiotic therapy | 5/16 (31%) 1: leukopenia 1: fungal BSI 3: sodium overload |
| Rieg, S. et al., 2017 [183] | Post hoc analysis of the INSTINCT prospective multicentre cohort study Patients with SAB | All-cause 30 d and 90 d mortality, death, or SAB-related late complications within 180 days | 964 BSI (452 monotherapy and 512 combination) FOS was used in 99/512 (19%) 121 (12.6%) IE [20/512 (4.4%) monotherapy, 101/452 (19.7%) combination] | MRSA 108/964 (11.2%) MSSA 856/964 (88.8%) | 5 g/8 h Median duration 14 d (IQR 7–26, range 1–66) | MSSA: FLU, VAN, TEC, DAP MRSA: VAN, TEIC, DAP, LNZ | Overall, 30 d mortality: monotherapy 82/443 (18.5%), combination 93/509 (18.3%), (p = 1) 90 d mortality: monotherapy 140/436 (32.1%), combination 156/503 (31%), (p = 0.87) SAB-related late complications within 180 d: monotherapy 25/428 (5.8%), combination 19/490 (3.9%), (p = 0.18) No specific outcomes in patients receiving FOS | No data reported |
| Pericas, J.M. et al., 2018 [181] | Open-label randomised clinical trial IMI + FOS vs. VAN for MRSA BSI | Primary endpoint: persistent bacteraemia at 7 d Secondary endpoints: negative BC at 72 h after the initiation of study treatment, relapse of BSI, mortality | 15 BSI 8 IE FOS + IMI (n = 8) (4 complicated BSI, 4 IE: 2 NVE, 2 PVE) VAN (n = 7) (3 complicated BSI, 1 NVE, 3 CIED-IE) | MRSA | 2 g/6 h EI group, VAN: mean 35.7 d (range 27–42), IMI + FOS: mean 18.2 d (range 4–51) Complicated bacteraemia VAN: mean 18.3 d (range 17–21), IMI + FOS: mean 27.2 d (range 15–42) | IMI 1 g/6 h VAN 30–45 mg/kg/24 h (divided into 2–3 doses, trough levels ≥ 15 mg/L) | Overall, all patients in the FOS + IMI arm had negative BC at 3 days Cure rates: IMI + FOS 4 (50%) VAN 3 (43%) In-hospital mortality: IMI + FOS 3 (37.5%), VAN 1 (14.2%) Persistent bacteriemia: IMI + FOS 0, VAN 1 (14.2%) Relapse: IMI + FOS 0, VAN 1 (14.2%) | IMI + FOS: 1 salt overload VAN: 1 renal toxicity |
| Rieg, S. et al., 2020 [182] | Post hoc analysis of the INSTINCT prospective multicentre cohort study Patients with SAB | All-cause 90 d mortality, death, or SAB-related late complications within 180 days | 578 BSI [313 combination with RIF (n = 242) or FOS (n = 58) and 265 monotherapy 129 IE, 23% NVE, 7,1% of CIED or vascular grafts or PVE | MSSA 250 (94%) monotherapy 264 (84%) combination MRSA 15 (6%) monotherapy 49 (16%) combination | 5 g/8 h Median 23 d (IQR 13–33) | MSSA: FLU or DAP MRSA: VAN, TEIC, DAP, LNZ | Overall, all-cause 90 d mortality: 190/565 (34%), Death or SAB-related late complications within 180 d: 45% [52% (132/255) monotherapy vs. 39% (115/297) combination], Combination therapy was associated with a better outcome than monotherapy (HR 0.65, 95% CI 0.46–0.92), especially in implanted foreign devices. IE subgroup: 90 d mortality: 16/32 (50%) monotherapy, 27/81 (33%) RIF, 4/11 (36%) FOS | No data reported |
| Pujol, M. et al., 2021 [10] | Randomised clinical trial DAP + FOS vs. DAP for MRSA BSI | Treatment success 6 weeks after the end of therapy | 155 BSI 18 left-side IE | MRSA | 2 g/6 h DAP + FOS: median 14 d (IQR 11–21) | DAP 10 mg/kg/24 h DAP Median 14 days (IQR 10–18.5) | Overall, treatment success °: DAP + FOS 40/74 (54.1%), DAP 34/81 (42.0%) (p = 0.135) Microbiological failure °°: DAP + FOS 0, DAP 9/81 (11.1%) (p = 0.003) Persistent bacteraemia at 7 d: DAP + FOS 0, DAP 5/81 (6.2%) Complicated bacteraemia: DAP + FOS 12/74 (16.2%), DAP 26/81 (32.1%) (p = 0.022) No differences were observed in patients with or without IE | DAP + FOS 13/74 (17.6%) DAP 4/81 (4.9%) (p = 0.018) |
| Aoyagi, S. et al., 1994 [179] | Case report | N/A | 1 IE on ventricular patch graft | MRSA | 300 mg/6 h (paediatric dosage) 24 d | IMI 125 mg/6 h (paediatric dosage) | Clearance of bacteraemia: 24 h from FOS start Symptom-free during 12 months of follow-up | No data reported |
| Chen, L.Y. et al., 2011 [184] | Case report | N/A | 1 CIED-IE plus osteomyelitis | DNS MRSA | 6 g/6 h 56 d | DAP 9 mg/kg/24 h, followed by 12 mg/kg/24 h | Clearance of bacteraemia: 7 d Symptom free during 12 months of follow-up | No AE reported |
| Mirò, J.M. et al., 2012 [185] | Case series Failure with high-dose DAP or VAN | N/A | 3 IE (1 aortic PVE, 2 left-sided NVE) | 1 MSSA (PVE) 2 MRSA (NVE) | 2 g/6 h 6 weeks | DAP 10 mg/kg/24 h | Clearance of bacteraemia Alive at 6 months (n = 1) and 12 months (n = 2) FU No need of surgery | No AE reported |
| Vergara-Lopez, S. et al., 2015 [186] | Case report | N/A | 1 Aortic NVE | MRSE + carbapenem-resistant Klebsiella oxytoca | 4 g/6 h 28 d | VAN (1 g/12 h) AMK (1 g/24 h) | Clearance of bacteraemia Complete disappearance of the vegetation at echocardiography | Self-limited hypokalaemia |
4. Oral Strategies
| Bacteria | Oral Antibiotic Strategies for Step-Down Treatment # | |||
|---|---|---|---|---|
| MSSA/ MS-CONS | Dicloxacillin + rifampicin/fusidic acid | Levofloxacin/moxifloxacin + rifampicin/fusidic acid | Linezolid monotherapy or linezolid + adjunctive therapy | TMP-SMX + adjunctive therapy |
| MRSA | Linezolid *° | |||
| MR CONS | Linezolid + levofloxacin/moxifloxacin | Levofloxacin/moxifloxacin + rifampicin/fusidic acid/clindamycin | Linezolid monotherapy or linezolid + rifampicin | TMP-SMX + adjunctive therapy |
| Oral Streptococci/ Streptococcus spp. | Amoxicillin monotherapy or amoxicillin + rifampicin | Moxifloxacin + rifampicin/clindamycin/amoxicillin | Linezolid monotherapy or linezolid + rifampicin/clindamycin/amoxicillin | Moxifloxacin + linezolid |
| E. faecalis | Amoxicillin/clavulanate + cefditoren ° or amoxicillin + rifampicin | Moxifloxacin + Amoxicillin/rifampicin | Linezolid monotherapy or linezolid + amoxicillin/rifampicin | Moxifloxacin + linezolid |
| GISA (hVISA, VISA, DNS) | NOT RECOMMENDED (No data available) | |||
| E. faecium | ||||
| VVR Enterococcus spp. | ||||
5. New Therapeutic Strategies: Considerations for Their Optimal Use in IE
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cahill, T.J.; Baddour, L.M.; Habib, G.; Hoen, B.; Salaun, E.; Pettersson, G.B.; Schäfers, H.J.; Prendergast, B.D. Challenges in Infective Endocarditis. J. Am. Coll. Cardiol. 2017, 69, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhan, Y.; Zhang, K.; Gao, Y.; Chen, L.; Zhan, J.; Chen, Z.; Zeng, Z. The Global, Regional, and National Burden and Trends of Infective Endocarditis from 1990 to 2019: Results From the Global Burden of Disease Study 2019. Front. Med. 2022, 9, 774224. [Google Scholar] [CrossRef] [PubMed]
- Selton-Suty, C.; Célard, M.; Le Moing, V.; Doco-Lecompte, T.; Chirouze, C.; Iung, B.; Strady, C.; Revest, M.; Vandenesch, F.; Bouvet, A.; et al. Preeminence of Staphylococcus aureus in Infective Endocarditis: A 1-Year Population-Based Survey. Clin. Infect. Dis. 2012, 54, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G.; Durack, D.T.; Selton-Suty, C.; Athan, E.; Bayer, A.S.; Chamis, A.L.; Dahl, A.; DiBernardo, L.; Durante-Mangoni, E.; Duval, X.; et al. The 2023 Duke-International Society for Cardiovascular Infectious Diseases Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023, 77, 518–526. [Google Scholar] [CrossRef]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the Management of Endocarditis: Developed by the Task Force on the Management of Endocarditis of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the Management of Infective Endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef] [PubMed]
- Gentile, I.; Buonomo, A.R.; Corcione, S.; Paradiso, L.; Giacobbe, D.R.; Bavaro, D.F.; Tiseo, G.; Sordella, F.; Bartoletti, M.; Palmiero, G.; et al. CEFTO-CURE Study: CEFTObiprole Clinical Use in Real-lifE—A Multi-Centre Experience in Italy. Int. J. Antimicrob. Agents 2023, 62, 106817. [Google Scholar] [CrossRef]
- Geriak, M.; Haddad, F.; Rizvi, K.; Rose, W.; Kullar, R.; LaPlante, K.; Yu, M.; Vasina, L.; Ouellette, K.; Zervos, M.; et al. Clinical Data on Daptomycin plus Ceftaroline versus Standard of Care Monotherapy in the Treatment of Methicillin-Resistant Staphylococcus aureus Bacteremia. Antimicrob. Agents Chemother. 2019, 63, e02483-18. [Google Scholar] [CrossRef]
- Hidalgo-Tenorio, C.; Sadyrbaeva-Dolgova, S.; Enríquez-Gómez, A.; Muñoz, P.; Plata-Ciezar, A.; Miró, J.M.; Alarcón, A.; Martínez-Marcos, F.J.; Loeches, B.; Escrihuela-Vidal, F.; et al. EN-DALBACEN 2.0 Cohort: Real-Life Study of Dalbavancin as Sequential/Consolidation Therapy in Patients with Infective Endocarditis Due to Gram-Positive Cocci. Int. J. Antimicrob. Agents 2023, 62, 106918. [Google Scholar] [CrossRef]
- Pujol, M.; Miró, J.-M.; Shaw, E.; Aguado, J.-M.; San-Juan, R.; Puig-Asensio, M.; Pigrau, C.; Calbo, E.; Montejo, M.; Rodriguez-Álvarez, R.; et al. Daptomycin Plus Fosfomycin Versus Daptomycin Alone for Methicillin-Resistant Staphylococcus aureus Bacteremia and Endocarditis: A Randomized Clinical Trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 72, 1517–1525. [Google Scholar] [CrossRef]
- Brownell, L.E.; Adamsick, M.L.; McCreary, E.K.; Vanderloo, J.P.; Ernst, E.J.; Jackson, E.R.; Schulz, L.T. Clinical Outcomes and Economic Impact of Oritavancin for Gram-Positive Infections: A Single Academic Medical Center Health System Experience. Drugs-Real World Outcomes 2020, 7, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Martí-Carvajal, A.J.; Dayer, M.; Conterno, L.O.; Gonzalez Garay, A.G.; Martí-Amarista, C.E. A Comparison of Different Antibiotic Regimens for the Treatment of Infective Endocarditis. Cochrane Database Syst. Rev. 2020, 5, CD009880. [Google Scholar] [CrossRef] [PubMed]
- Freling, S.; Wald-Dickler, N.; Banerjee, J.; Canamar, C.P.; Tangpraphaphorn, S.; Bruce, D.; Davar, K.; Dominguez, F.; Norwitz, D.; Krishnamurthi, G.; et al. Real-World Application of Oral Therapy for Infective Endocarditis: A Multicenter Retrospective, Cohort Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023, 77, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Tobudic, S.; Forstner, C.; Burgmann, H.; Lagler, H.; Ramharter, M.; Steininger, C.; Vossen, M.G.; Winkler, S.; Thalhammer, F. Dalbavancin as Primary and Sequential Treatment for Gram-Positive Infective Endocarditis: 2-Year Experience at the General Hospital of Vienna. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 67, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, L.; Rhomberg, P.R.; Fritsche, T.R.; Sader, H.S.; Jones, R.N. Bactericidal Activity of BAL9141, a Novel Parenteral Cephalosporin against Contemporary Gram-Positive and Gram-Negative Isolates. Diagn. Microbiol. Infect. Dis. 2004, 50, 73–75. [Google Scholar] [CrossRef]
- Bogdanovich, T.; Ednie, L.M.; Shapiro, S.; Appelbaum, P.C. Antistaphylococcal Activity of Ceftobiprole, a New Broad-Spectrum Cephalosporin. Antimicrob. Agents Chemother. 2005, 49, 4210–4219. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.P.; Park, S.-J.; Kim, H.S.; Kim, E.S.; Kim, M.-N.; Kim, S.-H.; Lee, S.-O.; Choi, S.-H.; Jeong, J.-Y.; Woo, J.H.; et al. In Vitro Activities of Ceftobiprole, Dalbavancin, Daptomycin, Linezolid, and Tigecycline against Methicillin-Resistant Staphylococcus aureus Blood Isolates: Stratified Analysis by Vancomycin MIC. Diagn. Microbiol. Infect. Dis. 2012, 73, 264–266. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Flamm, R.K.; Mendes, R.E.; Streit, J.M.; Smart, J.I.; Hamed, K.A.; Duncan, L.R.; Sader, H.S. Ceftobiprole Activity against Gram-Positive and -Negative Pathogens Collected from the United States in 2006 and 2016. Antimicrob. Agents Chemother. 2019, 63, e01566-18. [Google Scholar] [CrossRef]
- Hope, R.; Livermore, D.M.; Brick, G.; Lillie, M.; Reynolds, R. BSAC Working Parties on Resistance Surveillance Non-Susceptibility Trends among Staphylococci from Bacteraemias in the UK and Ireland, 2001–2006. J. Antimicrob. Chemother. 2008, 62 (Suppl. 2), ii65–ii74. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Flamm, R.K.; Duncan, L.R.; Shortridge, D.; Smart, J.I.; Hamed, K.A.; Mendes, R.E.; Sader, H.S. Ceftobiprole Activity When Tested against Contemporary Bacteria Causing Bloodstream Infections in the United States (2016–2017). Diagn. Microbiol. Infect. Dis. 2019, 94, 304–313. [Google Scholar] [CrossRef]
- López Díaz, M.C.; Ríos, E.; Rodríguez-Avial, I.; Simaluiza, R.J.; Picazo, J.J.; Culebras, E. In-Vitro Activity of Several Antimicrobial Agents against Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates Expressing Aminoglycoside-Modifying Enzymes: Potency of Plazomicin Alone and in Combination with Other Agents. Int. J. Antimicrob. Agents 2017, 50, 191–196. [Google Scholar] [CrossRef]
- Deshpande, L.M.; Jones, R.N. Bactericidal Activity and Synergy Studies of BAL9141, a Novel Pyrrolidinone-3-Ylidenemethyl Cephem, Tested against Streptococci, Enterococci and Methicillin-Resistant Staphylococci. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2003, 9, 1120–1124. [Google Scholar] [CrossRef]
- Rouse, M.S.; Steckelberg, J.M.; Patel, R. In Vitro Activity of Ceftobiprole, Daptomycin, Linezolid, and Vancomycin against Methicillin-Resistant Staphylococci Associated with Endocarditis and Bone and Joint Infection. Diagn. Microbiol. Infect. Dis. 2007, 58, 363–365. [Google Scholar] [CrossRef]
- Leonard, S.N.; Cheung, C.M.; Rybak, M.J. Activities of Ceftobiprole, Linezolid, Vancomycin, and Daptomycin against Community-Associated and Hospital-Associated Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2008, 52, 2974–2976. [Google Scholar] [CrossRef]
- Rodríguez-García, R.; Rodríguez-Esteban, M.Á.; García-Carús, E.; Telenti, M.; Fernández, J. In Vitro Activity of Ceftaroline and Ceftobiprole against Clinical Isolates of Gram-Positive Bacteria from Infective Endocarditis: Are These Drugs Potential Options for the Initial Management of This Disease? Diagn. Microbiol. Infect. Dis. 2020, 98, 115153. [Google Scholar] [CrossRef]
- Campanile, F.; Bongiorno, D.; Mongelli, G.; Zanghì, G.; Stefani, S. Bactericidal Activity of Ceftobiprole Combined with Different Antibiotics against Selected Gram-Positive Isolates. Diagn. Microbiol. Infect. Dis. 2019, 93, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Bongiorno, D.; Mongelli, G.; Stefani, S.; Campanile, F. Genotypic Analysis of Italian MRSA Strains Exhibiting Low-Level Ceftaroline and Ceftobiprole Resistance. Diagn. Microbiol. Infect. Dis. 2019, 95, 114852. [Google Scholar] [CrossRef]
- Peiffer-Smadja, N.; Guillotel, E.; Luque-Paz, D.; Maataoui, N.; Lescure, F.-X.; Cattoir, V. In Vitro Bactericidal Activity of Amoxicillin Combined with Different Cephalosporins against Endocarditis-Associated Enterococcus Faecalis Clinical Isolates. J. Antimicrob. Chemother. 2019, 74, 3511–3514. [Google Scholar] [CrossRef] [PubMed]
- Entenza, J.M.; Veloso, T.R.; Vouillamoz, J.; Giddey, M.; Majcherczyk, P.; Moreillon, P. In Vivo Synergism of Ceftobiprole and Vancomycin against Experimental Endocarditis Due to Vancomycin-Intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 3977–3984. [Google Scholar] [CrossRef] [PubMed]
- Tattevin, P.; Basuino, L.; Bauer, D.; Diep, B.A.; Chambers, H.F. Ceftobiprole Is Superior to Vancomycin, Daptomycin, and Linezolid for Treatment of Experimental Endocarditis in Rabbits Caused by Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 610–613. [Google Scholar] [CrossRef]
- Chambers, H.F. Evaluation of Ceftobiprole in a Rabbit Model of Aortic Valve Endocarditis Due to Methicillin-Resistant and Vancomycin-Intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Ednie, L.M.; Appelbaum, P.C. Antistaphylococcal Activity of ACHN-490 Tested Alone and in Combination with Other Agents by Time-Kill Assay. Antimicrob. Agents Chemother. 2010, 54, 2258–2261. [Google Scholar] [CrossRef] [PubMed]
- Barber, K.E.; Werth, B.J.; Ireland, C.E.; Stone, N.E.; Nonejuie, P.; Sakoulas, G.; Pogliano, J.; Rybak, M.J. Potent Synergy of Ceftobiprole plus Daptomycin against Multiple Strains of Staphylococcus aureus with Various Resistance Phenotypes. J. Antimicrob. Chemother. 2014, 69, 3006–3010. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.; Singh, K.V.; Panesso, D.; Murray, B.E. Time-Kill and Synergism Studies of Ceftobiprole against Enterococcus Faecalis, Including Beta-Lactamase-Producing and Vancomycin-Resistant Isolates. Antimicrob. Agents Chemother. 2007, 51, 2043–2047. [Google Scholar] [CrossRef]
- Oltolini, C.; Castiglioni, B.; Tassan Din, C.; Castiglioni, A.; Ossi, C.; La Canna, G.; Pajoro, U.; Scarpellini, P. Meticillin-Resistant Staphylococcus aureus Endocarditis: First Report of Daptomycin plus Ceftobiprole Combination as Salvage Therapy. Int. J. Antimicrob. Agents 2016, 47, 502–504. [Google Scholar] [CrossRef]
- Mahmoud, E.; Al Mansour, S.; Bosaeed, M.; Alharbi, A.; Alsaedy, A.; Aljohani, S.; Alalwan, B.; Alothman, A. Ceftobiprole for Treatment of MRSA Blood Stream Infection: A Case Series. Infect. Drug Resist. 2020, 13, 2667–2672. [Google Scholar] [CrossRef] [PubMed]
- Tascini, C.; Attanasio, V.; Ripa, M.; Carozza, A.; Pallotto, C.; Bernardo, M.; Francisci, D.; Oltolini, C.; Palmiero, G.; Scarpellini, P. Ceftobiprole for the Treatment of Infective Endocarditis: A Case Series. J. Glob. Antimicrob. Resist. 2020, 20, 56–59. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Kosar, J.; Baxter, M.; Dhami, R.; Borgia, S.; Irfan, N.; MacDonald, K.S.; Dow, G.; Lagacé-Wiens, P.; Dube, M.; et al. Real-Life Experience with Ceftobiprole in Canada: Results from the CLEAR (CanadianLEadership onAntimicrobialReal-Life Usage) Registry. J. Glob. Antimicrob. Resist. 2021, 24, 335–339. [Google Scholar] [CrossRef]
- Holland, T.L.; Cosgrove, S.E.; Doernberg, S.B.; Pavlov, O.; Titov, I.; Atanasov, B.; Gehr, M.A.; Engelhardt, M.; Hamed, K.; Ionescu, D.; et al. LB2302. Ceftobiprole Compared to Daptomycin with or without Optional Aztreonam for the Treatment of Complicated Staphylococcus aureus (SAB): Results of a Phase 3, Randomized, Double-Blind Trial (ERADICATE). Open Forum Infect. Dis. 2022, 9, ofac492.1892. [Google Scholar] [CrossRef]
- Giuliano, S.; Angelini, J.; D’Elia, D.; Geminiani, M.; Barison, R.D.; Giacinta, A.; Sartor, A.; Campanile, F.; Curcio, F.; Cotta, M.O.; et al. Ampicillin and Ceftobiprole Combination for the Treatment of Enterococcus Faecalis Invasive Infections: “The Times They Are A-Changin”. Antibiotics 2023, 12, 879. [Google Scholar] [CrossRef]
- Holland, T.L.; Cosgrove, S.E.; Doernberg, S.B.; Jenkins, T.C.; Turner, N.A.; Boucher, H.W.; Pavlov, O.; Titov, I.; Kosulnykov, S.; Atanasov, B.; et al. Ceftobiprole for Treatment of Complicated Staphylococcus aureus Bacteremia. N. Engl. J. Med. 2023, 389, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Jorgenson, M.R.; DePestel, D.D.; Carver, P.L. Ceftaroline Fosamil: A Novel Broad-Spectrum Cephalosporin with Activity against Methicillin-Resistant Staphylococcus aureus. Ann. Pharmacother. 2011, 45, 1384–1398. [Google Scholar] [CrossRef] [PubMed]
- Espedido, B.A.; Jensen, S.O.; van Hal, S.J. Ceftaroline Fosamil Salvage Therapy: An Option for Reduced-Vancomycin-Susceptible MRSA Bacteraemia. J. Antimicrob. Chemother. 2015, 70, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Rathi, S.; Jain, A. Ceftaroline: A Comprehensive Update. Int. J. Antimicrob. Agents 2011, 37, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Werth, B.J.; Barber, K.E.; Ireland, C.E.; Rybak, M.J. Evaluation of Ceftaroline, Vancomycin, Daptomycin, or Ceftaroline plus Daptomycin against Daptomycin-Nonsusceptible Methicillin-Resistant Staphylococcus aureus in an in Vitro Pharmacokinetic/Pharmacodynamic Model of Simulated Endocardial Vegetations. Antimicrob. Agents Chemother. 2014, 58, 3177–3181. [Google Scholar] [CrossRef] [PubMed]
- Dhand, A.; Bayer, A.S.; Pogliano, J.; Yang, S.-J.; Bolaris, M.; Nizet, V.; Wang, G.; Sakoulas, G. Use of Antistaphylococcal β-Lactams to Increase Daptomycin Activity in Eradicating Persistent Bacteremia Due to Methicillin-Resistant Staphylococcus aureus: Role of Enhanced Daptomycin Binding. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 53, 158–163. [Google Scholar] [CrossRef]
- Werth, B.J.; Vidaillac, C.; Murray, K.P.; Newton, K.L.; Sakoulas, G.; Nonejuie, P.; Pogliano, J.; Rybak, M.J. Novel Combinations of Vancomycin plus Ceftaroline or Oxacillin against Methicillin-Resistant Vancomycin-Intermediate Staphylococcus aureus (VISA) and Heterogeneous VISA. Antimicrob. Agents Chemother. 2013, 57, 2376–2379. [Google Scholar] [CrossRef]
- Tran, N.; Rybak, M.J. β-Lactam Combinations with Vancomycin Show Synergistic Activity against Vancomycin-Susceptible Staphylococcus aureus, Vancomycin-Intermediate S. Aureus (VISA), and Heterogeneous VISA. Antimicrob. Agents Chemother. 2018, 62, e00157-18. [Google Scholar] [CrossRef]
- Eliazar, J.; Johnson, T.; Chbib, C. Pre-Clinical Impact of the Synergistic Mechanism of Daptomycin and Ceftaroline on Patients with Methicillin-Resistant Staphylococcus aureus Bacteremia Infections. Curr. Rev. Clin. Exp. Pharmacol. 2021, 16, 296–299. [Google Scholar] [CrossRef]
- Hutton, M.A.; Sundaram, A.; Perri, M.B.; Zervos, M.J.; Herc, E.S. Assessment of Invitrosynergy of Daptomycin or Vancomycin plus Ceftaroline for Daptomycin Non-Susceptible Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2020, 98, 115126. [Google Scholar] [CrossRef]
- Werth, B.J.; Shireman, L.M. Pharmacodynamics of Ceftaroline plus Ampicillin against Enterococcus Faecalis in an In Vitro Pharmacokinetic/Pharmacodynamic Model of Simulated Endocardial Vegetations. Antimicrob. Agents Chemother. 2017, 61, e02235-16. [Google Scholar] [CrossRef] [PubMed]
- Thieme, L.; Klinger-Strobel, M.; Hartung, A.; Stein, C.; Makarewicz, O.; Pletz, M.W. In Vitro Synergism and Anti-Biofilm Activity of Ampicillin, Gentamicin, Ceftaroline and Ceftriaxone against Enterococcus Faecalis. J. Antimicrob. Chemother. 2018, 73, 1553–1561. [Google Scholar] [CrossRef]
- Cusumano, J.A.; Daffinee, K.E.; Piehl, E.C.; García-Solache, M.; Desbonnet, C.; Rice, L.B.; LaPlante, K.L. Meropenem plus Ceftaroline Is Active against Enterococcus Faecalis in an In Vitro Pharmacodynamic Model Using Humanized Dosing Simulations. Antimicrob. Agents Chemother. 2022, 66, e0042622. [Google Scholar] [CrossRef]
- Smith, J.R.; Barber, K.E.; Raut, A.; Aboutaleb, M.; Sakoulas, G.; Rybak, M.J. β-Lactam Combinations with Daptomycin Provide Synergy against Vancomycin-Resistant Enterococcus Faecalis and Enterococcus Faecium. J. Antimicrob. Chemother. 2015, 70, 1738–1743. [Google Scholar] [CrossRef] [PubMed]
- Mainardi, J.L.; Gutmann, L.; Acar, J.F.; Goldstein, F.W. Synergistic Effect of Amoxicillin and Cefotaxime against Enterococcus Faecalis. Antimicrob. Agents Chemother. 1995, 39, 1984–1987. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Moise, P.A.; Casapao, A.M.; Nonejuie, P.; Olson, J.; Okumura, C.Y.M.; Rybak, M.J.; Kullar, R.; Dhand, A.; Rose, W.E.; et al. Antimicrobial Salvage Therapy for Persistent Staphylococcal Bacteremia Using Daptomycin plus Ceftaroline. Clin. Ther. 2014, 36, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Jacqueline, C.; Amador, G.; Batard, E.; Le Mabecque, V.; Miègeville, A.-F.; Biek, D.; Caillon, J.; Potel, G. Comparison of Ceftaroline Fosamil, Daptomycin and Tigecycline in an Experimental Rabbit Endocarditis Model Caused by Methicillin-Susceptible, Methicillin-Resistant and Glycopeptide-Intermediate Staphylococcus aureus. J. Antimicrob. Chemother. 2011, 66, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Jacqueline, C.; Caillon, J.; Batard, E.; Le Mabecque, V.; Amador, G.; Ge, Y.; Biek, D.; Potel, G. Evaluation of the in Vivo Efficacy of Intramuscularly Administered Ceftaroline Fosamil, a Novel Cephalosporin, against a Methicillin-Resistant Staphylococcus aureus Strain in a Rabbit Endocarditis Model. J. Antimicrob. Chemother. 2010, 65, 2264–2265. [Google Scholar] [CrossRef]
- Jacqueline, C.; Caillon, J.; Le Mabecque, V.; Miègeville, A.-F.; Ge, Y.; Biek, D.; Batard, E.; Potel, G. In Vivo Activity of a Novel Anti-Methicillin-Resistant Staphylococcus aureus Cephalosporin, Ceftaroline, against Vancomycin-Susceptible and -Resistant Enterococcus Faecalis Strains in a Rabbit Endocarditis Model: A Comparative Study with Linezolid and Vancomycin. Antimicrob. Agents Chemother. 2009, 53, 5300–5302. [Google Scholar] [CrossRef]
- Jacqueline, C.; Caillon, J.; Le Mabecque, V.; Miègeville, A.-F.; Hamel, A.; Bugnon, D.; Ge, J.Y.; Potel, G. In Vivo Efficacy of Ceftaroline (PPI-0903), a New Broad-Spectrum Cephalosporin, Compared with Linezolid and Vancomycin against Methicillin-Resistant and Vancomycin-Intermediate Staphylococcus aureus in a Rabbit Endocarditis Model. Antimicrob. Agents Chemother. 2007, 51, 3397–3400. [Google Scholar] [CrossRef]
- García, P.; Moscoso, M.; Fernández, M.C.; Fuentes-Valverde, V.; Pérez, A.; Bou, G. Comparison of the in Vivo Efficacy of Ceftaroline Fosamil, Vancomycin and Daptomycin in a Murine Model of Methicillin-Resistant Staphylococcus aureus Bacteraemia. Int. J. Antimicrob. Agents 2023, 62, 106836. [Google Scholar] [CrossRef] [PubMed]
- Luther, M.K.; Rice, L.B.; LaPlante, K.L. Ampicillin in Combination with Ceftaroline, Cefepime, or Ceftriaxone Demonstrates Equivalent Activities in a High-Inoculum Enterococcus Faecalis Infection Model. Antimicrob. Agents Chemother. 2016, 60, 3178–3182. [Google Scholar] [CrossRef] [PubMed]
- EMA Zinforo. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zinforo (accessed on 11 September 2023).
- Brandariz-Núñez, D.; Suanzes, J.; Gutiérrez-Urbón, J.M.; Fernández-Oliveira, C.; Margusino, L.; Martín-Herranz, I. Incidence and Risk Factors for Mortality in Patients Treated with Combined Ceftaroline for Gram-Positive Infective Endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2022, 41, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Destache, C.J.; Guervil, D.J.; Kaye, K.S. Ceftaroline Fosamil for the Treatment of Gram-Positive Endocarditis: CAPTURE Study Experience. Int. J. Antimicrob. Agents 2019, 53, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Casapao, A.M.; Davis, S.L.; Barr, V.O.; Klinker, K.P.; Goff, D.A.; Barber, K.E.; Kaye, K.S.; Mynatt, R.P.; Molloy, L.M.; Pogue, J.M.; et al. Large Retrospective Evaluation of the Effectiveness and Safety of Ceftaroline Fosamil Therapy. Antimicrob. Agents Chemother. 2014, 58, 2541–2546. [Google Scholar] [CrossRef]
- Britt, R.S.; Evoy, K.E.; Lee, G.C.; Reveles, K.R.; Sorensen, K.M.; Jones, X.; Bollinger, M.; Frei, C.R. Early Use of Ceftaroline Fosamil in the United States Veterans Health Care System. Drugs 2017, 77, 1345–1351. [Google Scholar] [CrossRef]
- Zasowski, E.J.; Trinh, T.D.; Claeys, K.C.; Lagnf, A.M.; Bhatia, S.; Klinker, K.P.; Veve, M.P.; Estrada, S.J.; Johns, S.T.; Sawyer, A.J.; et al. Multicenter Cohort Study of Ceftaroline Versus Daptomycin for Treatment of Methicillin-Resistant Staphylococcus aureus Bloodstream Infection. Open Forum Infect. Dis. 2022, 9, ofab606. [Google Scholar] [CrossRef]
- McCreary, E.K.; Kullar, R.; Geriak, M.; Zasowski, E.J.; Rizvi, K.; Schulz, L.T.; Ouellette, K.; Vasina, L.; Haddad, F.; Rybak, M.J.; et al. Multicenter Cohort of Patients With Methicillin-Resistant Staphylococcus aureus Bacteremia Receiving Daptomycin Plus Ceftaroline Compared With Other MRSA Treatments. Open Forum Infect. Dis. 2020, 7, ofz538. [Google Scholar] [CrossRef]
- Ahmad, O.; Crawford, T.N.; Myint, T. Comparing the Outcomes of Ceftaroline Plus Vancomycin or Daptomycin Combination Therapy Versus Monotherapy in Adults with Complicated and Prolonged Methicillin-Resistant Staphylococcus aureus Bacteremia Initially Treated with Supplemental Ceftaroline. Infect. Dis. Ther. 2020, 9, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Penfield, N.; Oliver, N.T.; Hunter, A.; Rodriguez-Barradas, M. Daptomycin and Combination Daptomycin-Ceftaroline as Salvage Therapy for Persistent Methicillin-Resistant Staphylococcus aureus Bacteremia. Infect. Dis. Lond. Engl. 2018, 50, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Fabre, V.; Ferrada, M.; Buckel, W.R.; Avdic, E.; Cosgrove, S.E. Ceftaroline in Combination With Trimethoprim-Sulfamethoxazole for Salvage Therapy of Methicillin-Resistant Staphylococcus aureus Bacteremia and Endocarditis. Open Forum Infect. Dis. 2014, 1, ofu046. [Google Scholar] [CrossRef]
- Johnson, T.M.; Molina, K.C.; Miller, M.A.; Kiser, T.H.; Huang, M.; Mueller, S.W. Combination Ceftaroline and Daptomycin Salvage Therapy for Complicated Methicillin-Resistant Staphylococcus aureus Bacteraemia Compared with Standard of Care. Int. J. Antimicrob. Agents 2021, 57, 106310. [Google Scholar] [CrossRef]
- Kufel, W.D.; Parsels, K.A.; Blaine, B.E.; Steele, J.M.; Mahapatra, R.; Paolino, K.M.; Thomas, S.J. Vancomycin plus Ceftaroline for Persistent Methicillin-Resistant Staphylococcus aureus Bacteremia. Pharmacotherapy 2023, 43, 15–23. [Google Scholar] [CrossRef]
- Morrisette, T.; Lagnf, A.M.; Alosaimy, S.; Rybak, M.J. A Comparison of Daptomycin Alone and in Combination with Ceftaroline Fosamil for Methicillin-Resistant Staphylococcus aureus Bacteremia Complicated by Septic Pulmonary Emboli. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2020, 39, 2199–2203. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.; Huang, V.; Hartman, P.; Perri, M.B.; Moreno, D.; Zervos, M.J. Ceftaroline Fosamil Monotherapy for Methicillin-Resistant Staphylococcus aureus Bacteremia: A Comparative Clinical Outcomes Study. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2017, 57, 27–31. [Google Scholar] [CrossRef]
- Hornak, J.P.; Anjum, S.; Reynoso, D. Adjunctive Ceftaroline in Combination with Daptomycin or Vancomycin for Complicated Methicillin-Resistant Staphylococcus aureus Bacteremia after Monotherapy Failure. Ther. Adv. Infect. Dis. 2019, 6, 2049936119886504. [Google Scholar] [CrossRef] [PubMed]
- Polenakovik, H.M.; Pleiman, C.M. Ceftaroline for Meticillin-Resistant Staphylococcus aureus Bacteraemia: Case Series and Review of the Literature. Int. J. Antimicrob. Agents 2013, 42, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Tattevin, P.; Boutoille, D.; Vitrat, V.; Van Grunderbeeck, N.; Revest, M.; Dupont, M.; Alfandari, S.; Stahl, J.-P. Salvage Treatment of Methicillin-Resistant Staphylococcal Endocarditis with Ceftaroline: A Multicentre Observational Study. J. Antimicrob. Chemother. 2014, 69, 2010–2013. [Google Scholar] [CrossRef] [PubMed]
- Warren, E.F.; Crocker, R.J.; Tabor, B.; Pizzuti, M.; Tsai, Y.V.; Antosz, K.; Battle, S.; Ahuja, D.; Bookstaver, P.B. Successful Use of Nafcillin and Ceftaroline Combination Therapy for Persistent MSSA Bacteraemia and Endocarditis: A Case Series. JAC-Antimicrob. Resist. 2022, 5, dlac129. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Hung, W.-C.; Lo, S.-H.; Tseng, Y.-T.; Lu, P.-L. Successful Treatment with Daptomycin and Ceftaroline of Heterogeneous Vancomycin-Intermediate Staphylococcus aureus (hVISA) Endocarditis: A Case Report. J. Glob. Antimicrob. Resist. 2021, 27, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Jilani, T.N.; Masood, S.O. Ceftaroline Fosamil as an Alternative for a Severe Methicillin-Resistant Staphylococcus aureus Infection: A Case Report. Cureus 2018, 10, e3776. [Google Scholar] [CrossRef] [PubMed]
- Duss, F.-R.; Garcia de la Mària, C.; Croxatto, A.; Giulieri, S.; Lamoth, F.; Manuel, O.; Miró, J.M. Successful Treatment with Daptomycin and Ceftaroline of MDR Staphylococcus aureus Native Valve Endocarditis: A Case Report. J. Antimicrob. Chemother. 2019, 74, 2626–2630. [Google Scholar] [CrossRef] [PubMed]
- Sundaragiri, P.R.; Vallabhajosyula, S.; Haddad, T.M.; Esterbrooks, D.J. Tricuspid and Mitral Endocarditis Due to Methicillin-Resistant Staphylococcus aureus Exhibiting Vancomycin-Creep Phenomenon. BMJ Case Rep. 2015, 2015, bcr2015211974. [Google Scholar] [CrossRef]
- Cunha, B.A.; Gran, A. Successful Treatment of Meticillin-Resistant Staphylococcus aureus (MRSA) Aortic Prosthetic Valve Endocarditis with Prolonged High-Dose Daptomycin plus Ceftaroline Therapy. Int. J. Antimicrob. Agents 2015, 46, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Baxi, S.M.; Chan, D.; Jain, V. Daptomycin Non-Susceptible, Vancomycin-Intermediate Staphylococcus aureus Endocarditis Treated with Ceftaroline and Daptomycin: Case Report and Brief Review of the Literature. Infection 2015, 43, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Nonejuie, P.; Nizet, V.; Pogliano, J.; Crum-Cianflone, N.; Haddad, F. Treatment of High-Level Gentamicin-Resistant Enterococcus Faecalis Endocarditis with Daptomycin plus Ceftaroline. Antimicrob. Agents Chemother. 2013, 57, 4042–4045. [Google Scholar] [CrossRef]
- Jongsma, K.; Joson, J.; Heidari, A. Ceftaroline in the Treatment of Concomitant Methicillin-Resistant and Daptomycin-Non-Susceptible Staphylococcus aureus Infective Endocarditis and Osteomyelitis: Case Report. J. Antimicrob. Chemother. 2013, 68, 1444–1445. [Google Scholar] [CrossRef]
- Rose, W.E.; Schulz, L.T.; Andes, D.; Striker, R.; Berti, A.D.; Hutson, P.R.; Shukla, S.K. Addition of Ceftaroline to Daptomycin after Emergence of Daptomycin-Nonsusceptible Staphylococcus aureus during Therapy Improves Antibacterial Activity. Antimicrob. Agents Chemother. 2012, 56, 5296–5302. [Google Scholar] [CrossRef]
- Gritsenko, D.; Fedorenko, M.; Ruhe, J.J.; Altshuler, J. Combination Therapy With Vancomycin and Ceftaroline for Refractory Methicillin-Resistant Staphylococcus aureus Bacteremia: A Case Series. Clin. Ther. 2017, 39, 212–218. [Google Scholar] [CrossRef]
- Ho, T.T.; Cadena, J.; Childs, L.M.; Gonzalez-Velez, M.; Lewis, J.S. Methicillin-Resistant Staphylococcus aureus Bacteraemia and Endocarditis Treated with Ceftaroline Salvage Therapy. J. Antimicrob. Chemother. 2012, 67, 1267–1270. [Google Scholar] [CrossRef]
- Lin, J.C.; Aung, G.; Thomas, A.; Jahng, M.; Johns, S.; Fierer, J. The Use of Ceftaroline Fosamil in Methicillin-Resistant Staphylococcus aureus Endocarditis and Deep-Seated MRSA Infections: A Retrospective Case Series of 10 Patients. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2013, 19, 42–49. [Google Scholar] [CrossRef]
- Zasowski, E.J.; Trinh, T.D.; Claeys, K.C.; Casapao, A.M.; Sabagha, N.; Lagnf, A.M.; Klinker, K.P.; Davis, S.L.; Rybak, M.J. Multicenter Observational Study of Ceftaroline Fosamil for Methicillin-Resistant Staphylococcus aureus Bloodstream Infections. Antimicrob. Agents Chemother. 2017, 61, e02015-16. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.N.; Wardlow, L.C.; Coe, K.E.; Sobhanie, M.M.E. Clinical Outcomes With Definitive Treatment of Methicillin-Resistant Staphylococcus aureus Bacteremia With Retained Daptomycin and Ceftaroline Combination Therapy vs De-Escalation to Monotherapy With Vancomycin, Daptomycin, or Ceftaroline. Open Forum Infect. Dis. 2021, 8, ofab327. [Google Scholar] [CrossRef] [PubMed]
- Pani, A.; Colombo, F.; Agnelli, F.; Frantellizzi, V.; Baratta, F.; Pastori, D.; Scaglione, F. Off-Label Use of Ceftaroline Fosamil: A Systematic Review. Int. J. Antimicrob. Agents 2019, 54, 562–571. [Google Scholar] [CrossRef]
- Gatti, M.; Andreoni, M.; Pea, F.; Viale, P. Real-World Use of Dalbavancin in the Era of Empowerment of Outpatient Antimicrobial Treatment: A Careful Appraisal Beyond Approved Indications Focusing on Unmet Clinical Needs. Drug Des. Devel. Ther. 2021, 15, 3349–3378. [Google Scholar] [CrossRef]
- Zeng, D.; Debabov, D.; Hartsell, T.L.; Cano, R.J.; Adams, S.; Schuyler, J.A.; McMillan, R.; Pace, J.L. Approved Glycopeptide Antibacterial Drugs: Mechanism of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a026989. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Mendes, R.E.; Duncan, L.R.; Flamm, R.K.; Sader, H.S. Activity of Dalbavancin and Comparator Agents against Gram-Positive Cocci from Clinical Infections in the USA and Europe 2015-16. J. Antimicrob. Chemother. 2018, 73, 2748–2756. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Stefani, S.; Venditti, M.; Di Domenico, E.G. Biofilm-Related Infections in Gram-Positive Bacteria and the Potential Role of the Long-Acting Agent Dalbavancin. Front. Microbiol. 2021, 12, 749685. [Google Scholar] [CrossRef]
- Johnson, D.M.; Fritsche, T.R.; Sader, H.S.; Jones, R.N. Evaluation of Dalbavancin in Combination with Nine Antimicrobial Agents to Detect Enhanced or Antagonistic Interactions. Int. J. Antimicrob. Agents 2006, 27, 557–560. [Google Scholar] [CrossRef]
- Xhemali, X.; Smith, J.R.; Kebriaei, R.; Rice, S.A.; Stamper, K.C.; Compton, M.; Singh, N.B.; Jahanbakhsh, S.; Rybak, M.J. Evaluation of Dalbavancin Alone and in Combination with β-Lactam Antibiotics against Resistant Phenotypes of Staphylococcus aureus. J. Antimicrob. Chemother. 2019, 74, 82–86. [Google Scholar] [CrossRef]
- Candiani, G.; Abbondi, M.; Borgonovi, M.; Romanò, G.; Parenti, F. In-Vitro and in-Vivo Antibacterial Activity of BI 397, a New Semi-Synthetic Glycopeptide Antibiotic. J. Antimicrob. Chemother. 1999, 44, 179–192. [Google Scholar] [CrossRef]
- Lefort, A.; Pavie, J.; Garry, L.; Chau, F.; Fantin, B. Activities of Dalbavancin In Vitro and in a Rabbit Model of Experimental Endocarditis Due to Staphylococcus aureus with or without Reduced Susceptibility to Vancomycin and Teicoplanin. Antimicrob. Agents Chemother. 2004, 48, 1061–1064. [Google Scholar] [CrossRef]
- Volpicelli, L.; Venditti, M.; Oliva, A. Acute Bacterial Skin and Skin Structure Infections in Pediatric Patients: Potential Role of Dalbavancin. Expert Rev. Anti Infect. Ther. 2023, 21, 329–341. [Google Scholar] [CrossRef] [PubMed]
- EMA Xydalba. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xydalba (accessed on 11 September 2023).
- Bai, F.; Aldieri, C.; Cattelan, A.; Raumer, F.; Di Meco, E.; Moioli, M.C.; Tordato, F.; Morelli, P.; Borghi, F.; Rizzi, M.; et al. Efficacy and Safety of Dalbavancin in the Treatment of Acute Bacterial Skin and Skin Structure Infections (ABSSSIs) and Other Infections in a Real-Life Setting: Data from an Italian Observational Multicentric Study (DALBITA Study). Expert Rev. Anti Infect. Ther. 2020, 18, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Morrisette, T.; Miller, M.A.; Montague, B.T.; Barber, G.R.; McQueen, R.B.; Krsak, M. On- and off-Label Utilization of Dalbavancin and Oritavancin for Gram-Positive Infections. J. Antimicrob. Chemother. 2019, 74, 2405–2416. [Google Scholar] [CrossRef] [PubMed]
- Dinh, A.; Duran, C.; Pavese, P.; Khatchatourian, L.; Monnin, B.; Bleibtreu, A.; Denis, E.; Etienne, C.; Rouanes, N.; Mahieu, R.; et al. French National Cohort of First Use of Dalbavancin: A High Proportion of off-Label Use. Int. J. Antimicrob. Agents 2019, 54, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, S.; Krause, R.; Valentin, T.; Prattes, J.; Janata, O.; Lenger, A.; Bellmann-Weiler, R.; Weiss, G.; Zollner-Schwetz, I. Multicenter Clinical Experience of Real Life Dalbavancin Use in Gram-Positive Infections. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2019, 81, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Núñez, M.; Casas-Hidalgo, I.; García-Fumero, R.; Vallejo-Rodríguez, I.; Anguita-Santos, F.; Hernández-Quero, J.; Cabeza-Barrera, J.; Ruiz-Sancho, A. Dalbavancin Is a Novel Antimicrobial against Gram-Positive Pathogens: Clinical Experience beyond Labelled Indications. Eur. J. Hosp. Pharm. Sci. Pract. 2020, 27, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Durante-Mangoni, E.; Boccia, F.; Ursi, M.P.; Karruli, A.; Andini, R.; Galdo, M.; Zampino, R. Dalbavancin for Infective Endocarditis: A Single Centre Experience. J. Chemother. Florence Italy 2021, 33, 256–262. [Google Scholar] [CrossRef]
- Arrieta-Loitegui, M.; Caro-Teller, J.M.; Ortiz-Pérez, S.; López-Medrano, F.; San Juan-Garrido, R.; Ferrari-Piquero, J.M. Effectiveness, Safety and Cost Analysis of Dalbavancin in Clinical Practice. Eur. J. Hosp. Pharm. Sci. Pract. 2022, 29, 55–58. [Google Scholar] [CrossRef]
- Guleri, A.; More, R.; Sharma, R.; Wong, M.; Abdelrahman, A. Use of Dalbavancin in Infective Endocarditis: A Case Series. JAC-Antimicrob. Resist. 2021, 3, dlab099. [Google Scholar] [CrossRef]
- Taylor, K.; Williamson, J.; Luther, V.; Stone, T.; Johnson, J.; Gruss, Z.; Russ-Friedman, C.; Ohl, C.; Beardsley, J. Evaluating the Use of Dalbavancin for Off-Label Indications. Infect. Dis. Rep. 2022, 14, 266–272. [Google Scholar] [CrossRef]
- Bryson-Cahn, C.; Beieler, A.M.; Chan, J.D.; Harrington, R.D.; Dhanireddy, S. Dalbavancin as Secondary Therapy for Serious Staphylococcus aureus Infections in a Vulnerable Patient Population. Open Forum Infect. Dis. 2019, 6, ofz028. [Google Scholar] [CrossRef] [PubMed]
- Bork, J.T.; Heil, E.L.; Berry, S.; Lopes, E.; Davé, R.; Gilliam, B.L.; Amoroso, A. Dalbavancin Use in Vulnerable Patients Receiving Outpatient Parenteral Antibiotic Therapy for Invasive Gram-Positive Infections. Infect. Dis. Ther. 2019, 8, 171–184. [Google Scholar] [CrossRef]
- Ajaka, L.; Heil, E.; Schmalzle, S. Dalbavancin in the Treatment of Bacteremia and Endocarditis in People with Barriers to Standard Care. Antibiotics 2020, 9, 700. [Google Scholar] [CrossRef]
- Vazquez Deida, A.A.; Shihadeh, K.C.; Preslaski, C.R.; Young, H.L.; Wyles, D.L.; Jenkins, T.C. Use of a Standardized Dalbavancin Approach to Facilitate Earlier Hospital Discharge for Vulnerable Patients Receiving Prolonged Inpatient Antibiotic Therapy. Open Forum Infect. Dis. 2020, 7, ofaa293. [Google Scholar] [CrossRef]
- Veve, M.P.; Patel, N.; Smith, Z.A.; Yeager, S.D.; Wright, L.R.; Shorman, M.A. Comparison of Dalbavancin to Standard-of-Care for Outpatient Treatment of Invasive Gram-Positive Infections. Int. J. Antimicrob. Agents 2020, 56, 106210. [Google Scholar] [CrossRef]
- Lueking, R.; Wei, W.; Mang, N.S.; Ortwine, J.K.; Meisner, J. Evaluation of Dalbavancin Use on Clinical Outcomes, Cost-Savings, and Adherence at a Large Safety Net Hospital. Microbiol. Spectr. 2023, 11, e0238522. [Google Scholar] [CrossRef]
- Steele, J.M.; Seabury, R.W.; Hale, C.M.; Mogle, B.T. Unsuccessful Treatment of Methicillin-Resistant Staphylococcus aureus Endocarditis with Dalbavancin. J. Clin. Pharm. Ther. 2018, 43, 101–103. [Google Scholar] [CrossRef]
- Howard-Anderson, J.; Pouch, S.M.; Sexton, M.E.; Mehta, A.K.; Smith, A.L.; Lyon, G.M.; Friedman-Moraco, R. Left Ventricular Assist Device Infections and the Potential Role for Dalbavancin: A Case Report. Open Forum Infect. Dis. 2019, 6, ofz235. [Google Scholar] [CrossRef]
- Hakim, A.; Braun, H.; Thornton, D.; Strymish, J. Successful Treatment of Methicillin-Sensitive Staphylococcus aureus Tricuspid-Valve Endocarditis with Dalbavancin as an Outpatient in a Person Who Injects Drugs: A Case Report. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 91, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Teigell-Muñoz, F.J.; Mateos-González, M.; Bernal Hertfelder, E.; Sánchez de Torre, A.; García-Ferrón, M.; de Cáceres Velasco, C.; Bueno Muiño, C. A Dalbavancin for Successful Treatment of Infective Endocarditis Caused by Enterococcus Faecalis. Eur. J. Case Rep. Intern. Med. 2023, 10, 003654. [Google Scholar] [CrossRef]
- Kussmann, M.; Karer, M.; Obermueller, M.; Schmidt, K.; Barousch, W.; Moser, D.; Nehr, M.; Ramharter, M.; Poeppl, W.; Makristathis, A.; et al. Emergence of a Dalbavancin Induced Glycopeptide/Lipoglycopeptide Non-Susceptible Staphylococcus aureus during Treatment of a Cardiac Device-Related Endocarditis. Emerg. Microbes Infect. 2018, 7, 202. [Google Scholar] [CrossRef]
- Spaziante, M.; Franchi, C.; Taliani, G.; D’Avolio, A.; Pietropaolo, V.; Biliotti, E.; Esvan, R.; Venditti, M. Serum Bactericidal Activity Levels Monitor to Guide Intravenous Dalbavancin Chronic Suppressive Therapy of Inoperable Staphylococcal Prosthetic Valve Endocarditis: A Case Report. Open Forum Infect. Dis. 2019, 6, ofz427. [Google Scholar] [CrossRef]
- Hitzenbichler, F.; Mohr, A.; Camboni, D.; Simon, M.; Salzberger, B.; Hanses, F. Dalbavancin as Long-Term Suppressive Therapy for Patients with Gram-Positive Bacteremia Due to an Intravascular Source-a Series of Four Cases. Infection 2021, 49, 181–186. [Google Scholar] [CrossRef]
- Bouza, E.; Valerio, M.; Soriano, A.; Morata, L.; Carus, E.G.; Rodríguez-González, C.; Hidalgo-Tenorio, M.C.; Plata, A.; Muñoz, P.; Vena, A.; et al. Dalbavancin in the Treatment of Different Gram-Positive Infections: A Real-Life Experience. Int. J. Antimicrob. Agents 2018, 51, 571–577. [Google Scholar] [CrossRef]
- Cooper, M.M.; Preslaski, C.R.; Shihadeh, K.C.; Hawkins, K.L.; Jenkins, T.C. Multiple-Dose Dalbavancin Regimens as the Predominant Treatment of Deep-Seated or Endovascular Infections: A Scoping Review. Open Forum Infect. Dis. 2021, 8, ofab486. [Google Scholar] [CrossRef]
- Thomas, G.; Henao-Martínez, A.F.; Franco-Paredes, C.; Chastain, D.B. Treatment of Osteoarticular, Cardiovascular, Intravascular-Catheter-Related and Other Complicated Infections with Dalbavancin and Oritavancin: A Systematic Review. Int. J. Antimicrob. Agents 2020, 56, 106069. [Google Scholar] [CrossRef]
- Bouza, E.; Burillo, A. Oritavancin: A Novel Lipoglycopeptide Active against Gram-Positive Pathogens Including Multiresistant Strains. Int. J. Antimicrob. Agents 2010, 36, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Calic, D.; Schweizer, F.; Zelenitsky, S.; Adam, H.; Lagacé-Wiens, P.R.S.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; Karlowsky, J.A. New Lipoglycopeptides: A Comparative Review of Dalbavancin, Oritavancin and Telavancin. Drugs 2010, 70, 859–886. [Google Scholar] [CrossRef]
- Yan, Q.; Karau, M.J.; Patel, R. In Vitro Activity of Oritavancin against Planktonic and Biofilm States of Vancomycin-Susceptible and Vancomycin-Resistant Enterococci. Diagn. Microbiol. Infect. Dis. 2018, 91, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.G.; Lemaire, S.; Kahl, B.C.; Becker, K.; Proctor, R.A.; Denis, O.; Tulkens, P.M.; Van Bambeke, F. Pharmacodynamic Evaluation of the Activity of Antibiotics against Hemin- and Menadione-Dependent Small-Colony Variants of Staphylococcus aureus in Models of Extracellular (Broth) and Intracellular (THP-1 Monocytes) Infections. Antimicrob. Agents Chemother. 2012, 56, 3700–3711. [Google Scholar] [CrossRef]
- Wu, T.; Meyer, K.; Harrington, A.T.; Danziger, L.H.; Wenzler, E. In Vitro Activity of Oritavancin Alone or in Combination against Vancomycin-Susceptible and -Resistant Enterococci. J. Antimicrob. Chemother. 2019, 74, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Lagatolla, C.; Mehat, J.W.; La Ragione, R.M.; Luzzati, R.; Di Bella, S. In Vitro and In Vivo Studies of Oritavancin and Fosfomycin Synergism against Vancomycin-Resistant Enterococcus Faecium. Antibiotics 2022, 11, 1334. [Google Scholar] [CrossRef]
- Kaatz, G.W.; Seo, S.M.; Aeschlimann, J.R.; Houlihan, H.H.; Mercier, R.C.; Rybak, M.J. Efficacy of LY333328 against Experimental Methicillin-Resistant Staphylococcus aureus Endocarditis. Antimicrob. Agents Chemother. 1998, 42, 981–983. [Google Scholar] [CrossRef]
- Saleh-Mghir, A.; Lefort, A.; Petegnief, Y.; Dautrey, S.; Vallois, J.M.; Le Guludec, D.; Carbon, C.; Fantin, B. Activity and Diffusion of LY333328 in Experimental Endocarditis Due to Vancomycin-Resistant Enterococcus Faecalis. Antimicrob. Agents Chemother. 1999, 43, 115–120. [Google Scholar] [CrossRef]
- Lefort, A.; Saleh-Mghir, A.; Garry, L.; Carbon, C.; Fantin, B. Activity of LY333328 Combined with Gentamicin in Vitro and in Rabbit Experimental Endocarditis Due to Vancomycin-Susceptible or -Resistant Enterococcus Faecalis. Antimicrob. Agents Chemother. 2000, 44, 3017–3021. [Google Scholar] [CrossRef]
- EMA Tenkasi (Previously Orbactiv). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tenkasi-previously-orbactiv (accessed on 11 September 2023).
- Lupia, T.; De Benedetto, I.; Bosio, R.; Shbaklo, N.; De Rosa, F.G.; Corcione, S. Role of Oritavancin in the Treatment of Infective Endocarditis, Catheter- or Device-Related Infections, Bloodstream Infections, and Bone and Prosthetic Joint Infections in Humans: Narrative Review and Possible Developments. Life 2023, 13, 959. [Google Scholar] [CrossRef]
- Bloem, A.; Bax, H.I.; Yusuf, E.; Verkaik, N.J. New-Generation Antibiotics for Treatment of Gram-Positive Infections: A Review with Focus on Endocarditis and Osteomyelitis. J. Clin. Med. 2021, 10, 1743. [Google Scholar] [CrossRef]
- Ahiskali, A.; Rhodes, H. Oritavancin for the Treatment of Complicated Gram-Positive Infection in Persons Who Inject Drugs. BMC Pharmacol. Toxicol. 2020, 21, 73. [Google Scholar] [CrossRef]
- Johnson, J.A.; Feeney, E.R.; Kubiak, D.W.; Corey, G.R. Prolonged Use of Oritavancin for Vancomycin-Resistant Enterococcus Faecium Prosthetic Valve Endocarditis. Open Forum Infect. Dis. 2015, 2, ofv156. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.L.; Turner, M.S.; Frens, J.J.; Snider, C.B.; Smith, J.R. Real-World Experience with Oritavancin Therapy in Invasive Gram-Positive Infections. Infect. Dis. Ther. 2017, 6, 277–289. [Google Scholar] [CrossRef]
- Salcedo, D.A.T.; El-Herte, R.; Granada, M. Oritavancin for the Treatment of Infective Endocarditis Due to Gram-Positive Organism. Ann. Case Rep. 2018. [Google Scholar] [CrossRef]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Veganzones, J.; Montero, A.; Maseda, E. New Evidence on the Use of Fosfomycin for Bacteremia and Infectious Endocarditis. Rev. Esp. Quimioter. 2019, 32 (Suppl. S1), 25–29. [Google Scholar] [PubMed]
- Sojo-Dorado, J.; López-Hernández, I.; Rosso-Fernandez, C.; Morales, I.M.; Palacios-Baena, Z.R.; Hernández-Torres, A.; Merino de Lucas, E.; Escolà-Vergé, L.; Bereciartua, E.; García-Vázquez, E.; et al. Effectiveness of Fosfomycin for the Treatment of Multidrug-Resistant Escherichia Coli Bacteremic Urinary Tract Infections: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2137277. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Volpicelli, L.; Di Bari, S.; Curtolo, A.; Borrazzo, C.; Cogliati Dezza, F.; Cona, A.; Agrenzano, S.; Mularoni, A.; Trancassini, M.; et al. Effect of Ceftazidime/Avibactam plus Fosfomycin Combination on 30 Day Mortality in Patients with Bloodstream Infections Caused by KPC-Producing Klebsiella Pneumoniae: Results from a Multicentre Retrospective Study. JAC-Antimicrob. Resist. 2022, 4, dlac121. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Al Ismail, D.; Arcari, G.; Miele, M.C.; Casali, E.; Sacco, F.; Volpicelli, L.; De Angelis, M.; Mascellino, M.T.; Cancelli, F.; et al. Ceftazidime/Avibactam-Resistant Meropenem-Susceptible KPC-Producing Klebsiella Pneumoniae: Analysis of Cases and Evaluation of in Vitro Activity of Fosfomycin-Containing Combinations. J. Glob. Antimicrob. Resist. 2023, 33, 321–327. [Google Scholar] [CrossRef]
- Tseng, T.-C.; Chuang, Y.-C.; Yang, J.-L.; Lin, C.-Y.; Huang, S.-H.; Wang, J.-T.; Chen, Y.-C.; Chang, S.-C. The Combination of Daptomycin with Fosfomycin Is More Effective than Daptomycin Alone in Reducing Mortality of Vancomycin-Resistant Enterococcal Bloodstream Infections: A Retrospective, Comparative Cohort Study. Infect. Dis. Ther. 2023, 12, 589–606. [Google Scholar] [CrossRef]
- Antonello, R.M.; Principe, L.; Maraolo, A.E.; Viaggi, V.; Pol, R.; Fabbiani, M.; Montagnani, F.; Lovecchio, A.; Luzzati, R.; Di Bella, S. Fosfomycin as Partner Drug for Systemic Infection Management. A Systematic Review of Its Synergistic Properties from In Vitro and In Vivo Studies. Antibiotics 2020, 9, 500. [Google Scholar] [CrossRef]
- Antonello, R.M.; Canetti, D.; Riccardi, N. Daptomycin Synergistic Properties from In Vitro and In Vivo Studies: A Systematic Review. J. Antimicrob. Chemother. 2022, 78, 52–77. [Google Scholar] [CrossRef]
- García-de-la-Mària, C.; Gasch, O.; García-Gonzalez, J.; Soy, D.; Shaw, E.; Ambrosioni, J.; Almela, M.; Pericàs, J.M.; Tellez, A.; Falces, C.; et al. The Combination of Daptomycin and Fosfomycin Has Synergistic, Potent, and Rapid Bactericidal Activity against Methicillin-Resistant Staphylococcus aureus in a Rabbit Model of Experimental Endocarditis. Antimicrob. Agents Chemother. 2018, 62, e02633-17. [Google Scholar] [CrossRef] [PubMed]
- García-de-la-Mària, C.; Gasch, O.; Castañeda, X.; García-González, J.; Soy, D.; Cañas, M.-A.; Ambrosioni, J.; Almela, M.; Pericàs, J.M.; Téllez, A.; et al. Cloxacillin or Fosfomycin plus Daptomycin Combinations Are More Active than Cloxacillin Monotherapy or Combined with Gentamicin against MSSA in a Rabbit Model of Experimental Endocarditis. J. Antimicrob. Chemother. 2020, 75, 3586–3592. [Google Scholar] [CrossRef] [PubMed]
- Debbia, E.; Pesce, A.; Schito, G.C. In Vitro Activity of LY146032 Alone and in Combination with Other Antibiotics against Gram-Positive Bacteria. Antimicrob. Agents Chemother. 1988, 32, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Berti, A.D.; Sakoulas, G.; Nizet, V.; Tewhey, R.; Rose, W.E. β-Lactam Antibiotics Targeting PBP1 Selectively Enhance Daptomycin Activity against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 5005–5012. [Google Scholar] [CrossRef]
- Berti, A.D.; Theisen, E.; Sauer, J.-D.; Nonejuie, P.; Olson, J.; Pogliano, J.; Sakoulas, G.; Nizet, V.; Proctor, R.A.; Rose, W.E. Penicillin Binding Protein 1 Is Important in the Compensatory Response of Staphylococcus aureus to Daptomycin-Induced Membrane Damage and Is a Potential Target for β-Lactam-Daptomycin Synergy. Antimicrob. Agents Chemother. 2016, 60, 451–458. [Google Scholar] [CrossRef]
- Gasch, O.; Pillai, S.K.; Dakos, J.; Miyakis, S.; Moellering, R.C.; Eliopoulos, G.M. Daptomycin In Vitro Activity against Methicillin-Resistant Staphylococcus aureus Is Enhanced by d-Cycloserine in a Mechanism Associated with a Decrease in Cell Surface Charge. Antimicrob. Agents Chemother. 2013, 57, 4537–4539. [Google Scholar] [CrossRef]
- Mishra, N.N.; Lew, C.; Abdelhady, W.; Lapitan, C.K.; Proctor, R.A.; Rose, W.E.; Bayer, A.S. Synergy Mechanisms of Daptomycin-Fosfomycin Combinations in Daptomycin-Susceptible and -Resistant Methicillin-Resistant Staphylococcus aureus: In Vitro, Ex Vivo, and In Vivo Metrics. Antimicrob. Agents Chemother. 2022, 66, e0164921. [Google Scholar] [CrossRef]
- Courcol, R.J.; Martin, G.R. In-Vitro Activity of the Combination of Ceftriaxone and Fosfomycin against Staphylococci. J. Antimicrob. Chemother. 1987, 19, 276–278. [Google Scholar] [CrossRef]
- Duez, J.M.; Kohli, E.; Pechinot, A.; Tremeaux, J.C.; Kazmierczak, A. Combination between fosfomycin and oxacillin or cefotaxime against methicillin-resistant Staphylococci and Enterococci. Pathol. Biol. 1983, 31, 515–518. [Google Scholar]
- Utsui, Y.; Ohya, S.; Magaribuchi, T.; Tajima, M.; Yokota, T. Antibacterial Activity of Cefmetazole Alone and in Combination with Fosfomycin against Methicillin- and Cephem-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 1986, 30, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Grif, K.; Dierich, M.P.; Pfaller, K.; Miglioli, P.A.; Allerberger, F. In Vitro Activity of Fosfomycin in Combination with Various Antistaphylococcal Substances. J. Antimicrob. Chemother. 2001, 48, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Del Río, A.; García-de-la-Mària, C.; Entenza, J.M.; Gasch, O.; Armero, Y.; Soy, D.; Mestres, C.A.; Pericás, J.M.; Falces, C.; Ninot, S.; et al. Fosfomycin plus β-Lactams as Synergistic Bactericidal Combinations for Experimental Endocarditis Due to Methicillin-Resistant and Glycopeptide-Intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 2016, 60, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Guggenbichler, J.P.; Berchtold, D.; Allerberger, F.; Bonatti, H.; Hager, J.; Pfaller, W.; Dierich, M.P. In Vitro and in Vivo Effect of Antibiotics on Catheters Colonized by Staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 1992, 11, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.-J.; Chen, C.-C.; Zhang, C.-C.; Su, B.-A.; Li, C.-M.; Weng, T.-C.; Chiang, S.-R.; Ko, W.-C.; Chuang, Y.-C. In Vitro Efficacy of Fosfomycin-Based Combinations against Clinical Vancomycin-Resistant Enterococcus Isolates. Diagn. Microbiol. Infect. Dis. 2013, 77, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Farina, C.; Russello, G.; Chinello, P.; Pasticci, M.B.; Raglio, A.; Ravasio, V.; Rizzi, M.; Scarparo, C.; Vailati, F.; Suter, F.; et al. In Vitro Activity Effects of Twelve Antibiotics Alone and in Association against Twenty-Seven Enterococcus Faecalis Strains Isolated from Italian Patients with Infective Endocarditis: High in Vitro Synergistic Effect of the Association Ceftriaxone-Fosfomycin. Chemotherapy 2011, 57, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Moreno, M.; Trampuz, A.; Di Luca, M. Synergistic Antibiotic Activity against Planktonic and Biofilm-Embedded Streptococcus Agalactiae, Streptococcus Pyogenes and Streptococcus Oralis. J. Antimicrob. Chemother. 2017, 72, 3085–3092. [Google Scholar] [CrossRef]
- Vicente, M.V.; Olay, T.; Rodríguez, A. Experimental Endocarditis Caused by Streptococcus Sanguis: Single and Combined Antibiotic Therapy. Antimicrob. Agents Chemother. 1981, 20, 10–14. [Google Scholar] [CrossRef]
- Olay, T.; Rodríguez, A.; Oliver, L.E.; Vicente, M.V.; Quecedo, M.C. Interaction of Fosfomycin with Other Antimicrobial Agents: In Vitro and in Vivo Studies. J. Antimicrob. Chemother. 1978, 4, 569–576. [Google Scholar] [CrossRef]
- Rice, L.B.; Eliopoulos, C.T.; Yao, J.D.; Eliopoulos, G.M.; Moellering, R.C. In Vivo Activity of the Combination of Daptomycin and Fosfomycin Compared with Daptomycin Alone against a Strain of Enterococcus Faecalis with High-Level Gentamicin Resistance in the Rat Endocarditis Model. Diagn. Microbiol. Infect. Dis. 1992, 15, 173–176. [Google Scholar] [CrossRef]
- Garrigós, C.; Murillo, O.; Lora-Tamayo, J.; Verdaguer, R.; Tubau, F.; Cabellos, C.; Cabo, J.; Ariza, J. Fosfomycin-Daptomycin and Other Fosfomycin Combinations as Alternative Therapies in Experimental Foreign-Body Infection by Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Mihailescu, R.; Furustrand Tafin, U.; Corvec, S.; Oliva, A.; Betrisey, B.; Borens, O.; Trampuz, A. High Activity of Fosfomycin and Rifampin against Methicillin-Resistant Staphylococcus aureus Biofilm in Vitro and in an Experimental Foreign-Body Infection Model. Antimicrob. Agents Chemother. 2014, 58, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Furustrand Tafin, U.; Maiolo, E.M.; Jeddari, S.; Bétrisey, B.; Trampuz, A. Activities of Fosfomycin and Rifampin on Planktonic and Adherent Enterococcus Faecalis Strains in an Experimental Foreign-Body Infection Model. Antimicrob. Agents Chemother. 2014, 58, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Descourouez, J.L.; Jorgenson, M.R.; Wergin, J.E.; Rose, W.E. Fosfomycin Synergy in Vitro with Amoxicillin, Daptomycin, and Linezolid against Vancomycin-Resistant Enterococcus Faecium from Renal Transplant Patients with Infected Urinary Stents. Antimicrob. Agents Chemother. 2013, 57, 1518–1520. [Google Scholar] [CrossRef] [PubMed]
- EMA Fosfomycin-Containing Medicinal Products. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/fosfomycin-containing-medicinal-products (accessed on 28 September 2023).
- Aoyagi, S.; Kawara, T.; Mizoguchi, T.; Ando, F.; Yanai, T.; Yamamoto, E.; Suzuki, K. Methicillin-Resistant Staphylococcus aureus Endocarditis Following Patch Closure of a Ventricular Septal Defect: Report of a Case. Surg. Today 1994, 24, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Del Río, A.; Gasch, O.; Moreno, A.; Peña, C.; Cuquet, J.; Soy, D.; Mestres, C.A.; Suárez, C.; Pare, J.C.; Tubau, F.; et al. Efficacy and Safety of Fosfomycin plus Imipenem as Rescue Therapy for Complicated Bacteremia and Endocarditis Due to Methicillin-Resistant Staphylococcus aureus: A Multicenter Clinical Trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 59, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Pericàs, J.M.; Moreno, A.; Almela, M.; García-de-la-Mària, C.; Marco, F.; Muñoz, P.; Peña, C.; de Alarcón, A.; Del Río, A.; Eworo, A.; et al. Efficacy and Safety of Fosfomycin plus Imipenem versus Vancomycin for Complicated Bacteraemia and Endocarditis Due to Methicillin-Resistant Staphylococcus aureus: A Randomized Clinical Trial. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2018, 24, 673–676. [Google Scholar] [CrossRef]
- Rieg, S.; Ernst, A.; Peyerl-Hoffmann, G.; Joost, I.; Camp, J.; Hellmich, M.; Kern, W.V.; Kaasch, A.J.; Seifert, H. Combination Therapy with Rifampicin or Fosfomycin in Patients with Staphylococcus aureus Bloodstream Infection at High Risk for Complications or Relapse: Results of a Large Prospective Observational Cohort. J. Antimicrob. Chemother. 2020, 75, 2282–2290. [Google Scholar] [CrossRef]
- Rieg, S.; Joost, I.; Weiß, V.; Peyerl-Hoffmann, G.; Schneider, C.; Hellmich, M.; Seifert, H.; Kern, W.V.; Kaasch, A. Combination Antimicrobial Therapy in Patients with Staphylococcus aureus Bacteraemia-a Post Hoc Analysis in 964 Prospectively Evaluated Patients. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2017, 23, 406.e1–406.e8. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Huang, C.-H.; Kuo, S.-C.; Hsiao, C.-Y.; Lin, M.-L.; Wang, F.-D.; Fung, C.-P. High-Dose Daptomycin and Fosfomycin Treatment of a Patient with Endocarditis Caused by Daptomycin-Nonsusceptible Staphylococcus aureus: Case Report. BMC Infect. Dis. 2011, 11, 152. [Google Scholar] [CrossRef]
- Miró, J.M.; Entenza, J.M.; Del Río, A.; Velasco, M.; Castañeda, X.; Garcia de la Mària, C.; Giddey, M.; Armero, Y.; Pericàs, J.M.; Cervera, C.; et al. High-Dose Daptomycin plus Fosfomycin Is Safe and Effective in Treating Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus Endocarditis. Antimicrob. Agents Chemother. 2012, 56, 4511–4515. [Google Scholar] [CrossRef]
- Vergara-López, S.; Domínguez, M.C.; Conejo, M.C.; Pascual, Á.; Rodríguez-Baño, J. Prolonged Treatment with Large Doses of Fosfomycin plus Vancomycin and Amikacin in a Case of Bacteraemia Due to Methicillin-Resistant Staphylococcus Epidermidis and IMP-8 Metallo-β-Lactamase-Producing Klebsiella Oxytoca. J. Antimicrob. Chemother. 2015, 70, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Gould, F.K. Oral Antibiotics for Infective Endocarditis: A Clinical Review. J. Antimicrob. Chemother. 2020, 75, 2021–2027. [Google Scholar] [CrossRef]
- Stamboulian, D.; Bonvehi, P.; Arevalo, C.; Bologna, R.; Cassetti, I.; Scilingo, V.; Efron, E. Antibiotic Management of Outpatients with Endocarditis Due to Penicillin-Susceptible Streptococci. Rev. Infect. Dis. 1991, 13 (Suppl. S2), S160–S163. [Google Scholar] [CrossRef] [PubMed]
- Heldman, A.W.; Hartert, T.V.; Ray, S.C.; Daoud, E.G.; Kowalski, T.E.; Pompili, V.J.; Sisson, S.D.; Tidmore, W.C.; vom Eigen, K.A.; Goodman, S.N.; et al. Oral Antibiotic Treatment of Right-Sided Staphylococcal Endocarditis in Injection Drug Users: Prospective Randomized Comparison with Parenteral Therapy. Am. J. Med. 1996, 101, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Demonchy, E.; Dellamonica, P.; Roger, P.M.; Bernard, E.; Cua, E.; Pulcini, C. Audit of Antibiotic Therapy Used in 66 Cases of Endocarditis. Med. Mal. Infect. 2011, 41, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Tissot-Dupont, H.; Gouriet, F.; Oliver, L.; Jamme, M.; Casalta, J.-P.; Jimeno, M.-T.; Arregle, F.; Lavoute, C.; Hubert, S.; Philip, M.; et al. High-Dose Trimethoprim-Sulfamethoxazole and Clindamycin for Staphylococcus aureus Endocarditis. Int. J. Antimicrob. Agents 2019, 54, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Iversen, K.; Ihlemann, N.; Gill, S.U.; Madsen, T.; Elming, H.; Jensen, K.T.; Bruun, N.E.; Høfsten, D.E.; Fursted, K.; Christensen, J.J.; et al. Partial Oral versus Intravenous Antibiotic Treatment of Endocarditis. N. Engl. J. Med. 2019, 380, 415–424. [Google Scholar] [CrossRef]
- Pries-Heje, M.M.; Wiingaard, C.; Ihlemann, N.; Gill, S.U.; Bruun, N.E.; Elming, H.; Povlsen, J.A.; Madsen, T.; Jensen, K.T.; Fursted, K.; et al. Five-Year Outcomes of the Partial Oral Treatment of Endocarditis (POET) Trial. N. Engl. J. Med. 2022, 386, 601–602. [Google Scholar] [CrossRef]
- Attanasio, V.; Di Luca, M.; Carozza, A.; Severino, S.; Pallotto, C.; Capoluongo, N.; Palmiero, G.; Bernardo, M.; Tascini, C. Clinical Efficacy of Amoxicillin/Clavulanate plus Cefditoren as de-Escalation Combination Therapy for Endocarditis Due to Strongly Biofilm-Forming Enterococcus Faecalis. Infect. Dis. Lond. Engl. 2020, 52, 376–379. [Google Scholar] [CrossRef]
- Colli, A.; Campodonico, R.; Gherli, T. Early Switch from Vancomycin to Oral Linezolid for Treatment of Gram-Positive Heart Valve Endocarditis. Ann. Thorac. Surg. 2007, 84, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Lemaignen, A.; Bernard, L.; Tattevin, P.; Bru, J.-P.; Duval, X.; Hoen, B.; Brunet-Houdard, S.; Mainardi, J.-L.; Caille, A. Oral Switch versus Standard Intravenous Antibiotic Therapy in Left-Sided Endocarditis Due to Susceptible Staphylococci, Streptococci or Enterococci (RODEO): A Protocol for Two Open-Label Randomised Controlled Trials. BMJ Open 2020, 10, e033540. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.C.; Basuino, L.; Dip, E.C.; Chambers, H.F. Comparative Efficacies of Tedizolid Phosphate, Vancomycin, and Daptomycin in a Rabbit Model of Methicillin-Resistant Staphylococcus aureus Endocarditis. Antimicrob. Agents Chemother. 2015, 59, 3252–3256. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.V.; Arias, C.A.; Murray, B.E. Tedizolid as Step-Down Therapy Following Daptomycin versus Continuation of Daptomycin against Enterococci and Methicillin- and Vancomycin-Resistant Staphylococcus aureus in a Rat Endocarditis Model. Antimicrob. Agents Chemother. 2020, 64, e02303-19. [Google Scholar] [CrossRef]
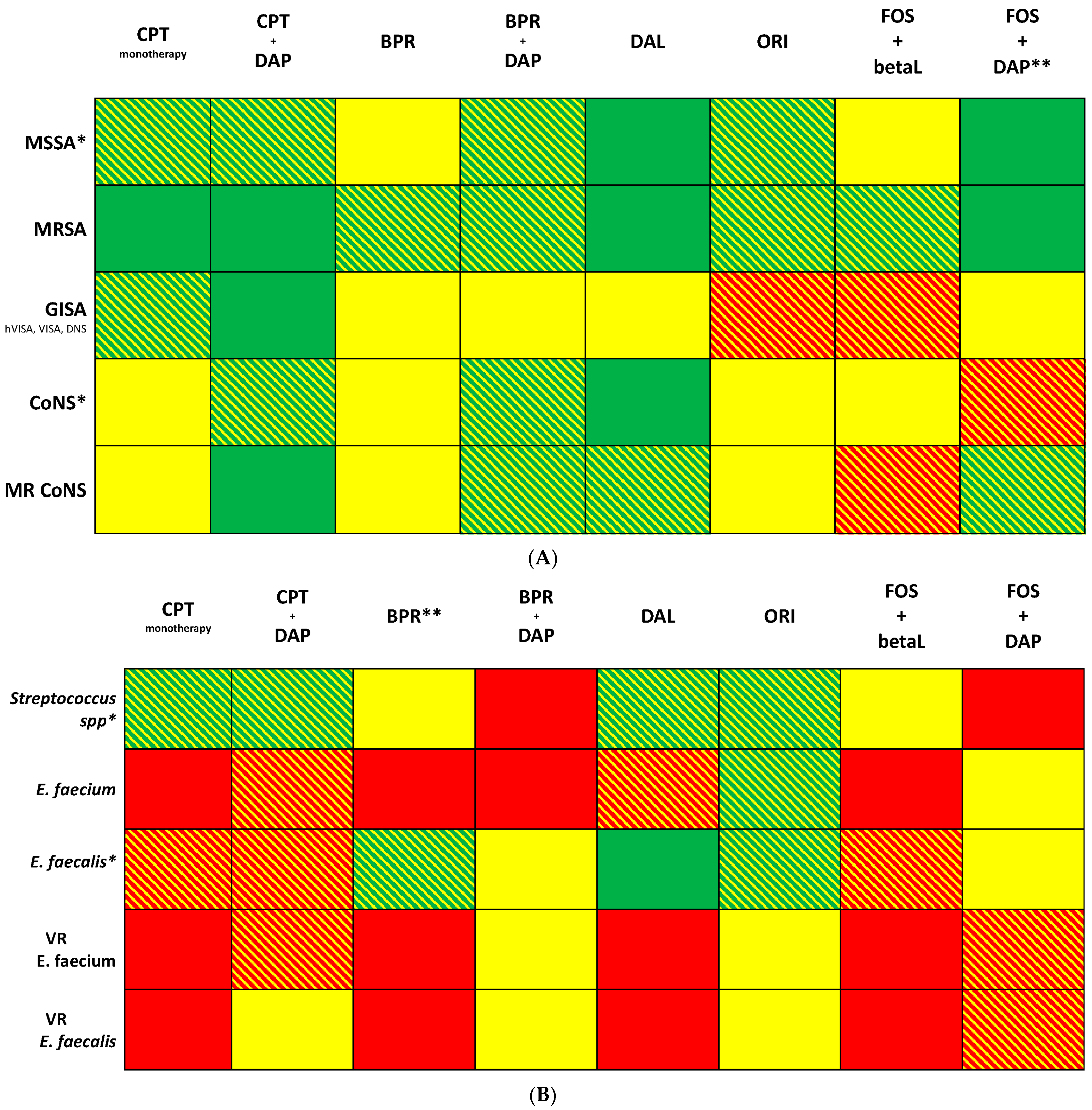
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, A.; Cogliati Dezza, F.; Cancelli, F.; Curtolo, A.; Falletta, A.; Volpicelli, L.; Venditti, M. New Antimicrobials and New Therapy Strategies for Endocarditis: Weapons That Should Be Defended. J. Clin. Med. 2023, 12, 7693. https://doi.org/10.3390/jcm12247693
Oliva A, Cogliati Dezza F, Cancelli F, Curtolo A, Falletta A, Volpicelli L, Venditti M. New Antimicrobials and New Therapy Strategies for Endocarditis: Weapons That Should Be Defended. Journal of Clinical Medicine. 2023; 12(24):7693. https://doi.org/10.3390/jcm12247693
Chicago/Turabian StyleOliva, Alessandra, Francesco Cogliati Dezza, Francesca Cancelli, Ambrogio Curtolo, Antonio Falletta, Lorenzo Volpicelli, and Mario Venditti. 2023. "New Antimicrobials and New Therapy Strategies for Endocarditis: Weapons That Should Be Defended" Journal of Clinical Medicine 12, no. 24: 7693. https://doi.org/10.3390/jcm12247693
APA StyleOliva, A., Cogliati Dezza, F., Cancelli, F., Curtolo, A., Falletta, A., Volpicelli, L., & Venditti, M. (2023). New Antimicrobials and New Therapy Strategies for Endocarditis: Weapons That Should Be Defended. Journal of Clinical Medicine, 12(24), 7693. https://doi.org/10.3390/jcm12247693





