Radiographic Evaluation and Changes in Bone Density of the Humeral Side after Reverse Total Shoulder Arthroplasty
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Evaluation Items
2.2.1. Radiographic Evaluation
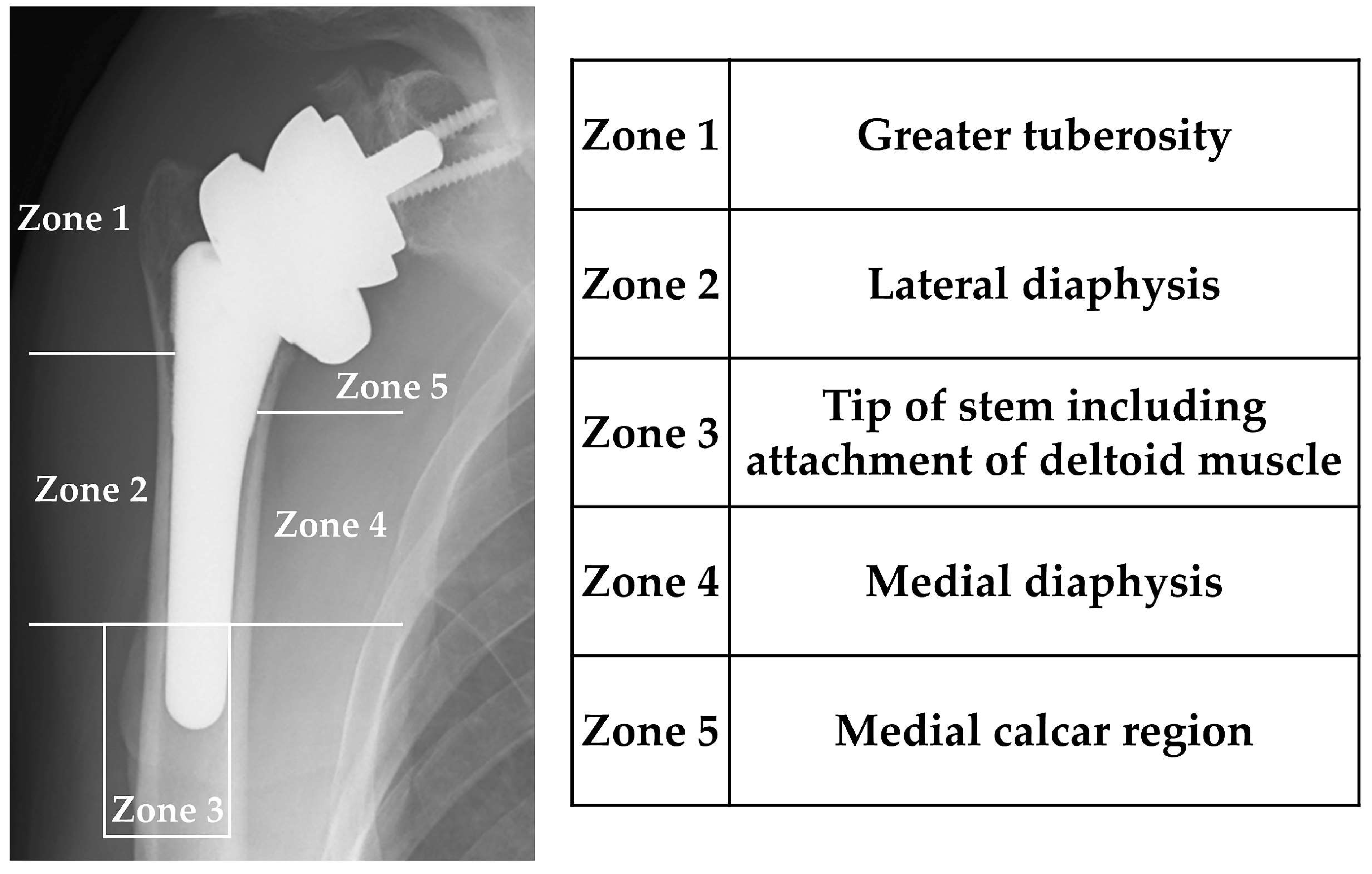
2.2.2. Postoperative Change in Bone Density around the Stem
Change Rate at 1 Year after Surgery
Factors Contributing to Bone Density in Each Zone at 1 Year Postoperatively
2.3. Statistical Analysis
3. Results
3.1. Evaluation at 1 Year after Surgery Using Plain Radiography
3.2. Bone Density Changes around the Stem
3.2.1. Rate of Change in Bone Density in Each Zone at 1 Year after Surgery, Compared to 2 Weeks after Surgery
3.2.2. Factors Associated with Bone Density at 1 Year after Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boileau, P. Complications and revision of reverse total shoulder arthroplasty. Orthop. Traumatol. Surg. Res. 2016, 102, S33–S43. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Suenaga, N.; Oizumi, N.; Yamaguchi, H.; Miyoshi, N.; Taniguchi, N.; Morita, S.; Munemoto, M.; Kurata, S.; Tanaka, Y. Humeral bone resorption after reverse shoulder arthroplasty using uncemented stem. JSES Int. 2020, 4, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Song, B.W.; Kim, S.H.; Choi, J.A.; Lee, J.W.; Chung, S.W.; Rhie, T.Y. The measurement of bone mineral density of bilateral proximal humeri using DXA in patients with unilateral rotator cuff tear. Osteoporos. Int. 2014, 25, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Song, B.W.; Lee, Y.S. Measurement of volumetric bone mineral density in proximal humerus using quantitative computed tomography in patients with unilateral rotator cuff tear. J. Shoulder Elbow. Surg. 2014, 23, 993–1002. [Google Scholar] [CrossRef]
- Sköldenberg, O.G.; Sjöö, H.; Kelly-Pettersson, P.; Bodén, H.; Eisler, T.; Stark, A.; Muren, O. Good stability but high periprosthetic bone mineral loss and late-occurring periprosthetic fractures with use of uncemented tapered femoral stems in patients with a femoral neck fracture. Acta Orthop. 2014, 85, 396–402. [Google Scholar] [CrossRef]
- Leonardsson, O.; Kärrholm, J.; Åkesson, K.; Garellick, G.; Rogmark, C. Higher risk of reoperation for bipolar and uncemented hemiarthroplasty. Acta Orthop. 2012, 83, 459–466. [Google Scholar] [CrossRef]
- Lindahl, H. Epidemiology of periprosthetic femur fracture around a total hip arthroplasty. Injury 2007, 38, 651–654. [Google Scholar] [CrossRef]
- Gundry, M.; Hopkins, S.; Knapp, K. A review on bone mineral density loss in total knee replacements leading to increased fracture risk. Clin. Rev. Bone Miner. Metab. 2017, 15, 162–174. [Google Scholar] [CrossRef]
- Inaba, Y.; Kobayashi, N.; Oba, M.; Ike, H.; Kubota, S.; Saito, T. Difference in postoperative periprosthetic bone mineral density changes between 3 major designs of uncemented stems: A 3-year follow-up study. J. Arthroplast. 2016, 31, 1836–1841. [Google Scholar] [CrossRef]
- Morita, A.; Kobayashi, N.; Choe, H.; Tezuka, T.; Higashihira, S.; Inaba, Y. Preoperative factors predicting the severity of BMD loss around the implant after total hip arthroplasty. BMC Musculoskelet. Disord. 2021, 22, 290. [Google Scholar] [CrossRef]
- Hayaishi, Y.; Miki, H.; Nishii, T.; Hananouchi, T.; Yoshikawa, H.; Sugano, N. Proximal femoral bone mineral density after resurfacing total hip arthroplasty and after standard stem-type cementless total hip arthroplasty, both having similar neck preservation and the same articulation type. J. Arthroplast. 2007, 22, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Pitto, R.P.; Bhargava, A.; Pandit, S.; Munro, J.T. Retroacetabular stress-shielding in THA. Clin. Orthop. Relat. Res. 2008, 466, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Holm, C.E.; Lind, T.; Flivik, G.; Odgaard, A.; Petersen, M.M. Bone remodeling and implant migration of uncemented femoral and cemented asymmetrical tibial components in total knee arthroplasty-DXA and RSA evaluation with 2-year follow up. Knee Surg. Relat. Res. 2021, 33, 25. [Google Scholar] [CrossRef] [PubMed]
- Jaroma, A.; Soininvaara, T.; Kröger, H. Periprosthetic tibial bone mineral density changes after total knee arthroplasty. Acta Orthop. 2016, 87, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Dincel, Y.M.; Sari, A.; Tekin, C.; Gunaydin, B.; Cetin, M.U.; Arslan, Y.Z. Changes in bone mineral density after total knee arthroplasty. Acta Ortop. Bras. 2020, 28, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Zhang, B.X.; Lade, J.A.; Pianta, R.M.; Unni, R.P.; Haw, C.S. Periprosthetic bone remodeling after novel short-stem neck-sparing total hip arthroplasty. J. Arthroplast. 2016, 31, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Kabata, T.; Kajino, Y.; Yamamuro, Y.; Taninaka, A.; Kataoka, T.; Saiki, Y.; Yanagi, Y.; Ima, M.; Iyobe, T.; et al. Influence of greater trochanteric bone density and three-dimensional morphology on perioperative greater trochanteric fracture following total hip arthroplasty via an anterolateral approach. BMC Musculoskelet. Disord. 2023, 24, 856. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, W.X.; Zeng, Y.; Ma, J.; Yang, J.; Shen, B. Comparison of Femoral Bone Mineral Density Changes around 3 Common Designs of Cementress Stems after Total Hip Arthroplasty-A Retrospective Cohort Study. Orthop. Surg. 2022, 14, 1059–1070. [Google Scholar] [CrossRef]
- Kweon, S.-H.; Park, J.S.; Park, B.H. Sarcopenia and Its Association With Change of Bone Mineral Density and Functional Outcome in Old-Aged Hip Arthroplasty Patients. Geriatr. Orthop. Surg. Rehabil. 2022, 13, 21514593221092880. [Google Scholar] [CrossRef]
- Melis, B.; DeFranco, M.; Lädermann, A.; Molé, D.; Favard, L.; Nérot, C.; Maynou, C.; Walch, G. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J. Bone Jt. Surg. Br. 2011, 93, 1240–1246. [Google Scholar] [CrossRef]
- Boileau, P.; Watkinson, D.; Hatzidakis, A.M.; Hovorka, I. Neer Award2005: The Grammont reverse shoulder prosthesis: Results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J. Shoulder Elb. Surg. 2006, 15, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Grey, B.; Rodseth, R.N.; Roche, S.J. Humeral stem loosening following reverse shoulder arthroplasty: A systematic review and meta-analysis. JBJS Rev. 2018, 6, e5. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Kang, J.S.; Moon, K.H. Primary Total Hip Arthroplasty Using Summit® Stems in Korean: Minimum Four-year Follow-up. Hip Pelvis 2017, 29, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Viamont-Guerra, M.R.; Ramos-Pascual, S.; Saffarini, M.; Sales, J.; Laude, F. Effect of femoral stem surface coating on clinical and radiographic outcomes of cementless primary total hip arthroplasty: A patient-matched retrospective study. Int. Orthop. 2023, 47, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Nakano, M.; Nakamura, Y.; Momose, T.; Sobajima, A.; Takahashi, J.; Nakata, K.; Nawata, M. Relationship between stress shielding and optimal femoral canal contact regions for short, tapered-wedge stem analyzed by 2D and 3D Systems in total hip arthroplasty. J. Clin. Med. 2023, 12, 3138. [Google Scholar] [CrossRef]
- Yokoya, S.; Harada, Y.; Sumimoto, Y.; Kikugawa, K.; Natsu, K.; Nakamura, Y.; Nagata, Y.; Negi, H.; Watanabe, C.; Adachi, N. Factors affecting stress shielding and osteolysis after reverse shoulder arthroplasty: A multicenter study in a Japanese population. J. Orthop. Sci. 2023, 27. epub ahead of print. [Google Scholar] [CrossRef]
- Nourissat, G.; Corsia, S.; Harris, H.W.; Bouché, P.A. Specific Design of a Press Fit Humeral Stem Provides Low Stress Shielding in Reverse shoulder Arthroplasty at minimum 5 years FU. J. Shoulder Elb. Arthroplast. 2022, 6, 24715492221112543. [Google Scholar] [CrossRef]
- Engh, C.A.; Massin, P.; Kathleen, E.; Suthers, K.E. Roentgenographic assessment of the biologic fixation of porous -surfaced femoral components. Clin. Orthop. Relat. Res. 1990, 257, 107–128. [Google Scholar] [CrossRef]
- Suzuki, T.; Sukezaki, F.; Shibuki, T.; Toyoshima, Y.; Nagai, T.; Inagaki, K. Teriparatide administration increases periprosthetic bone mineral density after total knee arthroplasty: A prospective study. J. Arthroplast. 2018, 33, 79–85. [Google Scholar] [CrossRef]
- Chernchujit, B.; Prasetia, R. The role of teriparatide in tuberosity healing after reverse shoulder arthroplasty in complex proximal humeral fragility fracture. J. Orthop. Surg. 2018, 26, 2309499017754104. [Google Scholar] [CrossRef]
- Morita, A.; Kobayashi, N.; Choe, H.; Ike, H.; Tezuka, T.; Higashihira, S.; Inaba, Y. Effect of switching administration of alendronate after teriparatide for the prevention of BMD loss around the implant after total hip arthroplasty, 2-year follow-up: A randomized controlled trial. J. Orthop. Surg. Res. 2020, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Nagoya, S.; Tateda, K.; Okazaki, S.; Kosukegawa, I.; Shimizu, J.; Yamashita, T. Restoration of proximal periprosthetic bone loss by denosumab in cementless total hip arthroplasty. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Raiss, P.; Schnetzke, M.; Wittmann, T.; Kilian, C.M.; Edwards, T.B.; Denard, P.J.; Neyton, L.; Godenèche, A.; Walch, G. Postoperative radiographic findings of an uncemented convertible short stem for anatomic and reverse shoulder arthroplasty. J Shoulder Elb. Surg. 2019, 28, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, F.; Gligor, A.; Bataga, T. Structured integration and alignment algorithm: A tool for personalized surgical treatment of tibial plateau fractures. J. Pers. Med. 2021, 11, 190. [Google Scholar] [CrossRef]

| Zone 1 | Zone 2 | Zone 3 | Zone 4 | Zone 5 | Total | |
|---|---|---|---|---|---|---|
| Bone resorption | 5 (13.5%) | 6 (16.2%) | 0 | 0 | 16 (43.2%) | 19/37 (51.4%) |
| Lucent line | 0 | 0 | 5 (13.5%) | 0 | 0 | 5/37 (13.5%) |
| Spot welds | 0 | 6 (20.7%) | 0 | 14 (48.2%) | 0 | 14/29 (48.2%) |
| Loosening | 0 | 0 | 0 | 0 | 0 | 0/37 |
| DEXA | 2 Weeks after Surgery | 1 Year after Surgery | Rate of Change (%) 1 |
|---|---|---|---|
| Zone 1 | 0.619 ± 0.164 | 0.565 ± 0.133 | −5.308 ± 23.349 |
| Zone 2 | 0.914 ± 0.206 | 0.860 ± 0.188 | −4.820 ± 20.461 |
| Zone 3 | 0.932 ± 0.205 | 0.879 ± 0.164 | −6.190 ± 12.794 |
| Zone 4 | 0.843 ± 0.174 | 0.901 ± 0.190 | 7.860 ± 16.285 |
| Zone 5 | 0.698 ± 0.182 | 0.521 ± 0.258 | −22.626 ± 34.326 |
| p Value | β (95% CI) | |
|---|---|---|
| Zone 1 | ||
| Sex (male vs. female) | 0.547 | −0.032 (−0.671 to 0.926) |
| Age | 0.621 | 0.002 (−0.007 to 0.012) |
| Cemented vs. non-cemented | 0.320 | −0.059 (−0.178 to 0.060) |
| Preoperative diagnosis | 0.317 | −0.047 (−0.141 to 0.047) |
| Preoperative DEXA | 0.016 * | 0.480 (0.095 to 0.866) |
| Zone 2 | ||
| Sex (male vs. female) | 0.169 | 0.101 (−0.046 to 0.249) |
| Age | 0.749 | 0.002 (−0.011 to 0.015) |
| Cemented vs. non-cemented | 0.416 | −0.056 (−0.193 to 0.082) |
| Preoperative diagnosis | 0.127 | −0.087 (−0.199 to 0.026) |
| Preoperative DEXA | 0.078 | 0.269 (−0.032 to 0.570) |
| Zone 3 | ||
| Sex (male vs. female) | 0.013 * | 0.110 (0.025 to 0.196) |
| Age | 0.346 | 0.004 (−0.005 to 0.012) |
| Cemented vs. non-cemented | 0.347 | −0.042 (0.132 to 0.048) |
| Preoperative diagnosis | 0.130 | −0.058 (−0.135 to 0.018) |
| Preoperative DEXA | <0.001 * | 0.503 (0.297 to 0.709) |
| Zone 4 | ||
| Sex (male vs. female) | 0.055 | 0.134 (−0.003 to 0.270) |
| Age | 0.910 | 0.001 (−0.012 to 0.014) |
| Cemented vs. non-cemented | 0.624 | 0.032 (−0.100 to 0.165) |
| Preoperative diagnosis | 0.619 | −0.026 (−0.130 to 0.079) |
| Preoperative DEXA | 0.047 * | 0.443 (0.007 to 0.879) |
| Zone 5 | ||
| Sex (male vs. female) | 0.679 | 0.043 (−0.168 to 0.254) |
| Age | 0.399 | −0.008 (−0.027 to 0.011) |
| Cemented vs. non-cemented | 0.601 | −0.059 (−0.285 to 0.168) |
| Preoperative diagnosis | 0.266 | −0.106 (−0.296 to 0.085) |
| Preoperative DEXA | 0.018 * | 0.808 (0.147 to 1.469) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soma, D.; Ichiseki, T.; Ueda, S.; Sakurai, M.; Kawahara, N. Radiographic Evaluation and Changes in Bone Density of the Humeral Side after Reverse Total Shoulder Arthroplasty. J. Clin. Med. 2023, 12, 7698. https://doi.org/10.3390/jcm12247698
Soma D, Ichiseki T, Ueda S, Sakurai M, Kawahara N. Radiographic Evaluation and Changes in Bone Density of the Humeral Side after Reverse Total Shoulder Arthroplasty. Journal of Clinical Medicine. 2023; 12(24):7698. https://doi.org/10.3390/jcm12247698
Chicago/Turabian StyleSoma, Daisuke, Toru Ichiseki, Shusuke Ueda, Masaru Sakurai, and Norio Kawahara. 2023. "Radiographic Evaluation and Changes in Bone Density of the Humeral Side after Reverse Total Shoulder Arthroplasty" Journal of Clinical Medicine 12, no. 24: 7698. https://doi.org/10.3390/jcm12247698





