Radiomics-Assisted Computed Tomography-Based Analysis to Evaluate Lung Morphology Characteristics after Congenital Diaphragmatic Hernia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Imaging Protocols
2.3. Image Reconstruction
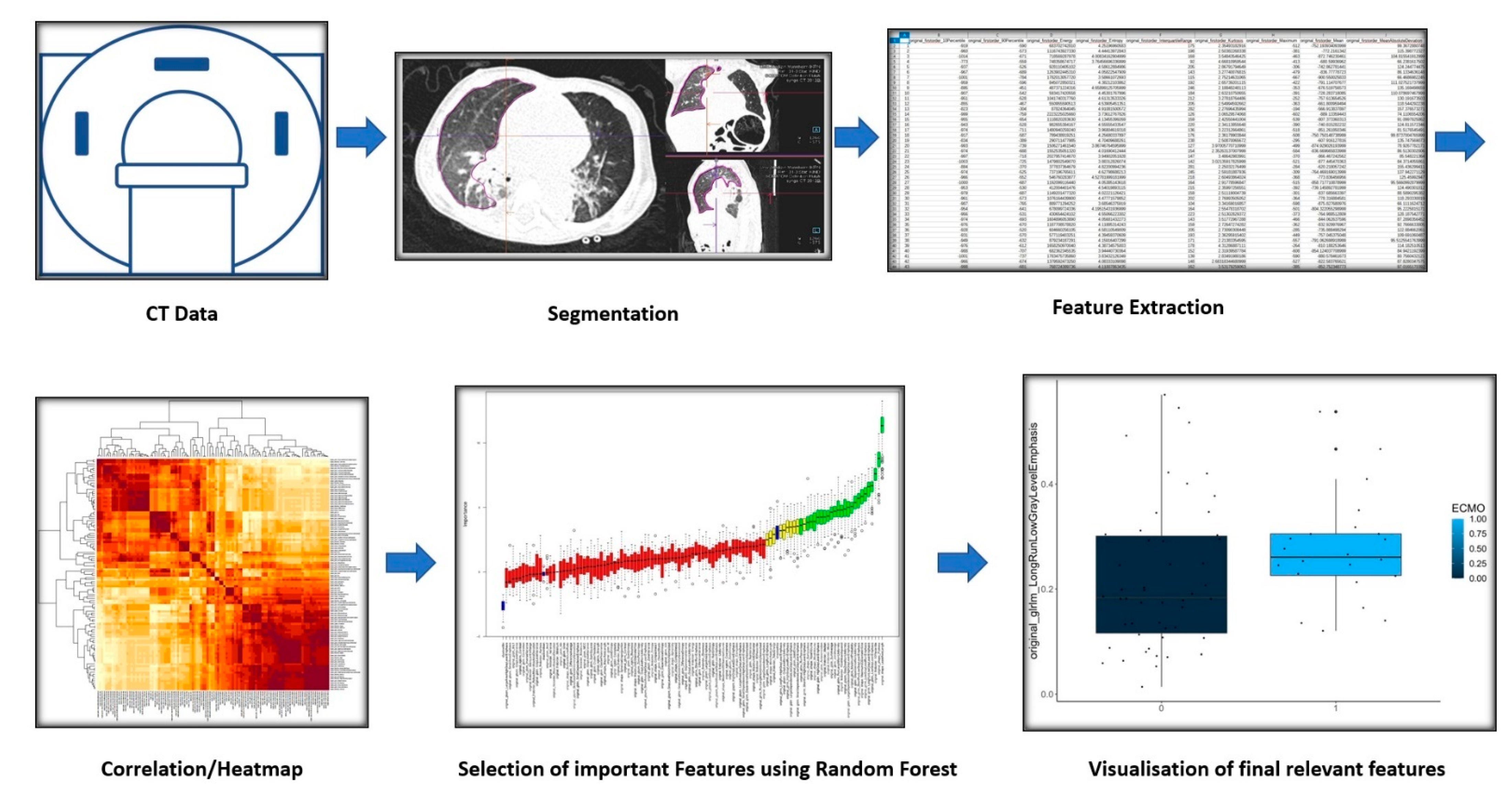
2.4. Segmentation and Analysis Software
2.5. Image Analysis and Segmentation
2.6. Feature Extraction
2.7. Clustering, Feature Selection, and Statistical Analysis
3. Results
3.1. Patient Collective and Extracted Features
3.2. Cluster Analysis
3.3. Variable Importance Assessment
3.4. Reduction of Feature Redundancy and Final Feature Selection
- A.
- Differences between the ipsilateral and contralateral lungs:
- B.
- Differences between the contralateral lungs in patients with and without ECMO treatment:
- C.
- Differences between the ipsilateral lungs in patients with and without ECMO treatment:
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greer, J.J.; Allan, D.W.; Babiuk, R.P.; Lemke, R.P. Recent Advances in Understanding the Pathogenesis of Nitrofen-Induced Congenital Diaphragmatic Hernia. Pediatr. Pulmonol. 2000, 29, 394–399. [Google Scholar] [CrossRef]
- Klaassens, M.; De Klein, A.; Tibboel, D. The Etiology of Congenital Diaphragmatic Hernia: Still Largely Unknown? Eur. J. Med. Genet. 2009, 52, 281–286. [Google Scholar] [CrossRef]
- Muratore, C.S.; Wilson, J.M. Congenital Diaphragmatic Hernia: Where Are We and Where Do We Go from Here? Semin. Perinatol. 2000, 24, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Longoni, M.; Pober, B.R.; High, F.A. Congenital Diaphragmatic Hernia Overview. In GeneReviews®; University of Washington: Seattle, WA, USA, 2006. [Google Scholar]
- Dumpa, V.; Chandrasekharan, P. Congenital Diaphragmatic Hernia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Snoek, K.G.; Reiss, I.K.M.; Greenough, A.; Capolupo, I.; Urlesberger, B.; Wessel, L.; Storme, L.; Deprest, J.; Schaible, T.; Van Heijst, A.; et al. Standardized Postnatal Management of Infants with Congenital Diaphragmatic Hernia in Europe: The CDH EURO Consortium Consensus—2015 Update. Neonatology 2016, 110, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Tennant, P.W.; Pearce, M.S.; Bythell, M.; Rankin, J. 20-Year Survival of Children Born with Congenital Anomalies: A Population-Based Study. Lancet 2010, 375, 649–656. [Google Scholar] [CrossRef]
- Badillo, A.; Gingalewski, C. Congenital Diaphragmatic Hernia: Treatment and Outcomes. Semin. Perinatol. 2014, 38, 92–96. [Google Scholar] [CrossRef]
- Tan, J.K.; Banton, G.; Minutillo, C.; Hall, G.L.; Wilson, A.; Murray, C.; Nathan, E.A.; Verheggen, M.; Ramsay, J.; Samnakay, N.; et al. Long-Term Medical and Psychosocial Outcomes in Congenital Diaphragmatic Hernia Survivors. Arch. Dis. Child. 2019, 104, 761–767. [Google Scholar] [CrossRef]
- Yamoto, M.; Nagata, K.; Terui, K.; Hayakawa, M.; Okuyama, H.; Amari, S.; Yokoi, A.; Masumoto, K.; Okazaki, T.; Inamura, N.; et al. Long-Term Outcomes of Congenital Diaphragmatic Hernia: Report of a Multicenter Study in Japan. Children 2022, 9, 856. [Google Scholar] [CrossRef]
- Bagolan, P.; Morini, F. Long-Term Follow up of Infants with Congenital Diaphragmatic Hernia. Semin. Pediatr. Surg. 2007, 16, 134–144. [Google Scholar] [CrossRef]
- Panitch, H.B.; Weiner, D.J.; Feng, R.; Perez, M.R.; Healy, F.; McDonough, J.M.; Rintoul, N.; Hedrick, H.L. Lung Function over the First 3 Years of Life in Children with Congenital Diaphragmatic Hernia: Lung Function in Congenital Diaphragmatic Hernia. Pediatr. Pulmonol. 2015, 50, 896–907. [Google Scholar] [CrossRef]
- Ijsselstijn, H.; Tibboel, D.; Hop, W.J.; Molenaar, J.C.; De Jongste, J.C. Long-Term Pulmonary Sequelae in Children with Congenital Diaphragmatic Hernia. Am. J. Respir. Crit. Care Med. 1997, 155, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Jancelewicz, T.; Vu, L.T.; Keller, R.L.; Bratton, B.; Lee, H.; Farmer, D.; Harrison, M.; Miniati, D.; Mackenzie, T.; Hirose, S.; et al. Long-Term Surgical Outcomes in Congenital Diaphragmatic Hernia: Observations from a Single Institution. J. Pediatr. Surg. 2010, 45, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, K.G.; Pober, B.R. Congenital Diaphragmatic Hernia and Pulmonary Hypoplasia: New Insights from Developmental Biology and Genetics. Am. J. Med. Genet. Part C Semin. Med. Genet. 2007, 145C, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, K.G.; Herron, B.J.; Vargas, S.O.; Huang, H.; Tevosian, S.G.; Kochilas, L.; Rao, C.; Pober, B.R.; Babiuk, R.P.; Epstein, J.A.; et al. Fog2 Is Required for Normal Diaphragm and Lung Development in Mice and Humans. PLoS Genet. 2005, 1, e10. [Google Scholar] [CrossRef] [PubMed]
- Harding, R. Fetal Pulmonary Development: The Role of Respiratory Movements. Equine Vet. J. 1997, 29, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Pober, B. Genetic Aspects of Human Congenital Diaphragmatic Hernia. Clin. Genet. 2008, 74, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Keijzer, R.; Puri, P. Congenital Diaphragmatic Hernia. Semin. Pediatr. Surg. 2010, 19, 180–185. [Google Scholar] [CrossRef]
- Mugford, M.; Elbourne, D.; Field, D. Extracorporeal Membrane Oxygenation for Severe Respiratory Failure in Newborn Infants. Cochrane Database Syst. Rev. 2008, 16, CD001340. [Google Scholar] [CrossRef]
- Thiagarajan, R.R.; Barbaro, R.P.; Rycus, P.T.; Mcmullan, D.M.; Conrad, S.A.; Fortenberry, J.D.; Paden, M.L. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017, 63, 60–67. [Google Scholar] [CrossRef]
- Debus, A.; Hagelstein, C.; Kilian, A.K.; Weiss, C.; Schönberg, S.O.; Schaible, T.; Neff, K.W.; Büsing, K.A. Fetal Lung Volume in Congenital Diaphragmatic Hernia: Association of Prenatal MR Imaging Findings with Postnatal Chronic Lung Disease. Radiology 2013, 266, 887–895. [Google Scholar] [CrossRef]
- Weis, M.; Zoellner, F.G.; Hagelstein, C.; Schoenberg, S.O.; Zahn, K.; Schaible, T.; Neff, K.W. Lung Perfusion MRI After Congenital Diaphragmatic Hernia Repair in 2-Year-Old Children With and Without Extracorporeal Membrane Oxygenation Therapy. Am. J. Roentgenol. 2016, 206, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Stoll-Dannenhauer, T.; Schwab, G.; Zahn, K.; Schaible, T.; Wessel, L.; Weiss, C.; Schoenberg, S.O.; Henzler, T.; Weis, M. Computed Tomography Based Measurements to Evaluate Lung Density and Lung Growth after Congenital Diaphragmatic Hernia. Sci. Rep. 2021, 11, 5035. [Google Scholar] [CrossRef] [PubMed]
- Thurlbeck, W.M.; Kida, K.; Langston, C.; Cowan, M.J.; Kitterman, J.A.; Tooley, W.; Bryan, H. Postnatal Lung Growth after Repair of Diaphragmatic. Thorax 1979, 34, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Parekh, V.; Jacobs, M.A. Radiomics: A New Application from Established Techniques. Expert Rev. Precis. Med. Drug Dev. 2016, 1, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; Van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting More Information from Medical Images Using Advanced Feature Analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; Bonilha, L.; Li, L.M.; Cendes, F. Texture Analysis of Medical Images. Clin. Radiol. 2004, 59, 1061–1069. [Google Scholar] [CrossRef]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in Medical Imaging—“How-to” Guide and Critical Reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2014. [Google Scholar]
- Kursa, M.B.; Jankowski, A.; Rudnicki, W.R. Boruta–A System for Feature Selection. Fundam. Informaticae 2010, 101, 271–285. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Soft. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- McCague, C.; Ramlee, S.; Reinius, M.; Selby, I.; Hulse, D.; Piyatissa, P.; Bura, V.; Crispin-Ortuzar, M.; Sala, E.; Woitek, R. Introduction to Radiomics for a Clinical Audience. Clin. Radiol. 2023, 78, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tam, L.; Wright, J.; Mohammadzadeh, M.; Han, M.; Chen, E.; Wagner, M.; Nemalka, J.; Lai, H.; Eghbal, A.; et al. Radiomics Can Distinguish Pediatric Supratentorial Embryonal Tumors, High-Grade Gliomas, and Ependymomas. AJNR Am. J. Neuroradiol. 2022, 43, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-C.; Kim, J.; Kim, K.; Byun, B.H.; Lim, I.; Kong, C.-B.; Song, W.S.; Koh, J.-S.; Woo, S.-K. Preliminary Radiogenomic Evidence for the Prediction of Metastasis and Chemotherapy Response in Pediatric Patients with Osteosarcoma Using 18F-FDG PET/CT, EZRIN, and KI67. Cancers 2021, 13, 2671. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, G.; Zhang, W.; He, J.; Sun, W.; Yang, M.; Sun, Y.; Peet, A. MRI-Based Whole-Tumor Radiomics to Classify the Types of Pediatric Posterior Fossa Brain Tumor. Neurochirurgie 2022, 68, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, W.; Wang, F.; He, L.; Liu, E. Early Recognition of Necrotizing Pneumonia in Children Based on Non-Contrast-Enhanced Computed Tomography Radiomics Signatures. Transl. Pediatr. 2021, 10, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Prayer, F.; Watzenböck, M.L.; Heidinger, B.H.; Rainer, J.; Schmidbauer, V.; Prosch, H.; Ulm, B.; Rubesova, E.; Prayer, D.; Kasprian, G. Fetal MRI Radiomics: Non-Invasive and Reproducible Quantification of Human Lung Maturity. Eur. Radiol. 2023, 33, 4205–4213. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, X.; Li, J.; Zhang, S.; Zhou, P.; Yu, X.; Ren, Y.; Wang, J.; Zhang, L.; Li, Y.; et al. A COVID-19 Risk Score Combining Chest CT Radiomics and Clinical Characteristics to Differentiate COVID-19 Pneumonia from Other Viral Pneumonias. Aging 2021, 13, 9186–9224. [Google Scholar] [CrossRef] [PubMed]
- Gülbay, M.; Özbay, B.O.; Mendi, B.A.R.; Baştuğ, A.; Bodur, H. A CT Radiomics Analysis of COVID-19-Related Ground-Glass Opacities and Consolidation: Is It Valuable in a Differential Diagnosis with Other Atypical Pneumonias? PLoS ONE 2021, 16, e0246582. [Google Scholar] [CrossRef]
- Stefano, A.; Gioè, M.; Russo, G.; Palmucci, S.; Torrisi, S.E.; Bignardi, S.; Basile, A.; Comelli, A.; Benfante, V.; Sambataro, G.; et al. Performance of Radiomics Features in the Quantification of Idiopathic Pulmonary Fibrosis from HRCT. Diagnostics 2020, 10, 306. [Google Scholar] [CrossRef]
- Liang, C.-H.; Liu, Y.-C.; Wan, Y.-L.; Yun, C.-H.; Wu, W.-J.; López-González, R.; Huang, W.-M. Quantification of Cancer-Developing Idiopathic Pulmonary Fibrosis Using Whole-Lung Texture Analysis of HRCT Images. Cancers 2021, 13, 5600. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Zhang, Z.; Yang, X.; Li, X.; Gao, Y.; Huang, K. A Novel CT-Based Radiomics Features Analysis for Identification and Severity Staging of COPD. Acad. Radiol. 2022, 29, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Cho, Y.H.; Lee, S.M.; Hwang, J.; Lee, J.S.; Oh, Y.-M.; Lee, S.-D.; Loh, L.-C.; Ong, C.-K.; Seo, J.B.; et al. Deep Radiomics-Based Survival Prediction in Patients with Chronic Obstructive Pulmonary Disease. Sci. Rep. 2021, 11, 15144. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Almendro, V.; Polyak, K. Intra-Tumour Heterogeneity: A Looking Glass for Cancer? Nat. Rev. Cancer 2012, 12, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Walleyo, A.; Debus, A.; Kehl, S.; Weiss, C.; Schönberg, S.O.; Schaible, T.; Büsing, K.A.; Neff, K.W. Periodic MRI Lung Volume Assessment in Fetuses with Congenital Diaphragmatic Hernia: Prediction of Survival, Need for ECMO, and Development of Chronic Lung Disease. Am. J. Roentgenol. 2013, 201, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.S.F.; Aerts, H.J.W.L. Applications and Limitations of Radiomics. Phys. Med. Biol. 2016, 61, R150–R166. [Google Scholar] [CrossRef] [PubMed]
- Hersh, C.P.; Washko, G.R.; Jacobson, F.L.; Gill, R.; Estepar, R.S.J.; Reilly, J.J.; Silverman, E.K. Interobserver Variability in the Determination of Upper Lobe-Predominant Emphysema. Chest 2007, 131, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.E.; Criner, G.J.; Moore, W.H.; Van Rikxoort, E.M.; Sciurba, F.C.; Shah, P.L.; Vliegenthart, R.; Welling, J.B.A.; Slebos, D.-J. HRCT Characteristics of Severe Emphysema Patients: Interobserver Variability among Expert Readers and Comparison with Quantitative Software. Eur. J. Radiol. 2021, 136, 109561. [Google Scholar] [CrossRef]
- Weis, M.; Henzler, T.; Nance, J.W.; Haubenreisser, H.; Meyer, M.; Sudarski, S.; Schoenberg, S.O.; Neff, K.W.; Hagelstein, C. Radiation Dose Comparison Between 70 kVp and 100 kVp With Spectral Beam Shaping for Non–Contrast-Enhanced Pediatric Chest Computed Tomography: A Prospective Randomized Controlled Study. Invest. Radiol. 2017, 52, 155–162. [Google Scholar] [CrossRef]
- Hagelstein, C.; Henzler, T.; Haubenreisser, H.; Meyer, M.; Sudarski, S.; Schoenberg, S.O.; Neff, K.W.; Weis, M. Ultra-High Pitch Chest Computed Tomography at 70 kVp Tube Voltage in an Anthropomorphic Pediatric Phantom and Non-Sedated Pediatric Patients: Initial Experience with 3rd Generation Dual-Source CT. Z. Med. Phys. 2016, 26, 349–361. [Google Scholar] [CrossRef]
- Akinkuotu, A.C.; Cruz, S.M.; Abbas, P.I.; Lee, T.C.; Welty, S.E.; Olutoye, O.O.; Cassady, C.I.; Mehollin-Ray, A.R.; Ruano, R.; Belfort, M.A.; et al. Risk-Stratification of Severity for Infants with CDH: Prenatal versus Postnatal Predictors of Outcome. J. Pediatr. Surg. 2016, 51, 44–48. [Google Scholar] [CrossRef]
- Brindle, M.E.; Cook, E.F.; Tibboel, D.; Lally, P.A.; Lally, K.P. A Clinical Prediction Rule for the Severity of Congenital Diaphragmatic Hernias in Newborns. Pediatrics 2014, 134, e413–e419. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.M.; DiGeronimo, R.J.; Yoder, B.A. Congenital Diaphragmatic Hernia: A Simplified Postnatal Predictor of Outcome. J. Pediatr. Surg. 2007, 42, 510–516. [Google Scholar] [CrossRef] [PubMed]




| Compared Variables | Patient Group | Selected Variables | p -Value |
|---|---|---|---|
| Ipsi- vs contralateral | All (n = 144) | original_firstorder_10Percentile | <0.001 |
| original_gldm_LowGrayLevelEmphasis | <0.001 | ||
| original_glrlm_LongRunLowGrayLevelEmphasis | <0.001 | ||
| original_shape_Flatness | <0.001 | ||
| original_shape_LeastAxisLength | <0.001 | ||
| original_shape_Maximum2DDiameterSlice | 0.005 | ||
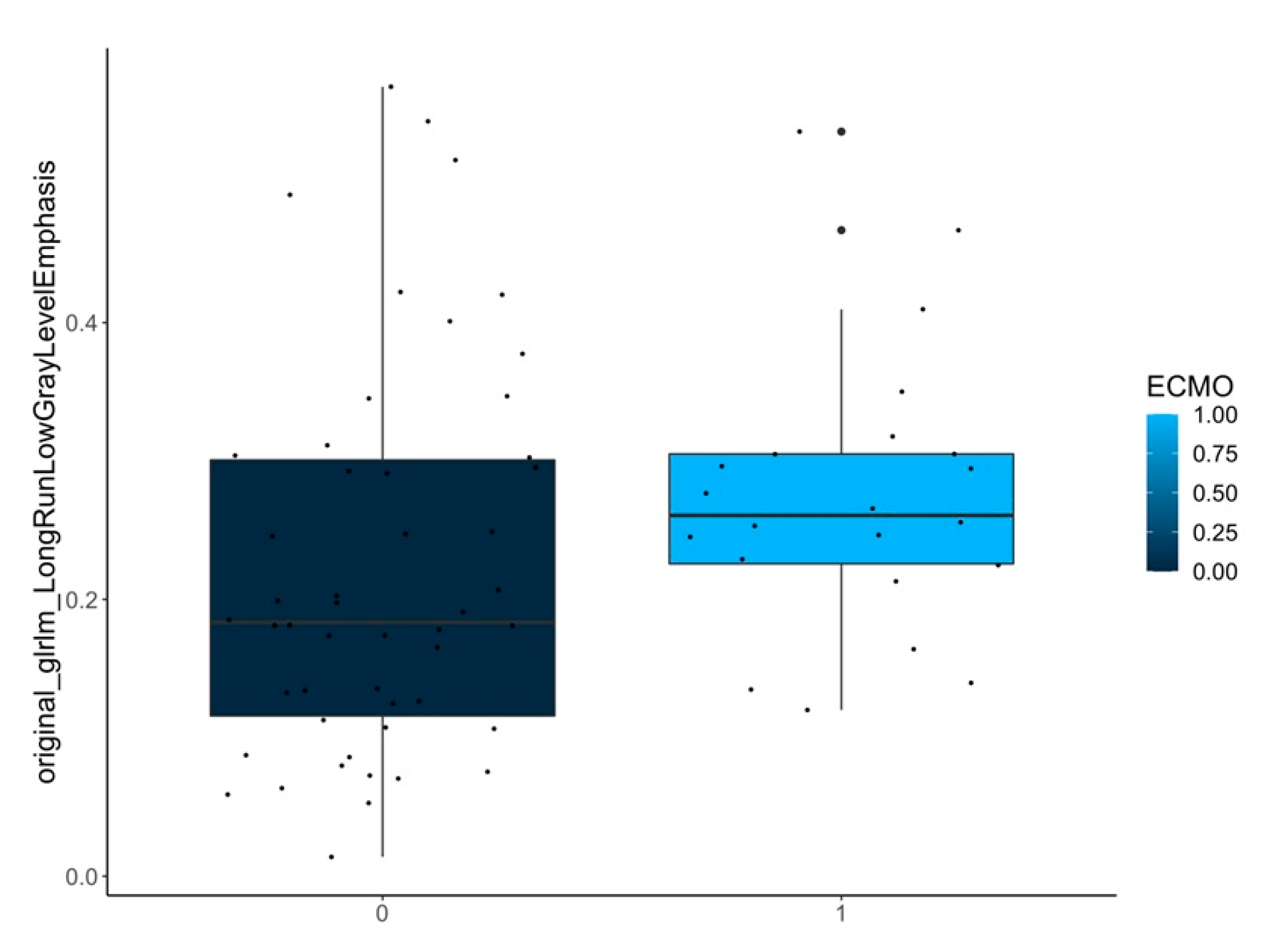
| ECMO yes/no | contralateral-lungs (n = 72) | original_shape_MinorAxisLength | 0.003 |
| original_shape_Maximum2DDiameterSlice | 0.003 | ||
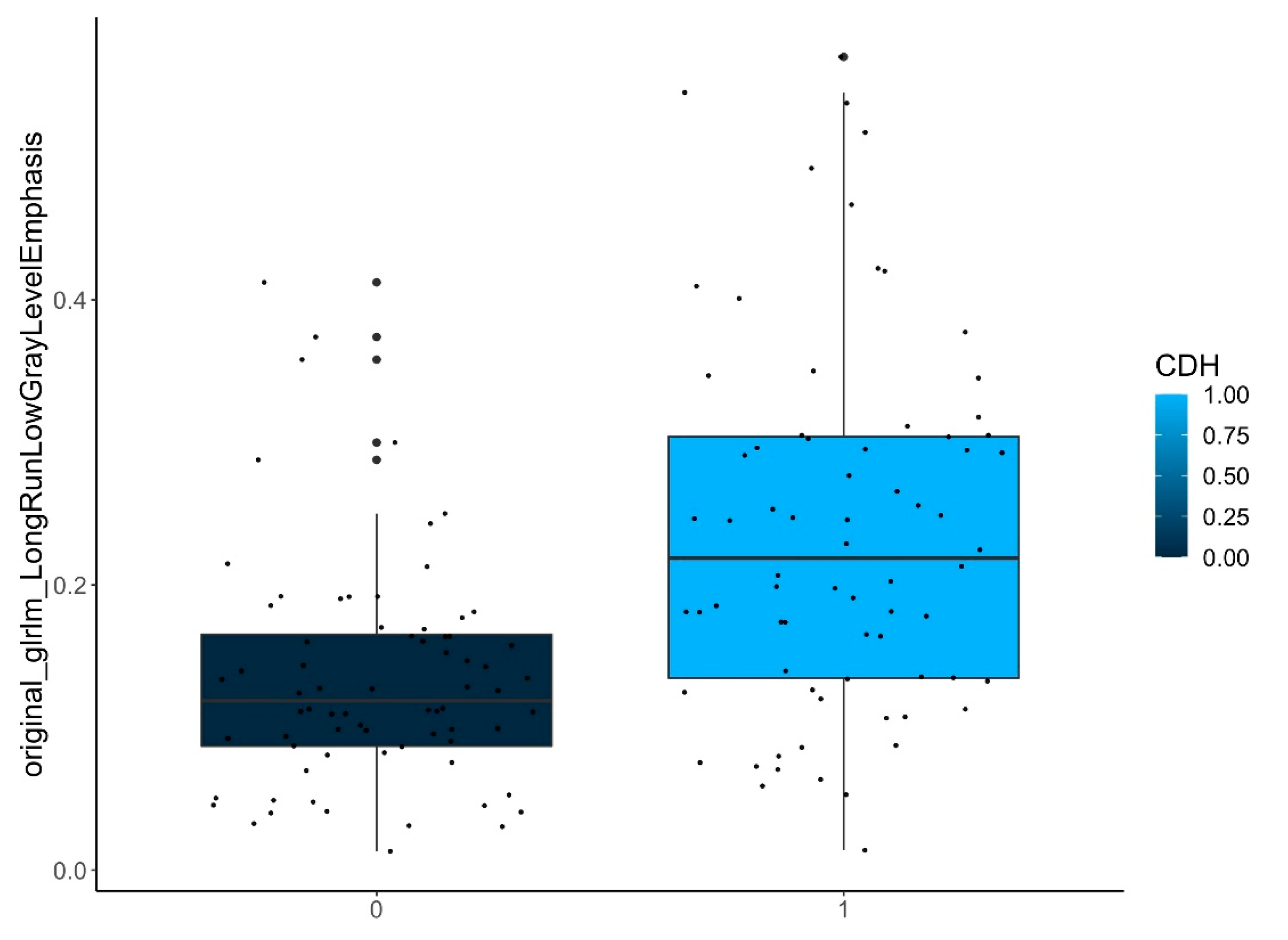
| ECMO yes/no | ipsilateral-lungs (n = 72) | original_glrlm_LongRunLowGrayLevelEmphasis | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virlan, S.-V.; Froelich, M.F.; Thater, G.; Rafat, N.; Elrod, J.; Boettcher, M.; Schoenberg, S.O.; Weis, M. Radiomics-Assisted Computed Tomography-Based Analysis to Evaluate Lung Morphology Characteristics after Congenital Diaphragmatic Hernia. J. Clin. Med. 2023, 12, 7700. https://doi.org/10.3390/jcm12247700
Virlan S-V, Froelich MF, Thater G, Rafat N, Elrod J, Boettcher M, Schoenberg SO, Weis M. Radiomics-Assisted Computed Tomography-Based Analysis to Evaluate Lung Morphology Characteristics after Congenital Diaphragmatic Hernia. Journal of Clinical Medicine. 2023; 12(24):7700. https://doi.org/10.3390/jcm12247700
Chicago/Turabian StyleVirlan, Silviu-Viorel, Matthias F. Froelich, Greta Thater, Neysan Rafat, Julia Elrod, Michael Boettcher, Stefan O. Schoenberg, and Meike Weis. 2023. "Radiomics-Assisted Computed Tomography-Based Analysis to Evaluate Lung Morphology Characteristics after Congenital Diaphragmatic Hernia" Journal of Clinical Medicine 12, no. 24: 7700. https://doi.org/10.3390/jcm12247700





