Short-Term Outcome of Robotic versus Laparoscopic Hysterectomy for Endometrial Cancer in Women with Diabetes: Analysis of the US Nationwide Inpatient Sample
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Ethics Statement
2.3. Study Population
2.4. Study Variables and Outcome Measures
2.5. Postoperative Complications
2.6. Other Variables
2.7. Statistical Analysis
3. Results
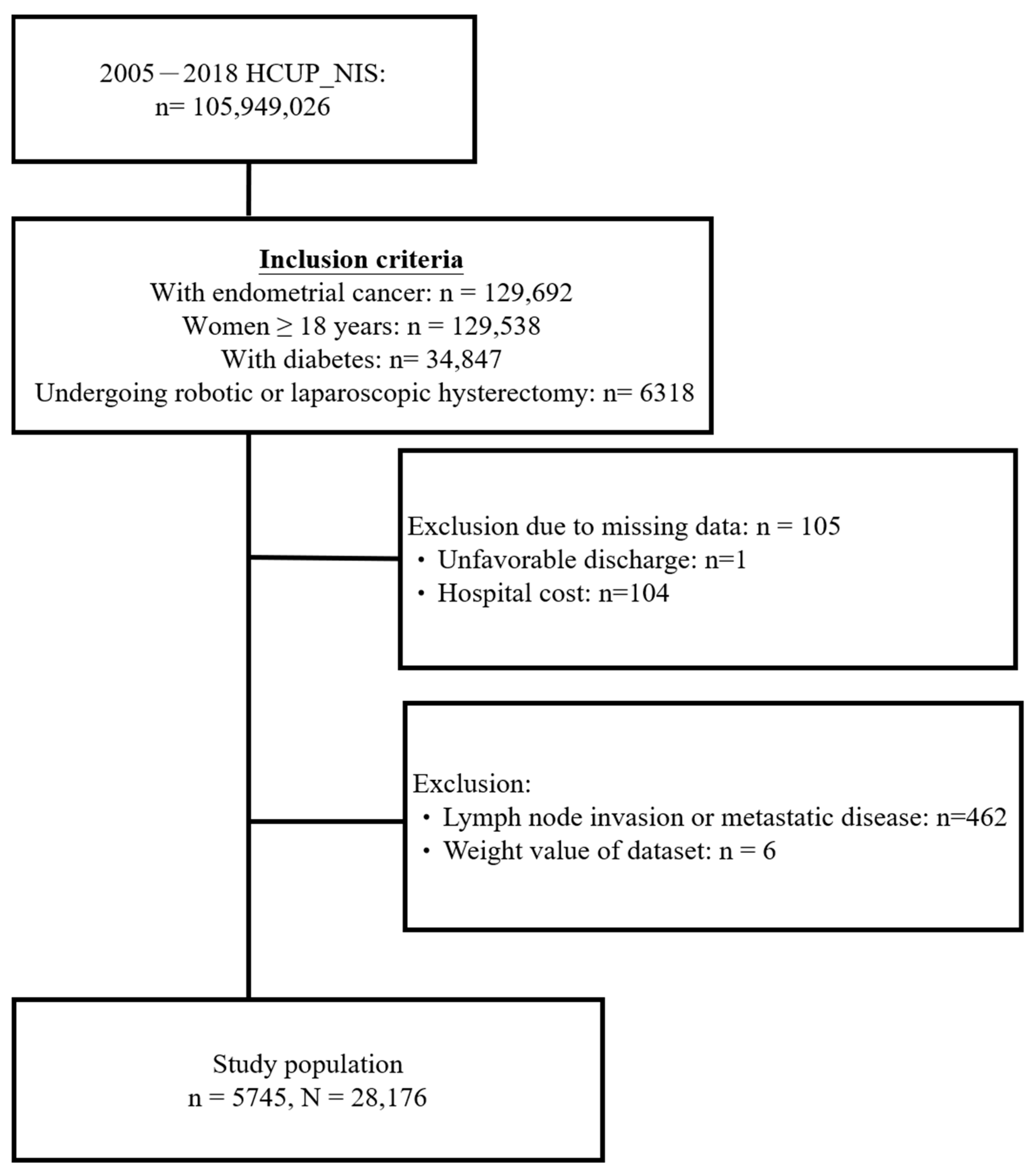
3.1. Study Population Selection
3.2. Characteristics of Women with Diabetes Undergoing Robotic or Pure Laparoscopic Hysterectomy for EC
3.3. In-Hospital Outcomes of Women with Diabetes Undergoing Robotic or Pure Laparoscopic Hysterectomy for EC
3.4. Associations between the Types of Minimally Invasive Hysterectomy and In-Hospital Outcomes
3.5. Associations between Types of Minimally Invasive Hysterectomy and In-Hospital Outcomes, Stratified by Age and Obesity Status
4. Discussion
4.1. Summary of Main Results
4.2. Results in the Context of Published Literature
4.3. Strengths and Weaknesses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Habeshian, T.S.; Zhang, J.; Peeri, N.C.; Du, M.; De Vivo, I.; Setiawan, V.W. Differential trends in rising endometrial cancer incidence by age, race, and ethnicity. JNCI Cancer Spectr. 2023, 7, pkad001. [Google Scholar] [CrossRef]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gong, T.T.; Liu, F.H.; Jiang, Y.T.; Sun, H.; Ma, X.X.; Zhao, Y.H.; Wu, Q.J. Global, Regional, and National Burden of Endometrial Cancer, 1990–2017: Results from the Global Burden of Disease Study, 2017. Front. Oncol. 2019, 9, 1440. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, C.M.; Backes, F.J.; O’Malley, D.; Bixel, K.L.; Suarez, A.A.; Fowler, J.M.; Copeland, L.J.; Goodfellow, P.J.; Cohn, D.E. Endometrial Cancer: Who Lives, Who Dies, Can We Improve Their Story? Oncologist 2021, 26, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.S.; Tolikas, A.C.; Miliaras, D.E. Hysterectomy-current methods and alternatives for benign indications. Obstet. Gynecol. Int. 2010, 2010, 356740. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, M.; Kobayashi, E.; Terai, Y.; Yamashita, T.; Terao, Y.; Nomura, H.; Asada, H.; Hoshiba, T.; Mikami, M.; Mandai, M.; et al. Questionnaire survey regarding current status of minimally invasive surgery for endometrial cancer in Japan: A cross-sectional survey for JSGOE members. J. Obstet. Gynaecol. Res. 2023, 49, 2370–2378. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Yu, Y.L.; Ryu, J.; Ju, Y.J.; Kang, S. How Do Patients Value the Benefit of Minimally Invasive Surgery in Cancer Treatment? Value Health 2022, 25, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Qu, S. The Relationship between Diabetes Mellitus and Cancers and Its Underlying Mechanisms. Front. Endocrinol. 2022, 13, 800995. [Google Scholar] [CrossRef]
- Saed, L.; Varse, F.; Baradaran, H.R.; Moradi, Y.; Khateri, S.; Friberg, E.; Khazaei, Z.; Gharahjeh, S.; Tehrani, S.; Sioofy-Khojine, A.B.; et al. The effect of diabetes on the risk of endometrial Cancer: An updated a systematic review and meta-analysis. BMC Cancer 2019, 19, 527. [Google Scholar] [CrossRef]
- Friberg, E.; Mantzoros, C.S.; Wolk, A. Diabetes and risk of endometrial cancer: A population-based prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 276–280. [Google Scholar] [CrossRef]
- Njoku, K.; Agnew, H.J.; Crosbie, E.J. Impact of Type 2 Diabetes Mellitus on Endometrial Cancer Survival: A Prospective Database Analysis. Front. Oncol. 2022, 12, 899262. [Google Scholar] [CrossRef]
- Wang, J.; Chen, K.; Li, X.; Jin, X.; An, P.; Fang, Y.; Mu, Y. Postoperative adverse events in patients with diabetes undergoing orthopedic and general surgery. Medicine 2019, 98, e15089. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, A.; Cao, J.; Liu, Y.; Lou, J.; Li, H.; Ma, Y.; Song, Y.; Mi, W.; Liu, J. Association of Diabetes Mellitus with Postoperative Complications and Mortality after Non-Cardiac Surgery: A Meta-Analysis and Systematic Review. Front. Endocrinol. 2022, 13, 841256. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, M.C.; Simpson, A.N.; Dossa, F.; Liani, V.; Kaur, Y.; Acuna, S.A.; Robertson, D.; Satkunaratnam, A.; Bernardini, M.Q.; Ferguson, S.E.; et al. Laparoscopic and robotic hysterectomy in endometrial cancer patients with obesity: A systematic review and meta-analysis of conversions and complications. Am. J. Obstet. Gynecol. 2019, 221, 410–428.e19. [Google Scholar] [CrossRef] [PubMed]
- Eoh, K.J.; Nam, E.J.; Kim, S.W.; Shin, M.; Kim, S.J.; Kim, J.A.; Kim, Y.T. Nationwide Comparison of Surgical and Oncologic Outcomes in Endometrial Cancer Patients Undergoing Robotic, Laparoscopic, and Open Surgery: A Population-Based Cohort Study. Cancer Res. Treat. 2021, 53, 549–557. [Google Scholar] [CrossRef]
- Uwins, C.; Patel, H.; Prakash Bhandoria, G.; Butler-Manuel, S.; Tailor, A.; Ellis, P.; Chatterjee, J. Laparoscopic and Robotic Surgery for Endometrial and Cervical Cancer. Clin. Oncol. (R. Coll. Radiol.) 2021, 33, e372–e382. [Google Scholar] [CrossRef] [PubMed]
- NIS Database Documentation; Department of Health and Human Services, Agency for Healthcare Research and Quality: Rockville, MD, USA, 2022.
- Ognjanović, B.I.; Marković, S.D.; Pavlović, S.Z.; Žikić, R.V.; Stajn, A.S.; Saičić, Z.S. Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: Protective effect of selenium. Physiol. Res. 2008, 57, 403–411. [Google Scholar] [CrossRef]
- Available online: https://documentation.sas.com/doc/en/statcdc/14.2/statug/statug_surveyreg_overview.htm (accessed on 1 October 2023).
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; Ntzani, E.E.; Ioannidis, J.P. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ 2015, 350, g7607. [Google Scholar] [CrossRef] [PubMed]
- Friberg, E.; Orsini, N.; Mantzoros, C.S.; Wolk, A. Diabetes mellitus and risk of endometrial cancer: A meta-analysis. Diabetologia 2007, 50, 1365–1374. [Google Scholar] [CrossRef]
- Wartko, P.D.; Beck, T.L.; Reed, S.D.; Mueller, B.A.; Hawes, S.E. Association of endometrial hyperplasia and cancer with a history of gestational diabetes. Cancer Causes Control 2017, 28, 819–828. [Google Scholar] [CrossRef]
- Dalsgaard, T.; Jensen, M.D.; Hartwell, D.; Mosgaard, B.J.; Jørgensen, A.; Jensen, B.R. Robotic Surgery Is Less Physically Demanding Than Laparoscopic Surgery: Paired Cross Sectional Study. Ann. Surg. 2020, 271, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.L.; Mogensen, O.; Wu, C.; Lund, K.; Iachina, M.; Korsholm, M.; Jensen, P.T. Nationwide Introduction of Minimally Invasive Robotic Surgery for Early-Stage Endometrial Cancer and Its Association with Severe Complications. JAMA Surg. 2019, 154, 530–538. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Su, P.Y.; Hao, J.H.; Sun, Y.H. The role of preexisting diabetes mellitus on incidence and mortality of endometrial cancer: A meta-analysis of prospective cohort studies. Int. J. Gynecol. Cancer 2013, 23, 294–303. [Google Scholar] [CrossRef]
- Nagle, C.M.; Crosbie, E.J.; Brand, A.; Obermair, A.; Oehler, M.K.; Quinn, M.; Leung, Y.; Spurdle, A.B.; Webb, P.M. The association between diabetes, comorbidities, body mass index and all-cause and cause-specific mortality among women with endometrial cancer. Gynecol. Oncol. 2018, 150, 99–105. [Google Scholar] [CrossRef]
- Sebranek, J.J.; Lugli, A.K.; Coursin, D.B. Glycaemic control in the perioperative period. Br. J. Anaesth. 2013, 111 (Suppl. S1), i18–i34. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.N.; Jonsdottir, G.M.; Jorgensen, S.; Shah, N.; Einarsson, J.I. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS J. Soc. Laparoendosc. Surg. 2012, 16, 519–524. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 5745) | Robotic (n = 3668) | Pure Laparoscopic (n = 2077) | p-Value |
|---|---|---|---|---|
| Demography | ||||
| Age, years | 64.2 ± 0.15 | 64.3 ± 0.18 | 64.1 ± 0.24 | 0.374 |
| 18–49 | 482 (8.4) | 279 (7.6) | 203 (9.8) | 0.003 |
| 50–59 | 1347 (23.4) | 854 (23.2) | 493 (23.8) | |
| 60–69 | 2146 (37.4) | 1418 (38.7) | 728 (35.1) | |
| 70–79 | 1297 (22.6) | 833 (22.7) | 464 (22.3) | |
| ≥80 | 473 (8.2) | 284 (7.7) | 189 (9.0) | |
| Race | ||||
| White | 3691 (69.9) | 2454 (71.4) | 1237 (67.2) | 0.003 |
| Black | 572 (10.9) | 372 (10.8) | 200 (10.9) | |
| Hispanic | 608 (11.6) | 384 (11.3) | 224 (12.3) | |
| Other | 399 (7.6) | 224 (6.5) | 175 (9.6) | |
| Missing | 475 | 234 | 241 | |
| Insurance status | ||||
| Medicare/Medicaid | 3462 (60.4) | 2244 (61.2) | 1218 (58.8) | <0.001 |
| Private including HMO | 1985 (34.6) | 1278 (34.9) | 707 (34.0) | |
| Self-pay/no-charge/other | 291 (5.1) | 143 (3.9) | 148 (7.2) | |
| Missing | 7 | 3 | 4 | |
| Household income | ||||
| Q1 | 1475 (26.2) | 911 (25.3) | 564 (28.0) | <0.001 |
| Q2 | 1417 (25.1) | 947 (26.2) | 470 (23.1) | |
| Q3 | 1426 (25.3) | 949 (26.4) | 477 (23.4) | |
| Q4 | 1319 (23.3) | 801 (22.1) | 518 (25.6) | |
| Missing | 108 | 60 | 48 | |
| Smoking | 1041 (18.2) | 727 (19.9) | 314 (15.2) | <0.001 |
| Obesity | 2877 (50.3) | 2014 (55.0) | 863 (41.9) | <0.001 |
| Study years (NIS dataset) | ||||
| 2005–2009 | 1132 (19.2) | 235 (6.3) | 897 (42.4) | <0.001 |
| 2010–2015 | 3401 (59.3) | 2519 (68.4) | 882 (42.8) | |
| 2016–2018 | 1212 (21.5) | 914 (25.2) | 298 (14.8) | |
| Weekend admission | 154 (2.7) | 106 (2.9) | 48 (2.3) | 0.130 |
| Hospital bed size | ||||
| Small | 533 (9.1) | 313 (8.4) | 220 (10.2) | 0.229 |
| Medium | 1192 (21.0) | 744 (20.6) | 448 (21.6) | |
| Large | 3978 (70.0) | 2577 (71.0) | 1401 (68.2) | |
| Missing | 42 | 34 | 8 | |
| Hospital region | ||||
| Northeast | 1339 (23.5) | 766 (20.9) | 573 (28.1) | <0.001 |
| South | 1264 (21.9) | 935 (25.3) | 329 (15.9) | |
| Midwest | 1799 (31.1) | 1148 (31.2) | 651 (30.9) | |
| West | 1343 (23.5) | 819 (22.6) | 524 (25.1) | |
| Hospital location/teaching status | ||||
| Rural | 101 (1.7) | 31 (0.8) | 70 (3.4) | <0.001 |
| Urban nonteaching | 1085 (18.7) | 629 (17.1) | 456 (21.4) | |
| Urban teaching | 4517 (79.6) | 2974 (82.0) | 1543 (75.2) | |
| Missing | 42 | 34 | 8 | |
| Major comorbidities | ||||
| CKD | 234 (4.1) | 177 (4.9) | 57 (2.8) | <0.001 |
| Ischemic heart disease | 736 (12.8) | 481 (13.1) | 255 (12.2) | 0.289 |
| Congestive heart failure | 364 (6.3) | 233 (6.4) | 131 (6.3) | 0.934 |
| Atrial fibrillation | 431 (7.5) | 282 (7.7) | 149 (7.1) | 0.449 |
| Anemia | 540 (9.4) | 351 (9.6) | 189 (9.1) | 0.545 |
| COPD | 752 (13.1) | 488 (13.2) | 264 (12.7) | 0.571 |
| Cerebrovascular disease | 138 (2.4) | 94 (2.6) | 44 (2.1) | 0.229 |
| Peripheral vascular disease | 132 (2.3) | 95 (2.6) | 37 (1.8) | 0.042 |
| Severe liver disease | 30 (0.5) | 23 (0.6) | 7 (0.3) | 0.141 |
| Rheumatic disease | 101 (1.8) | 66 (1.8) | 35 (1.7) | 0.752 |
| Coagulopathy | 105 (1.8) | 72 (2.0) | 33 (1.6) | 0.268 |
| Diabetes with chronic complications | 537 (9.4) | 400 (11.0) | 137 (6.7) | <0.001 |
| Emergency admission | 626 (10.9) | 353 (9.7) | 273 (13.2) | 0.001 |
| CCI | ||||
| 0 | 3821 (66.4) | 2380 (64.8) | 1441 (69.4) | <0.001 |
| 1 | 1102 (19.2) | 717 (19.5) | 385 (18.5) | |
| 2+ | 822 (14.4) | 571 (15.6) | 251 (12.1) | |
| Total (n = 5745) | Robotic (n = 3668) | Pure Laparoscopic (n = 2077) | p-Value | |
|---|---|---|---|---|
| Complications, any | 1050 (18.3) | 688 (18.8) | 362 (17.4) | 0.203 |
| In-hospital death | 13 (0.2) | 7 (0.2) | 6 (0.3) | 0.476 |
| AMI | 22 (0.4) | 11 (0.3) | 11 (0.5) | 0.201 |
| CVA | 56 (1.0) | 43 (1.2) | 13 (0.6) | 0.021 |
| VTE | 44 (0.8) | 23 (0.6) | 21 (1.0) | 0.094 |
| Pneumonia | 49 (0.9) | 30 (0.8) | 19 (0.9) | 0.660 |
| Sepsis | 85 (1.5) | 54 (1.5) | 31 (1.5) | 0.928 |
| Surgical site infection | 21 (0.4) | 16 (0.4) | 5 (0.2) | 0.239 |
| Major blood loss | 577 (10.0) | 358 (9.8) | 219 (10.5) | 0.377 |
| Respiratory failure/mechanical ventilation | 265 (4.6) | 177 (4.8) | 88 (4.2) | 0.270 |
| Wound dehiscence | 25 (0.4) | 13 (0.4) | 12 (0.6) | 0.223 |
| AKI | 243 (4.3) | 176 (4.8) | 67 (3.2) | 0.004 |
| UTI | 41 (0.7) | 25 (0.7) | 16 (0.8) | 0.688 |
| LOS a | 2.32 ± 0.05 | 2.19 ± 0.04 | 2.57 ± 0.10 | <0.001 |
| Unfavorable discharge a | 278 (4.9) | 165 (4.6) | 113 (5.5) | 0.103 |
| Total hospital costs | 53,120.7 ± 799.0 | 58,055.8 ± 915.3 | 44,253.8 ± 1255.5 | <0.001 |
| Complication, Any | Unfavorable Discharge a | LOS a | Total Hospital Costs, US Dollars | |
|---|---|---|---|---|
| aOR b (95% CI) | aOR b (95% CI) | aBeta b (95% CI) | aBeta b (95% CI) | |
| Pure laparoscopic | Ref. | Ref. | Ref. | Ref. |
| Robot-assisted | 0.88 (0.75, 1.04) | 0.63 (0.46, 0.85) | −0.46 (−0.57, −0.35) | 6129.93 (4448.74, 7811.12) |
| Number of Women b | Complication, Any | Unfavorable Discharge a | LOS a | |
|---|---|---|---|---|
| aOR c (95% CI) | aOR c (95% CI) | aBeta c (95% CI) | ||
| Age | ||||
| <60 | 1617 | 0.92 (0.66, 1.29) | 0.93 (0.27, 3.15) | −0.35 (−0.43, −0.26) |
| >60 | 3506 | 0.87 (0.72, 1.05) | 0.60 (0.44, 0.80) | −0.50 (−0.65, −0.35) |
| Obesity status | ||||
| Obese | 3587 | 0.88 (0.71, 1.11) | 0.65 (0.43, 0.98) | −0.42 (−0.54, −0.30) |
| Non-obese | 2536 | 0.92 (0.73, 1.16) | 0.62 (0.41, 0.94) | −0.45 (−0.62, −0.28) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.-P.; Tseng, C.-J. Short-Term Outcome of Robotic versus Laparoscopic Hysterectomy for Endometrial Cancer in Women with Diabetes: Analysis of the US Nationwide Inpatient Sample. J. Clin. Med. 2023, 12, 7713. https://doi.org/10.3390/jcm12247713
Shen H-P, Tseng C-J. Short-Term Outcome of Robotic versus Laparoscopic Hysterectomy for Endometrial Cancer in Women with Diabetes: Analysis of the US Nationwide Inpatient Sample. Journal of Clinical Medicine. 2023; 12(24):7713. https://doi.org/10.3390/jcm12247713
Chicago/Turabian StyleShen, Huang-Pin, and Chih-Jen Tseng. 2023. "Short-Term Outcome of Robotic versus Laparoscopic Hysterectomy for Endometrial Cancer in Women with Diabetes: Analysis of the US Nationwide Inpatient Sample" Journal of Clinical Medicine 12, no. 24: 7713. https://doi.org/10.3390/jcm12247713





