SCAI Staging Application for Acute Myocardial Infarction-Related Cardiogenic Shock at a Single-Center Russian Registry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical, Laboratory, and Instrumental Data
2.3. Statistical Analysis
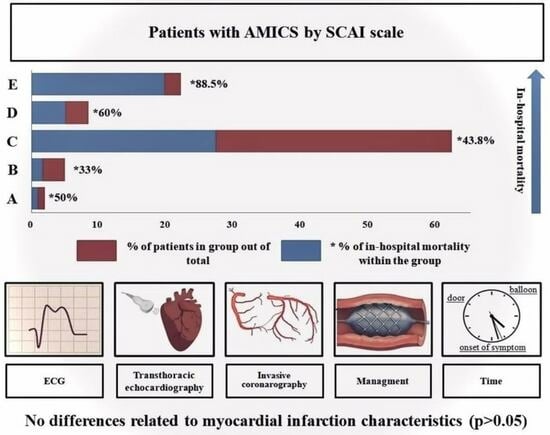
3. Results
3.1. Clinical Data
3.2. Comparison of Patient Characteristics Depending on the CS stage Classification on the SCAI Scale
3.3. Associations between MI Characteristics and SCAI Shock Stage
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alonso, D.R.; Scheidt, S.; Post, M.; Killip, T. Pathophysiology of cardiogenic shock. Quantification of myocardial necrosis, clinical, pathologic and electrocardiographic correlations. Circulation 1973, 48, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S. Phenotyping and Hemodynamic Assessment in Cardiogenic Shock: From Physiology to Clinical Application. Cardiol. Ther. 2022, 11, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Thangam, M.; Luke, A.A.; Johnson, D.Y.; Amin, A.P.; Lasala, J.; Huang, K.; Maddox, K.E.J. Sociodemographic differences in utilization and outcomes for temporary cardiovascular mechanical support in the setting of cardiogenic shock. Am. Heart J. 2021, 236, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Strom, J.B.; Zhao, Y.; Shen, C.; Chung, M.; Pinto, D.S.; Popma, J.J.; Yeh, R.W. National trends, predictors of use, and in-hospital outcomes in mechanical circulatory support for cardiogenic shock. EuroIntervention 2018, 13, e2152–e2159. [Google Scholar] [CrossRef] [PubMed]
- Ni Hici, T.; Boardman, H.M.; Baig, K.; Stafford, J.L.; Cernei, C.; Bodger, O.; Westaby, S. Mechanical assist devices for acute cardiogenic shock. Cochrane Database Syst. Rev. 2020, 6, CD013002. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, N.; Patel, N.; Saouma, S.; Anugu, V.R.; Anugula, D.; Asti, D.; Mehta, V.; Kumar, V.; Atti, V.; Edla, S.; et al. Utilization of the Impella for hemodynamic support during percutaneous intervention and cardiogenic shock: An insight. Expert Rev. Med. Devices 2017, 14, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Panteleev, O.O.; Ryabov, V.V. Cardiogenic shock: What’s new? Sib. J. Clin. Exp. Med. 2021, 36, 45–51. [Google Scholar] [CrossRef]
- Stretch, R.; Sauer, C.M.; Yuh, D.D.; Bonde, P. National trends in the utilization of short-term mechanical circulatory support: Incidence, outcomes, and cost analysis. J. Am. Coll. Cardiol. 2014, 64, 1407–1415. [Google Scholar] [CrossRef]
- Vyshlov, V.V.; Panteleev, O.O.; Ryabov, V.V. Intra-aortic balloon pump in patients with myocardial infarction and cardiogenic shock of stages A and B. Kardiologiia 2022, 62, 68–72. [Google Scholar] [CrossRef]
- Kaddoura, R.; Elbdri, S. Current evidence in the diagnosis and management of cardiogenic shock complicating acute coronary syndrome. Rev. Cardiovasc. Med. 2021, 22, 691–715. [Google Scholar] [CrossRef]
- Ghajar, A.; Ordonez, C.P.; Philips, B.; Pinzon, P.Q.; Fleming, L.M.; Motiwala, S.R.; Sriwattanakomen, R.; Ho, J.E.; Grandin, E.W.; Sabe, M.; et al. Cardiogenic shock related cardiovascular disease mortality trends in US population: Heart failure vs. acute myocardial infarction as contributing causes. Int. J. Cardiol. 2022, 367, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.D.; Bohula, E.A.; Morrow, D.A. Epidemiology and causes of cardiogenic shock. Curr. Opin. Crit. Care 2021, 27, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Miranda, C.Y.; Hall, S.A. Cardiogenic Shock in Patients with Advanced Chronic Heart Failure. Methodist DeBakey Cardiovasc. J. 2020, 16, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Zweck, E.; Thayer, K.L.; Helgestad, O.K.L.; Kanwar, M.; Ayouty, M.; Garan, A.R.; Hernandez-Montfort, J.; Mahr, C.; Wencker, D.; Sinha, S.S.; et al. Phenotyping Cardiogenic Shock. J. Am. Heart Assoc. 2021, 10, e020085. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; Soussi, S.; Lawler, P.R.; Kennedy, J.N.; Kashani, K.B. Validation of cardiogenic shock phenotypes in a mixed cardiac intensive care unit population. Catheter. Cardiovasc. Interv. 2022, 99, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Rivera, M.R.; Shaikh, P.; Kumar, A.; May, A.; Mahtta, D.; Jentzer, J.; Civitello, A.; Katz, J.; Naidu, S.S.; et al. Key Concepts Surrounding Cardiogenic Shock. Curr. Probl. Cardiol. 2022, 47, 101303. [Google Scholar] [CrossRef] [PubMed]
- Palacios Ordonez, C.; Garan, A.R. The landscape of cardiogenic shock: Epidemiology and current definitions. Curr. Opin. Cardiol. 2022, 37, 236–240. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure [published correction appears in Eur Heart J. 2021 Oct 14]. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Kapur, N.K.; Kanwar, M.; Sinha, S.S.; Thayer, K.L.; Garan, A.R.; Hernandez-Montfort, J.; Zhang, Y.; Li, B.; Baca, P.; Dieng, F.; et al. Criteria for Defining Stages of Cardiogenic Shock Severity. J. Am. Coll. Cardiol. 2022, 80, 185–198. [Google Scholar] [CrossRef]
- Freund, A.; Pöss, J.; de Waha-Thiele, S.; Meyer-Saraei, R.; Fuernau, G.; Eitel, I.; Feistritzer, H.-J.; Rubini, M.; Huber, K.; Windecker, S.; et al. Comparison of risk prediction models in infarct-related cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 890–897. [Google Scholar] [CrossRef]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Grines, C.L.; Marshall, J.J. It’s not shocking that the SCAI shock classification works. Catheter. Cardiovasc. Interv. 2020, 96, 1143–1144. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.S.; Baran, D.A.; Jentzer, J.C.; Hollenberg, S.M.; van Diepen, S.; Basir, M.B.; Grines, C.L.; Diercks, D.B.; Hall, S.; Kapur, N.K.; et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J. Am. Coll. Cardiol. 2022, 79, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, J.C.; van Diepen, S.; Barsness, G.W.; Henry, T.D.; Menon, V.; Rihal, C.S.; Naidu, S.S.; Baran, D.A. Cardiogenic Shock Classification to Predict Mortality in the Cardiac Intensive Care Unit. J. Am. Coll. Cardiol. 2019, 74, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Baran, D.A.; Long, A.; Badiye, A.P.; Stelling, K. Prospective validation of the SCAI shock classification: Single center analysis. Catheter. Cardiovasc. Interv. 2020, 96, 1339–1347. [Google Scholar] [CrossRef]
- Morici, N.; Frea, S.; Bertaina, M.; Sacco, A.; Corrada, E.; Dini, C.S.; Briani, M.; Tedeschi, M.; Saia, F.; Colombo, C.; et al. SCAI stage reclassification at 24 h predicts outcome of cardiogenic shock: Insights from the Altshock-2 registry. Catheter. Cardiovasc. Interv. 2023, 101, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Hanson, I.D.; Tagami, T.; Mando, R.; Balla, A.K.; Dixon, S.R.; Timmis, S.; Almany, S.; Naidu, S.S.; Baran, D.; Lemor, A.; et al. SCAI shock classification in acute myocardial infarction: Insights from the National Cardiogenic Shock Initiative. Catheter. Cardiovasc. Interv. 2020, 96, 1137–1142. [Google Scholar] [CrossRef]
- González-Pacheco, H.; Gopar-Nieto, R.; Araiza-Garaygordobil, D.; Briseño-Cruz, J.L.; Eid-Lidt, G.; Ortega-Hernandez, J.A.; Sierra-Lara, D.; Altamirano-Castillo, A.; Mendoza-García, S.; Manzur-Sandoval, D.; et al. Application of the SCAI classification to admission of patients with cardiogenic shock: Analysis of a tertiary care center in a middle-income country. PLoS ONE 2022, 17, e0273086. [Google Scholar] [CrossRef]
- Domienik-Karłowicz, J.; Kupczyńska, K.; Michalski, B.; Kapłon-Cieślicka, A.; Darocha, S.; Dobrowolski, P.; Wybraniec, M.; Wańha, W.; Jaguszewski, M. Fourth universal definition of myocardial infarction. Selected messages from the European Society of Cardiology document and lessons learned from the new guidelines on ST-segment elevation myocardial infarction and non-ST-segment elevation-acute coronary syndrome. Cardiol. J. 2021, 28, 195–201. [Google Scholar] [CrossRef]
- Kolte, D.; Khera, S.; Aronow, W.S.; Mujib, M.; Palaniswamy, C.; Sule, S.; Jain, D.; Gotsis, W.; Ahmed, A.; Frishman, W.H.; et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J. Am. Heart Assoc. 2014, 3, e000590. [Google Scholar] [CrossRef]
- Helgestad, O.K.; Josiassen, J.; Hassager, C.; Jensen, L.O.; Holmvang, L.; Sørensen, A.; Frydland, M.; Lassen, A.T.; Udesen, N.L.; Schmidt, H.; et al. Temporal trends in incidence and patient characteristics in cardiogenic shock following acute myocardial infarction from 2010 to 2017: A Danish cohort study. Eur. J. Heart Fail. 2019, 21, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Beesley, S.J.; Weber, G.; Sarge, T.; Nikravan, S.; Grissom, C.K.; Lanspa, M.J.; Shahul, S.; Brown, S.M. Septic Cardiomyopathy. Crit. Care Med. 2018, 46, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Cuinet, J.; Garbagnati, A.; Rusca, M.; Yerly, P.; Schneider, A.G.; Kirsch, M.; Liaudet, L. Cardiogenic shock elicits acute inflammation, delayed eosinophilia, and depletion of immune cells in most severe cases. Sci. Rep. 2020, 10, 7639. [Google Scholar] [CrossRef] [PubMed]
- Parenica, J.; Jarkovsky, J.; Malaska, J.; Mebazaa, A.; Gottwaldova, J.; Helanova, K.; Litzman, J.; Dastych, M.; Tomandl, J.; Spinar, J.; et al. Infectious Complications and Immune/Inflammatory Response in Cardiogenic Shock Patients: A Prospective Observational Study. Shock 2017, 47, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Meisel, S.R.; Pauzner, H.; Shechter, M.; Zeidan, Z.; David, D. Peripheral monocytosis following acute myocardial infarction: Incidence and its possible role as a bedside marker of the extent of cardiac injury. Cardiology 1998, 90, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, Y.; Anzai, T.; Yoshikawa, T.; Asakura, Y.; Takahashi, T.; Ishikawa, S.; Mitamura, H.; Ogawa, S. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction:a possible role for left ventricular remodeling. J. Am. Coll. Cardiol. 2002, 39, 241–246. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, Q.; Hu, T. Association of Lymphocyte to Monocyte Ratio and Risk of in-Hospital Mortality in Patients with Cardiogenic Shock: A Propensity Score Matching Study. Int. J. Gen. Med. 2021, 14, 4459–4468. [Google Scholar] [CrossRef]
- Riese, U.; Brenner, S.; Döcke, W.D.; Prösch, S.; Reinke, P.; Oppert, M.; Volk, H.-D.; Platzer, C. Catecholamines induce IL-10 release in patients suffering from acute myocardial infarction by transactivating its promoter in monocytic but not in T-cells. Mol. Cell Biochem. 2000, 212, 45–50. [Google Scholar] [CrossRef]
- De Werra, I.; Zanetti, G.; Jaccard, C.; Chioléro, R.; Schaller, M.D.; Yersin, B.; Glauser, M.P.; Calandra, T.; Heumann, D. CD14 expression on monocytes and TNF alpha production in patients with septic shock, cardiogenic shock or bacterial pneumonia. Swiss Med. Wkly. 2001, 131, 35–40. [Google Scholar] [CrossRef]





| Stage | Characteristics |
|---|---|
| At risk (A) |
|
| Beginning (B) |
|
| Classic (C) |
|
| Deteriorating (D) |
|
| Extremis (E) |
|
| Term | Definition |
| Large acute MI |
|
| Hypotension/tachycardia | Presence of any of the following criteria:
|
| Hypoperfusion | Presence of any following criteria:
|
| Deterioration | Presence of all following criteria:
|
| RS | Presence of any of the following criteria:
|
| Parameter | N | A + B (n = 8) | C (n = 73) | D (n = 10) | E (n = 26) | p Value | Fisher’s Exact Test |
|---|---|---|---|---|---|---|---|
| Demographic data | |||||||
| Age, years | 65.5 (58; 75.5) | 73 (66; 81) | 80.5 (73.3; 82) | 80 (78.3; 86.5) | 0.009 | ||
| Male, n (%) | 47 | 0 (0) | 34 (46.6) | 5 (50) | 8 (30.8) | 0.008 | 0.005 |
| Female, n (%) | 70 | 8 (100) | 39 (53.4) | 5 (50) | 18 (69.2) | ||
| Comorbidity | |||||||
| Respiratory disease, n (%) | 116 | 1 (12.5) | 17 (23.6) | 1 (10) | 5 (19.2) | 0.698 | 0.812 |
| Urinary system diseases, n (%) | 117 | 0 (0) | 23 (31.5) | 4 (40) | 4 (15.4) | 0.093 | 0.086 |
| Gastrointestinal diseases, n (%) | 117 | 6 (75) | 50 (68.5) | 8 (80) | 13 (50.9) | 0.229 | 0.257 |
| Oncology, n (%) | 115 | 0 (0) | 4 (5.6) | 0 (0) | 0 (0) | 0.463 | 0.785 |
| Cerebrovascular disease, n (%) | 116 | 2 (25) | 11 (15.3) | 0 (0) | 4 (15.4) | 0.487 | 0.5 |
| CAD risk factors | |||||||
| Smoking history, n (%) | 79 | 4 (57.1) | 21 (39.6) | 1 (11.1) | 1 (10) | 0.07 | 0.073 |
| Alcohol consumption, n (%) | 84 | 1 (14.3) | 5 (8.9) | 0 (0) | 0 (0) | 0.489 | 0.554 |
| Hypertension history, n (%) | 117 | 7 (87.5) | 69 (94.5) | 9 (90) | 24 (92.3) | 0.849 | |
| Diabetes, n (%) | 117 | 1 (12.5) | 25 (34.2) | 5 (50) | 3 (11.5) | 0.185 | 0.083 |
| Intensive care measures | |||||||
| MV, n (%) | 117 | 3 (37.5) | 52 (71.2) | 9 (90) | 26 (100) | <0.001 | <0.001 |
| IABP, n (%) | 110 | 0 (0) | 7 (10.4) | 10 (100) | 10 (38.5) | <0.001 | <0.001 |
| Inotropes, n (%) | 117 | 4 (50) | 46 (63) | 9 (90) | 22 (84.6) | 0.054 | 0.047 |
| RRT, n (%) | 109 | 0 (0) | 7 (10.1) | 1 (10) | 3 (13) | 0.8 | 0.945 |
| Blood transfusion, n (%) | 117 | 1 (12.5) | 14 (19.2) | 4 (40) | 5 (19.2) | 0.432 | 0.491 |
| PCI, n (%) | 111 | 5 (62.5) | 49 (70) | 8 (80) | 14 (60.9) | 0.903 | 0.806 |
| Duration of intensive care measures | |||||||
| MV duration, days | 90 | 1 (1; 1) | 3 (1; 7) | 2 (2; 8) | 1 (1; 4) | 0.07 | |
| ICU LOS, days | 119 | 1.5 (1; 5) | 5 (2; 13) | 9.5 (4; 25.3) | 1 (1; 5) | <0.001 | |
| In-hospital LOS days | 117 | 5 (1; 10.3) | 10 (3; 16) | 10.5 (5.3; 25.3) | 1 (1; 5) | <0.001 | |
| IABP duration, hours | 24 | NaN | 61 (49; 61) | 45 (30; 47) | 129 (84; 664) | 0.084 | |
| Haemotransfusion, doses | 24 | NaN | 2.9 (2; 3.5) | 6.3 (1.8; 8.5) | 2 (1; 2) | 0.404 | |
| Duration of RRT, min | 7 | NaN | 3 (2.8; 3) | NaN | 3 (2.5; 3.5) | 0.693 | |
| Clinical data | |||||||
| GCS, score | 113 | 15 (15; 15) | 13 (10; 15) | 15 (14; 15) | 8 (6.8; 12) | <0.001 | |
| SBP, mm Hg | 114 | 96.5 (86.3; 131) | 90 (76.5; 106) | 91.5 (89.3; 94.8) | 70 (60; 86) | <0.001 | |
| Mean BP, mm Hga | 115 | 72.2 (64.8; 93.8) | 69 (53; 82) | 71 (65; 85) | 50 (44; 60) | 0.001 | |
| HR, beats per minute | 116 | 87 (77; 100) | 87 (65; 108) | 101(94; 117) | 99 (70; 116) | 0.406 | |
| RR, per minute | 103 | 18 (17.8; 19.3) | 18 (16; 22) | 20 (17; 24) | 18 (16; 20) | 0.754 | |
| CVP, mm Hg | 83 | 7 (6; 8.25) | 12 (9; 16) | 12 (9; 13) | 16 (10; 18) | 0.062 | |
| PHv | 95 | 7.39 (7.36; 7.44) | 7.3 (7.27; 7.34) | 7.29 (7.26; 7.31) | 7.14 (7.06; 7.18) | <0.001 | |
| Laboratory (first 24 h) | |||||||
| Lactate, mmol/L | 104 | 1.7 (1.3; 1.7) | 3.4 (2.4; 5.6) | 4.8 (4.2; 6) | 8.6 (6.9; 11.4) | <0.001 | |
| Platelet, 103/microL | 117 | 222 (188; 263) | 248 (190; 296) | 202 (187.8; 269.8) | 184 (139.3; 238) | 0.032 | |
| RBC count, 106/microL | 117 | 4.46 (4.1; 4.51) | 4.38 (3.97; 5.05) | 4.5 (4.28; 4.7) | 4.19 (3.64; 4.9) | 0.472 | |
| Hemoglobin, g/dL | 117 | 132 (119; 143) | 133 (115;144) | 129 (123.3; 135.8) | 119 (103.3; 137.8) | 0.32 | |
| Hematocrit, % | 117 | 0.37 (0.35;0.42) | 0.39 (0.34; 0.43) | 0.38 (0.35; 0.4) | 0.36 (0.31; 0.43) | 0.609 | |
| WBC, 109/microL | 117 | 12.2 (10.7; 13.9) | 13.7 (10.4; 16) | 11.5 (10.1; 14.6) | 13.7 (8.8; 17.6) | 0.856 | |
| Monocytes, 109/microL | 117 | 1.15 (0.96; 1.23) | 0.98 (0.68; 1.32) | 0.53 (0.44; 0.68) | 0.78 (0.49: 0.94) | 0.005 | |
| Creatinine, mcmol/L | 117 | 86.5 (76; 112) | 131 (97; 166) | 134.5 (104;172.5) | 142.5 (116; 188) | 0.028 | |
| eGFR according to CKD-EPI, mL/min/1.73 m2 | 115 | 72 (56.3; 86) | 39.5 (28.3; 56) | 37 (28.5; 46.5) | 35 (23; 47) | 0.006 | |
| Total protein, g/dL | 74 | 64.5 (60.9; 69.4) | 66 (59.4; 72) | 65 (59.3; 68.8) | 62.4 (53.9; 65) | 0.417 | |
| Glucose, mmol/L | 116 | 7.73 (6.59; 8.87) | 10.6 (8.5; 15.8) | 11 (9.2; 14.5) | 13.2 (8.2; 16.7) | 0.235 | |
| TBil, mcmoll/L | 80 | 10.6 (6.88; 24.2) | 14.2 (10; 21.7) | 19 (13; 28.4) | 16.2 (11.3; 43.4) | 0.612 | |
| Echocardiography at admission | |||||||
| SV, ml | 53 | 60 (43.5; 65) | 41 (36; 52) | 37 (31; 38) | 41.5 (32; 52.3) | 0.504 | |
| MM, g/ml | 42 | 267 (209; 288) | 211 (180; 248) | NaN | 204 (174.3; 272.8) | 0.891 | |
| MMI | 42 | 142 (114; 143) | 112 (99; 128) | NaN | 112 (103; 151) | 0.815 | |
| IVC, mm | 51 | 16 (15; 17) | 20.5 (17.4; 22.1) | 20 (17.8; 20) | 19 (18; 22.5) | 0.43 | |
| LA, mL | 53 | 81.2 (73.8; 88.6) | 61.5 (43.5; 92) | 41 (40; 47) | 52.6 (43.3; 72) | 0.344 | |
| RA, mL | 34 | 70.2 (59.3; 81) | 59.7 (42; 88.3) | NaN | 53 (40; 70) | 0.604 | |
| Mortality, n (%) | 117 | 3 (37.5) | 32 (43.8) | 6 (60%) | 23 (88.5) | 0.002 | <0.001 |
| Risk scales | |||||||
| ORBI, score | 79 | 10.5 (8.5; 12.5) | 17 (12; 18.3) | 19 (15.5; 22.5) | 19 (14; 22) | 0.054 | |
| ORBI, % | 79 | 9 (5.2; 15.6) | 35.4 (12.4; 45.6) | 54.2 (28.3; 72.7) | 47 (21.7; 70) | 0.045 | |
| SOFA, score (at admission) | 27 | 5 (5; 5) | 10 (6; 12.5) | 10.5 (10; 11) | 14.5 (11; 15) | 0.129 | |
| GRACE, % | 117 | 7.5 (6; 16.3) | 30 (12; 50) | 29.5 (14; 53.3) | 60 (40; 80) | <0.001 | |
| CRUSADE, % | 117 | 9.3 (6.5; 10.4) | 13.6 (10.7; 19.5) | 15.5 (9; 19.5) | 19.5 (16.7; 19.5) | 0.002 | |
| GENEVA, score | 116 | 4 (1; 5) | 4 (1; 6) | 6 (4.3; 6) | 5 (1; 6) | 0.136 | |
| Dosage of vasopressors | |||||||
| Dopamine dosage, mcg/kg/min | 60 | 5 (4; 6) | 5 (5; 10) | 7 (3.5;8) | 10 (6; 13.8) | 0.113 | |
| Epinephrine dosage, mcg/kg/min | 11 | NaN | 0.05 (0.02; 0.1) | NaN | 0.1 (0.1; 0.2) | 0.146 | |
| Nonepinephrine dosage, mcg/kg/min | 51 | 0.05 (0.05; 0.05) | 0.25 (0.18; 0.5) | 0.3 (0.15; 0.6) | 0.4 (0.25; 0.9) | 0.095 | |
| VIS at admission | 84 | 5 (2.5; 6) | 10 (5; 29) | 23.5 (8.5; 46) | 40 (10; 62) | 0.023 | |
| CPR prior to hospital arrival | 116 | 0 (0) | 3 (4.2) | 0 (0) | 4 (15.4) | 0.106 | 0.140 |
| Without CPR | 116 | 5 (62.5) | 45 (62.5) | 9 (90) | 11 (42.3) | 0.106 | 0.140 |
| CPR in hospital | 116 | 3 (37.5) | 24 (33.3) | 1 (10) | 11 (42.3) | 0.106 | 0.140 |
| Levels 2,3,4,5 | Predictor | OR | 95% CI | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2–5 | Constant | 0.0 | 0.0–0.0 | 0.00 | ||||||
| MV: 1—yes, 2—no; 2–1 | 2.14 × 1081 | 2.67 × 1078–1.72 × 1084 | 0.00 | |||||||
| Lactate at the admission >2 mmol/L: 1—yes, 2—no | 0.0 | 0.0–0.0 | 0.00 | |||||||
| Monocytes at the admission | 6.63 × 1016 | 6.73 × 1013–6.54 × 1019 | 0.00 | |||||||
| SBP | 0.51 | 0.24–1.06 | 0.07 | |||||||
| pHv | 1.30 × 1019 | 1.72 × 1014–9.91 × 1023 | 1.64 × 10−14 | |||||||
| spO2/FioO2 | 0.67 | 0.57–0.79 | 1.81 × 10−6 | |||||||
| IABP—1, without IABP—2; 2–1 | 0.0 | 0.0–0.0 | 0.00 | |||||||
| 3–5 | Constant | 0.0 | 0.0–0.0 | 0.00 | ||||||
| MV: 1—yes, 2—no; 2–1 | 2.24 × 1045 | 2.79 × 1042–1.80 × 1048 | 0.00 | |||||||
| Lactate at the admission >2 mmol/L: 1—yes, 2—no | 0.66 | 0.41–1.07 | 0.09 | |||||||
| Monocytes at the admission | 34.39 | 1.65–716.91 | 0.02 | |||||||
| SBP | 1.04 | 0.99–1.10 | 0.16 | |||||||
| pHv | 2.45 × 107 | 8.24 × 106– 7.30 × 107 | 0.00 | |||||||
| spO2/FioO2 | 1.01 | 0.99–1.02 | 0.31 | |||||||
| IABP—1, without IABP—2; 2–1 | 6.71 | 0.31–147.54 | 0.23 | |||||||
| 4–5 | Constant | 0.0 | 0.0–0.0 | 0.00 | ||||||
| MV: 1—yes, 2—no; 2–1 | 0.00 | 9.85 × 1052–9.85 × 1052 | 9.85 × 1052 | |||||||
| Lactate at the admission >2 mmol/L: 1—yes, 2—no | 1.34 | 0.27–6.67 | 0.72 | |||||||
| Monocytes at admission | 0.0 | 0.0–0.0 | 0.00 | |||||||
| SBP | 1.33 | 1.16–1.52 | 4.35 × 10−5 | |||||||
| pHv | 233.67 | 42.14–1295.62 | 4.35 × 10−10 | |||||||
| spO2/FioO2 | 0.98 | 0.95–1.00 | 0.76 | |||||||
| IABP—1, without IABP—2; 2–1 | 0.0 | 0.0–0.0 | 0.00 | |||||||
| Model Fit Measures | ||||||||||
| Overall Model Test | ||||||||||
| Model | Deviance | AIC | R²N | χ² | df | p | ||||
| 1 | 36.59 | 84.58 | 0.78 | 102.28 | 21 | 1.1383 × 10−12 | ||||
| Omnibus Likelihood Ratio Tests | ||||||||||
| Predictor | χ² | Df | p | |||||||
| MV: 1—yes, 2—no | 5.60 | 3 | 0.13 | |||||||
| Lactate at the admission >2 mmol/L: 1—yes, 2—no | 5.49 | 3 | 0.14 | |||||||
| Monocytes at admission | 6.09 | 3 | 0.11 | |||||||
| SBP | 6.245 | 3 | 0.10 | |||||||
| pHv | 22.19 | 3 | 5.94 × 10−5 | |||||||
| IABP—1, without IABP—2 | 6.56 | 3 | 0.09 | |||||||
| spO2/FioO2 | −5.24 | 3 | 1.00 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryabov, V.V.; Panteleev, O.O.; Kercheva, M.A.; Gorokhovsky, A.A.; Syrkina, A.G.; Margolis, N.Y. SCAI Staging Application for Acute Myocardial Infarction-Related Cardiogenic Shock at a Single-Center Russian Registry. J. Clin. Med. 2023, 12, 7739. https://doi.org/10.3390/jcm12247739
Ryabov VV, Panteleev OO, Kercheva MA, Gorokhovsky AA, Syrkina AG, Margolis NY. SCAI Staging Application for Acute Myocardial Infarction-Related Cardiogenic Shock at a Single-Center Russian Registry. Journal of Clinical Medicine. 2023; 12(24):7739. https://doi.org/10.3390/jcm12247739
Chicago/Turabian StyleRyabov, Vyacheslav V., Oleg O. Panteleev, Maria A. Kercheva, Alexei A. Gorokhovsky, Anna G. Syrkina, and Natalia Y. Margolis. 2023. "SCAI Staging Application for Acute Myocardial Infarction-Related Cardiogenic Shock at a Single-Center Russian Registry" Journal of Clinical Medicine 12, no. 24: 7739. https://doi.org/10.3390/jcm12247739
APA StyleRyabov, V. V., Panteleev, O. O., Kercheva, M. A., Gorokhovsky, A. A., Syrkina, A. G., & Margolis, N. Y. (2023). SCAI Staging Application for Acute Myocardial Infarction-Related Cardiogenic Shock at a Single-Center Russian Registry. Journal of Clinical Medicine, 12(24), 7739. https://doi.org/10.3390/jcm12247739