Review of Clinical Applications of Dual-Energy CT in Patients after Endovascular Aortic Repair
Abstract
:1. Introduction
2. Dual-Energy Acquisition Techniques
2.1. Rapid-Kilovoltage Switching DECT
2.2. Dual-Source DECT
2.3. Split-Filter DECT
2.4. Multilayer Detector CT
3. Dual-Energy CT Postprocessing Techniques
3.1. Material Decomposition
3.2. Virtual Noncontrast and Iodine Mapping
3.3. Virtual Noncalcium
3.4. Virtual Monoenergetic Images (VMI), Noise Optimization
4. Applications of DECT in Patients after EVAR
4.1. Radiation Dose Reduction
4.2. Contrast Agent Volume Reduction
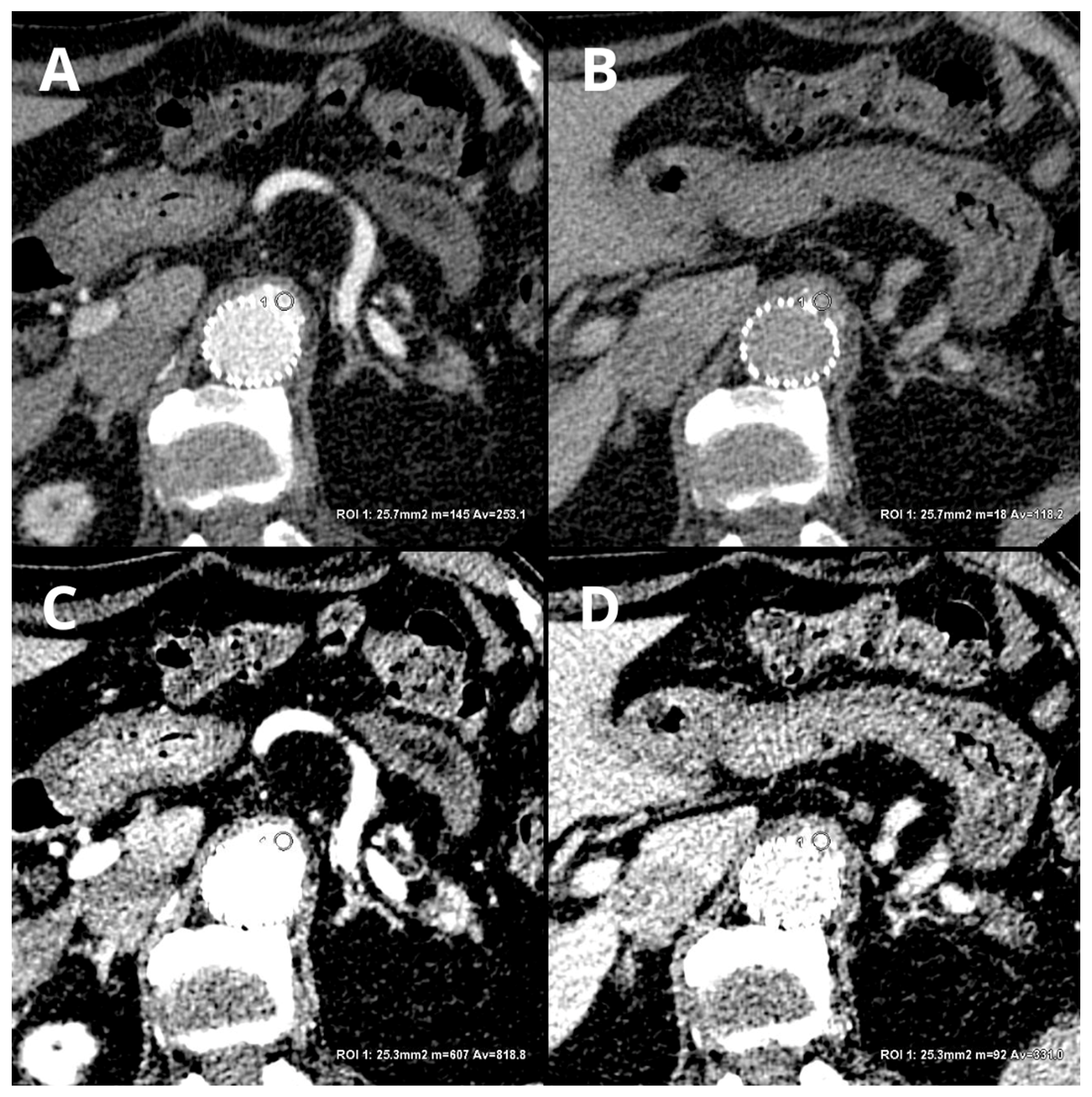
4.3. Endoleak Detection
4.4. Metal Artifact Reduction
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hounsfield, G.N. Computerized transverse axial scanning (tomography): I. Description of system. Br. J. Radiol. 1973, 46, 1016–1022. [Google Scholar] [CrossRef]
- Stather, P.; Sidloff, D.; Rhema, I.; Choke, E.; Bown, M.; Sayers, R. A review of current reporting of abdominal aortic aneurysm mortality and prevalence in the literature. Eur. J. Vasc. Endovasc. Surg. 2014, 47, 240–242. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef]
- Lederle, F.A.; Johnson, G.R.; Wilson, S.E.; Chute, E.P.; Littooy, F.N.; Bandyk, D.; Krupski, W.C.; Barone, G.W.; Acher, C.W.; Ballard, D.J. Prevalence and associations of abdominal aortic aneurysm detected through screening. Ann. Intern. Med. 1997, 126, 441–449. [Google Scholar] [CrossRef]
- Schlösser, F.; Gusberg, R.; Dardik, A.; Lin, P.; Verhagen, H.; Moll, F.; Muhs, B. Aneurysm Rupture after EVAR: Can the Ultimate Failure be Predicted? Eur. J. Vasc. Endovasc. Surg. 2009, 37, 15–22. [Google Scholar] [CrossRef]
- Schermerhorn, M.L.; Buck, D.B.; O’malley, A.J.; Curran, T.; McCallum, J.C.; Darling, J.; Landon, B.E. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N. Engl. J. Med. 2015, 373, 328–338. [Google Scholar] [CrossRef]
- Karaolanis, G.I.; Antonopoulos, C.N.; Georgakarakos, E.; Lianos, G.D.; Mitsis, M.; Glantzounis, G.K.; Giannoukas, A.; Kouvelos, G. Colour Duplex and/or Contrast-Enhanced Ultrasound Compared with Computed Tomography Angiography for Endoleak Detection after Endovascular Abdominal Aortic Aneurysm Repair: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 3628. [Google Scholar] [CrossRef]
- Mirza, T.; Karthikesalingam, A.; Jackson, D.; Walsh, S.; Holt, P.; Hayes, P.; Boyle, J. Duplex Ultrasound and Contrast-Enhanced Ultrasound Versus Computed Tomography for the Detection of Endoleak after EVAR: Systematic Review and Bivariate Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2010, 39, 418–428. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- Alvarez, R.E.; MacOvski, A. Energy-selective reconstructions in X-ray computerised tomography. Phys. Med. Biol. 1976, 21, 733–744. [Google Scholar] [CrossRef]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and multi-energy CT: Principles, technical approaches, and clinical applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Connolly, M.J.; McInnes, M.D.F.; El-Khodary, M.; McGrath, T.A.; Schieda, N. Diagnostic accuracy of virtual non-contrast enhanced dual-energy CT for diagnosis of adrenal adenoma: A systematic review and meta-analysis. Eur. Radiol. 2017, 27, 4324–4335. [Google Scholar] [CrossRef]
- Lehti, L.; Söderberg, M.; Höglund, P.; Nyman, U.; Gottsäter, A.; Wassélius, J. Reliability of virtual non-contrast computed tomography angiography: Comparing it with the real deal. Acta Radiol. Open 2018, 7, 2058460118790115. [Google Scholar] [CrossRef]
- Takahashi, N.; Hartman, R.P.; Vrtiska, T.J.; Kawashima, A.; Primak, A.N.; Dzyubak, O.P.; Mandrekar, J.N.; Fletcher, J.G.; McCollough, C.H. Dual-energy CT iodine-subtraction virtual unenhanced technique to detect urinary stones in an iodine-filled collecting system: A phantom study. Am. J. Roentgenol. 2008, 190, 1169–1173. [Google Scholar] [CrossRef]
- Graser, A.; Johnson, T.R.C.; Hecht, E.M.; Becker, C.R.; Leidecker, C.; Staehler, M.; Stief, C.G.; Hildebrandt, H.; Godoy, M.C.B.; Finn, M.E.; et al. Dual-energy CT in patients suspected of having renal masses: Can virtual nonenhanced images replace true nonenhanced images? Radiology 2009, 252, 433–440. [Google Scholar] [CrossRef]
- Phan, C.; Yoo, A.; Hirsch, J.; Nogueira, R.; Gupta, R. Differentiation of hemorrhage from iodinated contrast in different intracranial compartments using dual-energy head CT. Am. J. Neuroradiol. 2012, 33, 1088–1094. [Google Scholar] [CrossRef]
- Lehti, L.; Söderberg, M.; Höglund, P.; Wassélius, J. Comparing Arterial- and Venous-Phase Acquisition for Optimization of Virtual Noncontrast Images from Dual-Energy Computed Tomography Angiography. J. Comput. Assist. Tomogr. 2019, 43, 770–774. [Google Scholar] [CrossRef]
- Kazimierczak, W.; Kazimierczak, N.; Serafin, Z. Quality of virtual-non-contrast phases derived from arterial and delayed phases of fast-kVp switching dual-energy CT in patients after endovascular aortic repair. Int. J. Cardiovasc. Imaging 2023, 39, 1805–1813. [Google Scholar] [CrossRef]
- Heye, T.; Nelson, R.C.; Ho, L.M.; Marin, D.; Boll, D.T. Dual-energy CT applications in the abdomen. AJR Am. J. Roentgenol. 2012, 199, S64–S70. [Google Scholar] [CrossRef]
- Virarkar, M.K.; Vulasala, S.S.R.; Gupta, A.V.; Gopireddy, D.; Kumar, S.; Hernandez, M.; Lall, C.; Bhosale, P. Virtual Non-contrast Imaging in The Abdomen and The Pelvis: An Overview. In Seminars in Ultrasound, CT and MRI; Elsevier: Amsterdam, The Netherlands, 2022; Volume 43. [Google Scholar]
- Ascenti, G.; Sofia, C.; Mazziotti, S.; Silipigni, S.; D’Angelo, T.; Pergolizzi, S.; Scribano, E. Dual-energy CT with iodine quantification in distinguishing between bland and neoplastic portal vein thrombosis in patients with hepatocellular carcinoma. Clin. Radiol. 2016, 71, 938.e1–938.e9. [Google Scholar] [CrossRef]
- Ascenti, G.; Mazziotti, S.; Lamberto, S.; Bottari, A.; Caloggero, S.; Racchiusa, S.; Mileto, A.; Scribano, E. Dual-energy CT for detection of endoleaks after endovascular abdominal aneurysm repair: Usefulness of colored iodine overlay. Am. J. Roentgenol. 2011, 196, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Mannil, M.; Ramachandran, J.; de Martini, I.V.; Wegener, S.; Schmidt, B.; Flohr, T.; Krauss, B.; Valavanis, A.; Alkadhi, H.; Winklhofer, S. Modified Dual-Energy Algorithm for Calcified Plaque Removal: Evaluation in Carotid Computed Tomography Angiography and Comparison with Digital Subtraction Angiography. Investig. Radiol. 2017, 52, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Silvennoinen, H.; Ikonen, S.; Soinne, L.; Railo, M.; Valanne, L. CT angiographic analysis of carotid artery stenosis: Comparison of manual assessment, semiautomatic vessel analysis, and digital subtraction angiography. Am. J. Neuroradiol. 2007, 28, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Yu, T.; Duan, X.; Peng, Y.; Zhai, R. Determination of the optimal energy level in spectral CT imaging for displaying abdominal vessels in pediatric patients. Eur. J. Radiol. 2014, 83, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.J.; Ramirez-Giraldo, J.C. Dual-energy CT in children: Imaging algorithms and clinical applications. Radiology 2019, 291, 286–297. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Caruso, D.; Schoepf, U.J.; De Santis, D.; Muscogiuri, G.; Albrecht, M.H.; Meinel, F.G.; Wichmann, J.L.; Burchett, P.F.; Varga-Szemes, A.; et al. A noise-optimized virtual monoenergetic reconstruction algorithm improves the diagnostic accuracy of late hepatic arterial phase dual-energy CT for the detection of hypervascular liver lesions. Eur. Radiol. 2018, 28, 3393–3404. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Xu, L.; Bai, X.; Lu, X.; Yu, S.; Sun, H.; Jin, Z. Utilisation of virtual non-contrast images and virtual mono-energetic images acquired from dual-layer spectral CT for renal cell carcinoma: Image quality and radiation dose. Insights Imaging 2022, 13, 12. [Google Scholar] [CrossRef]
- Ko, S.M.; Choi, J.W.; Song, M.G.; Shin, J.K.; Chee, H.K.; Chung, H.W.; Kim, D.H. Myocardial perfusion imaging using adenosine-induced stress dual-energy computed tomography of the heart: Comparison with cardiac magnetic resonance imaging and conventional coronary angiography. Eur. Radiol. 2010, 21, 26–35. [Google Scholar] [CrossRef]
- Wichmann, J.L.; Bauer, R.W.; Doss, M.; Stock, W.; Lehnert, T.; Bodelle, B.; Frellesen, C.; Vogl, T.J.; Kerl, J.M. Diagnostic accuracy of late iodine-enhancement dual-energy computed tomography for the detection of chronic myocardial infarction compared with late gadolinium-enhancement 3-T magnetic resonance imaging. Investig. Radiol. 2013, 48, 851–856. [Google Scholar] [CrossRef]
- Grant, K.L.; Flohr, T.G.; Krauss, B.; Sedlmair, M.; Thomas, C.; Schmidt, B. Assessment of an Advanced Image-Based Technique to Calculate Virtual Monoenergetic Computed Tomographic Images from a Dual-Energy Examination to Improve Contrast-To-Noise Ratio in Examinations Using Iodinated Contrast Media. Investig. Radiol. 2014, 49, 586–592. [Google Scholar] [CrossRef]
- Zeng, Y.; Geng, D.; Zhang, J. Noise-optimized virtual monoenergetic imaging technology of the third-generation dual-source computed tomography and its clinical applications. Quant. Imaging Med. Surg. 2021, 11, 4627–4643. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, J.L.; Gillott, M.R.; De Cecco, C.N.; Mangold, S.; Varga-Szemes, A.; Yamada, R.; Otani, K.; Canstein, C.M.; Fuller, S.R.B.; Vogl, T.J.; et al. Dual-Energy Computed Tomography Angiography of the Lower Extremity Runoff. Investig. Radiol. 2016, 51, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.K.; Dendy, P. Spiral CT: How much does radiation dose matter? Lancet 1998, 352, 1082–1083. [Google Scholar] [CrossRef] [PubMed]
- Einstein, A.J.; Henzlova, M.J.; Rajagopalan, S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007, 298, 317–323. [Google Scholar] [CrossRef] [PubMed]
- de Jong, P.A.; Mayo, J.R.; Golmohammadi, K.; Nakano, Y.; Lequin, M.H.; Tiddens, H.A.W.M.; Aldrich, J.; Coxson, H.O.; Sin, D.D. Estimation of cancer mortality associated with repetitive computed tomography scanning. Am. J. Respir. Crit Care Med. 2006, 173, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Hall, E.J. Computed Tomography—An Increasing Source of Radiation Exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Elliston, C.D. Estimated radiation on risks potentially associated with full-body CT screening. Radiology 2004, 232, 735–738. [Google Scholar] [CrossRef] [PubMed]
- White, H.A.; MacDonald, S. Estimating risk associated with radiation exposure during follow-up after endovascular aortic repair (EVAR). J. Cardiovasc. Surg. 2010, 51, 95. [Google Scholar]
- Lehti, L.; Nyman, U.; Söderberg, M.; Björses, K.; Gottsäter, A.; Wassélius, J. 80-kVp CT angiography for endovascular aneurysm repair follow-up with halved contrast medium dose and preserved diagnostic quality. Acta Radiol. 2016, 57, 279–286. [Google Scholar] [CrossRef]
- Grajo, J.R.; Sahani, D.V. Dual-Energy CT of the Abdomen and Pelvis: Radiation Dose Considerations. J. Am. Coll. Radiol. 2018, 15, 1128–1132. [Google Scholar] [CrossRef]
- Weinman, J.P.; Mirsky, D.M.; Jensen, A.M.; Stence, N.V. Dual energy head CT to maintain image quality while reducing dose in pediatric patients. Clin. Imaging 2019, 55, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.J.; Curtis, W.A.; Ramirez-Giraldo, J.C. Effects of dual-energy technique on radiation exposure and image quality in pediatric body CT. Am. J. Roentgenol. 2016, 207, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Goo, H.W. Initial experience of dual-energy lung perfusion CT using a dual-source CT system in children. Pediatr. Radiol. 2010, 40, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Primak, A.N.; Giraldo, J.C.R.; Eusemann, C.D.; Schmidt, B.; Kantor, B.; Fletcher, J.G.; McCollough, C.H. Dual-source dual-energy CT with additional tin filtration: Dose and image quality evaluation in phantoms and in vivo. Am. J. Roentgenol. 2010, 195, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Macari, M.; Chandarana, H.; Schmidt, B.; Lee, J.; Lamparello, P.; Babb, J. Abdominal aortic aneurysm: Can the arterial phase at CT evaluation after endovascular repair be eliminated to reduce radiation dose? Radiology 2006, 241, 908–914. [Google Scholar] [CrossRef]
- Chandarana, H.; Godoy, M.C.B.; Vlahos, I.; Graser, A.; Babb, J.; Leidecker, C.; Macari, M. Abdominal aorta: Evaluation with dual-source dual-energy multidetector CT after endovascular repair of aneurysms-initial observations. Radiology 2008, 249, 692–700. [Google Scholar] [CrossRef]
- Flors, L.; Leiva-Salinas, C.; Norton, P.T.; Patrie, J.T.; Hagspiel, K.D. Endoleak detection after endovascular repair of thoracic aortic aneurysm using dual-source dual-energy CT: Suitable scanning protocols and potential radiation dose reduction. Am. J. Roentgenol. 2013, 200, 451–460. [Google Scholar] [CrossRef]
- Stolzmann, P.; Frauenfelder, T.; Pfammatter, T.; Peter, N.; Scheffel, H.; Lachat, M.; Schmidt, B.; Marincek, B.; Alkadhi, H.; Schertler, T. Endoleaks after endovascular abdominal aortic aneurysm repair: Detection with dual-energy dual-source CT. Radiology 2008, 249, 682–691. [Google Scholar] [CrossRef]
- Buffa, V.; Solazzo, A.; D’auria, V.; Del Prete, A.; Vallone, A.; Luzietti, M.; Madau, M.; Grassi, R.; Miele, V. Dual-source dual-energy CT: Dose reduction after endovascular abdominal aortic aneurysm repair. Radiol. Medica 2014, 119, 934–941. [Google Scholar] [CrossRef]
- Kazimierczak, W.; Kazimierczak, N.; Lemanowicz, A.; Nowak, E.; Migdalski, A.; Jawien, A.; Jankowski, T.; Serafin, Z. Improved Detection of Endoleaks in Virtual Monoenergetic Images in Dual-Energy CT Angiography Following EVAR. Acad. Radiol. 2023, 30, 2813–2824. [Google Scholar] [CrossRef]
- Iezzi, R.; Cotroneo, A.R.; Filippone, A.; Di Fabio, F.; Quinto, F.; Colosimo, C.; Bonomo, L. Multidetector CT in abdominal aortic aneurysm treated with endovascular repair: Are unenhanced and delayed phase enhanced images effective for endoleak detection? Radiology 2006, 241, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Glebova, N.O.; Selvarajah, S.; Orion, K.C.; Black, J.H.; Malas, M.B.; Perler , B.A.; Abularrage , C.J. Fenestrated endovascular repair of abdominal aortic aneurysms is associated with increased morbidity but comparable mortality with infrarenal endovascular aneurysm repair. J. Vasc. Surg. 2015, 61, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Troisi, N.; Donas, K.P.; Austermann, M.; Tessarek, J.; Umscheid, T.; Torsello, G. Secondary procedures after aortic aneurysm repair with fenestrated and branched endografts. J. Endovasc. Ther. 2011, 18, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Sommer, W.H.; Becker, C.R.; Haack, M.; Rubin, G.D.; Weidenhagen, R.; Schwarz, F.; Nikolaou, K.; Reiser, M.F.; Johnson, T.R.; Clevert, D.A. Time-resolved CT angiography for the detection and classification of endoleaks. Radiology 2012, 263, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, S.W.; Charagundla, S.R. Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology 2007, 243, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, R.; Cotroneo, A.R.; Filippone, A.; Santoro, M.; Basilico, R.; Storto, M.L. Multidetector-row computed tomography angiography in abdominal aortic aneurysm treated with endovascular repair: Evaluation of optimal timing of delayed phase imaging for the detection of low-flow endoleaks. J. Comput. Assist. Tomogr. 2008, 32, 609–615. [Google Scholar] [CrossRef]
- Javor, D.; Wressnegger, A.; Unterhumer, S.; Kollndorfer, K.; Nolz, R.; Beitzke, D.; Loewe, C. Endoleak detection using single-acquisition split-bolus dual-energy computer tomography (DECT). Eur. Radiol. 2017, 27, 1622–1630. [Google Scholar] [CrossRef]
- Boos, J.; Fang, J.; Heidinger, B.H.; Raptopoulos, V.; Brook, O.R. Dual energy CT angiography: Pros and cons of dual-energy metal artifact reduction algorithm in patients after endovascular aortic repair. Abdom. Radiol. 2017, 42, 749–758. [Google Scholar] [CrossRef]
- Iezzi, R.; Carchesio, F.; Posa, A.; Colosimo, C.; Bonomo, L. Post-EVAR split-bolus CT angiography using dual-energy CT: All you need in a single scan! In EuroSafe Imaging; ESR: Vienna, Austria, 2017. [Google Scholar]
- Mehran, R.; Nikolsky, E. Contrast-induced nephropathy: Definition, epidemiology, and patients at risk. Kidney Int. 2006, 69, S11–S15. [Google Scholar] [CrossRef]
- van der Molen, A.J.; Thomsen, H.S.; Morcos, S.K. Effect of iodinated contrast media on thyroid function in adults. Eur. Radiol. 2004, 14, 902–907. [Google Scholar]
- Katayama, H.; Yamaguchi, K.; Kozuka, T.; Takashima, T.; Seez, P.; Matsuura, K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology 1990, 175, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Hayashida, K.; Mouillet, G.; Hovasse, T.; Chevalier, B.; Oguri, A.; Watanabe, Y.; Dubois-Randé, J.-L.; Morice, M.-C.; Lefèvre, T.; et al. Prognostic value of chronic kidney disease after transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 2013, 62, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Kane, G.C.; Doyle, B.J.; Lerman, A.; Barsness, G.W.; Best, P.J.; Rihal, C.S. Ultra-Low Contrast Volumes Reduce Rates of Contrast-Induced Nephropathy in Patients with Chronic Kidney Disease Undergoing Coronary Angiography. J. Am. Coll. Cardiol. 2008, 51, 89–90. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; McDonald, J.S.; Bida, J.P.; Carter, R.E.; Fleming, C.J.; Misra, S.; Williamson, E.E.; Kallmes, D.F.; Paltiel, H.J.; Gilligan, L.A.; et al. Intravenous contrast material-induced nephropathy: Causal or coincident phenomenon? Radiology 2013, 267, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Huda, W.; Scalzetti, E.M.; Levin, G. Technique factors and image quality as functions of patient weight at abdominal CT. Radiology 2000, 217, 430–435. [Google Scholar] [CrossRef] [PubMed]
- van Hamersvelt, R.W.; Eijsvoogel, N.G.; Mihl, C.; de Jong, P.A.; Schilham, A.M.R.; Buls, N.; Das, M.; Leiner, T.; Willemink, M.J. Contrast agent concentration optimization in CTA using low tube voltage and dual-energy CT in multiple vendors: A phantom study. Int. J. Cardiovasc. Imaging 2018, 34, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, P.; Leipsic, J.A.; Capunay, C.; Deviggiano, A.; Vallejos, J.; Goldsmit, A.; Rodriguez-Granillo, G.A. Monochromatic image reconstruction by dual energy imaging allows half iodine load computed tomography coronary angiography. Eur. J. Radiol. 2015, 84, 1915–1920. [Google Scholar] [CrossRef]
- Godoy, M.C.; Heller, S.L.; Naidich, D.P.; Assadourian, B.; Leidecker, C.; Schmidt, B.; Vlahos, I. Dual-energy MDCT: Comparison of pulmonary artery enhancement on dedicated CT pulmonary angiography, routine and low contrast volume studies. Eur. J. Radiol. 2011, 79, e11–e17. [Google Scholar] [CrossRef]
- Nijhof, W.; Baltussen, E.; Kant, I.; Jager, G.; Slump, C.; Rutten, M. Low-dose CT angiography of the abdominal aorta and reduced contrast medium volume: Assessment of image quality and radiation dose. Clin. Radiol. 2016, 71, 64–73. [Google Scholar] [CrossRef]
- Dubourg, B.; Caudron, J.; Lestrat, J.-P.; Bubenheim, M.; Lefebvre, V.; Godin, M.; Tron, C.; Eltchaninoff, H.; Bauer, F.; Dacher, J.-N. Single-source dual-energy CT angiography with reduced iodine load in patients referred for aortoiliofemoral evaluation before transcatheter aortic valve implantation: Impact on image quality and radiation dose. Eur. Radiol. 2014, 24, 2659–2668. [Google Scholar] [CrossRef]
- Martin, S.S.; Albrecht, M.H.; Wichmann, J.L.; Hüsers, K.; Scholtz, J.-E.; Booz, C.; Bodelle, B.; Bauer, R.W.; Metzger, S.C.; Vogl, T.J.; et al. Value of a noise-optimized virtual monoenergetic reconstruction technique in dual-energy CT for planning of transcatheter aortic valve replacement. Eur. Radiol. 2017, 27, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Maturen, K.E.; Kaza, R.K.; Liu, P.S.; Quint, L.E.; Khalatbari, S.H.; Platt, J.F. “Sweet spot” for endoleak detection: Optimizing contrast to noise using low kev reconstructions from fast-switch kVp dual-energy CT. J. Comput. Assist. Tomogr. 2012, 36, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Wichmann, J.L.; Weyer, H.; Scholtz, J.-E.; Leithner, D.; Spandorfer, A.; Bodelle, B.; Jacobi, V.; Vogl, T.J.; Albrecht, M.H. Endoleaks after endovascular aortic aneurysm repair: Improved detection with noise-optimized virtual monoenergetic dual-energy CT. Eur. J. Radiol. 2017, 94, 125–132. [Google Scholar] [CrossRef]
- Skawran, S.; Angst, F.; Blüthgen, C.; Eberhard, M.; Kälin, P.; Kobe, A.; Nagy, D.; Szucs-Farkas, Z.; Alkadhi, H.; Euler, A. Dual-Energy Low-keV or Single-Energy Low-kV CT for Endoleak Detection? Investig. Radiol. 2020, 55, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, S.; Perisinakis, K.; Kontopodis, N.; Papadakis, A.E.; Maris, T.G.; Ioannou, C.V.; Karantanas, A.; Tsetis, D. Dual-energy CT angiography in imaging surveillance of endovascular aneurysm repair—Preliminary study results. Eur. J. Radiol. 2022, 148, 110165. [Google Scholar] [CrossRef] [PubMed]
- Ragusi, M.A.A.D.; van der Meer, R.W.; Joemai, R.M.S.; van Schaik, J.; van Rijswijk, C.S.P. Evaluation of CT Angiography Image Quality Acquired with Single-Energy Metal Artifact Reduction (SEMAR) Algorithm in Patients after Complex Endovascular Aortic Repair. Cardiovasc. Interv. Radiol 2018, 41, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, W.; Nowak, E.; Kazimierczak, N.; Jankowski, T.; Jankowska, A.; Serafin, Z. The value of metal artifact reduction and iterative algorithms in dual energy CT angiography in patients after complex endovascular aortic aneurysm repair. Heliyon 2023, 9, e20700. [Google Scholar] [CrossRef]
- Albrecht, M.H.; De Cecco, C.N.; Nance, J.W.; Varga-Szemes, A.; De Santis, D.; Eid, M.; Tesche, C.; Apfaltrer, G.; Doeberitz, P.L.v.K.; Jacobs, B.; et al. Cardiac Dual-Energy CT Applications and Clinical Impact. Curr. Radiol. Rep. 2017, 5, 42. [Google Scholar] [CrossRef]
- Mangold, S.; Cannaó, P.M.; Schoepf, U.J.; Wichmann, J.L.; Canstein, C.; Fuller, S.R.; Muscogiuri, G.; Varga-Szemes, A.; Nikolaou, K.; De Cecco, C.N. Impact of an advanced image-based monoenergetic reconstruction algorithm on coronary stent visualization using third generation dual-source dual-energy CT: A phantom study. Eur. Radiol. 2016, 26, 1871–1878. [Google Scholar] [CrossRef]
- Hickethier, T.; Baeßler, B.; Kroeger, J.R.; Doerner, J.; Pahn, G.; Maintz, D.; Michels, G.; Bunck, A.C. Monoenergetic reconstructions for imaging of coronary artery stents using spectral detector CT: In-vitro experience and comparison to conventional images. J. Cardiovasc. Comput. Tomogr. 2017, 11, 33–39. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Darnell, A.; Rengo, M.; Muscogiuri, G.; Bellini, D.; Ayuso, C.; Laghi, A. Dual-energy CT: Oncologic applications. AJR Am. J. Roentgenol. 2012, 199, S98. [Google Scholar] [CrossRef] [PubMed]
- Leithner, D.; Wichmann, J.L.; Vogl, T.J.; Trommer, J.; Martin, S.S.; Scholtz, J.-E.; Bodelle, B.; De Cecco, C.N.; Duguay, T.; Nance, J.W.; et al. Virtual Monoenergetic Imaging and Iodine Perfusion Maps Improve Diagnostic Accuracy of Dual-Energy Computed Tomography Pulmonary Angiography with Suboptimal Contrast Attenuation. Investig. Radiol. 2017, 52, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.P.; Antunes, C.; Curvo-Semedo, L. Pros and Cons of Dual-Energy CT Systems: “One Does Not Fit All”. Tomography 2023, 9, 195–216. [Google Scholar] [CrossRef]
- Goo, H.W.; Goo, J.M. Dual-energy CT: New horizon in medical imaging. Korean J. Radiol. 2017, 18, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Katsura, M.; Sato, J.; Akahane, M.; Kunimatsu, A.; Abe, O. Current and novel techniques for metal artifact reduction at CT: Practical guide for radiologists. Radiographics 2018, 38, 450–461. [Google Scholar] [CrossRef]
- Forghani, R.; De Man, B.; Gupta, R. Dual-Energy Computed Tomography: Physical Principles, Approaches to Scanning, Usage, and Implementation: Part 2. Neuroimaging Clin. N. Am. 2017, 27, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Toia, G.V.; Mileto, A.; Wang, C.L.; Sahani, D.V. Quantitative dual-energy CT techniques in the abdomen. Abdom. Radiol. 2022, 47, 3003–3018. [Google Scholar] [CrossRef]
- Parakh, A.; An, C.; Lennartz, S.; Rajiah, P.; Yeh, B.M.; Simeone, F.J.; Sahani, D.V.; Kambadakone, A.R. Recognizing and minimizing artifacts at dual-energy CT. Radiographics 2021, 41, 509–523. [Google Scholar] [CrossRef]
- Petritsch, B.; Pannenbecker, P.; Weng, A.M.; Grunz, J.-P.; Veldhoen, S.; Bley, T.A.; Kosmala, A. Split-filter dual-energy CT pulmonary angiography for the diagnosis of acute pulmonary embolism: A study on image quality and radiation dose. Quant. Imaging Med. Surg. 2021, 11, 1817–1827. [Google Scholar] [CrossRef]
- Wortman, J.R.; Sodickson, A.D. Pearls, Pitfalls, and Problems in Dual-Energy Computed Tomography Imaging of the Body. Radiol. Clin. N. Am. 2018, 56, 625–640. [Google Scholar] [CrossRef]
- Wichmann, J.L.; Hardie, A.D.; Schoepf, U.J.; Felmly, L.M.; Perry, J.D.; Varga-Szemes, A.; Mangold, S.; Caruso, D.; Canstein, C.; Vogl, T.J.; et al. Single- and dual-energy CT of the abdomen: Comparison of radiation dose and image quality of 2nd and 3rd generation dual-source CT. Eur. Radiol. 2017, 27, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Söderberg, M.; Nilsson, M.; Lindvall, H.; Christoffersen, C.; Leander, P. Evaluation of image quality and radiation dose of abdominal dual-energy CT. Acta Radiol. 2018, 59, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Harder, A.M.D.; Willemink, M.J.; de Ruiter, Q.M.; Schilham, A.M.; Krestin, G.P.; Leiner, T.; de Jong, P.A.; Budde, R.P. Achievable dose reduction using iterative reconstruction for chest computed tomography: A systematic review. Eur. J. Radiol. 2015, 84, 2307–2313. [Google Scholar] [CrossRef] [PubMed]
- Parakh, A.; Macri, F.; Sahani, D. Dual-Energy Computed Tomography: Dose Reduction, Series Reduction, and Contrast Load Reduction in Dual-Energy Computed Tomography. Radiol. Clin. N. Am. 2018, 56, 601–624. [Google Scholar] [CrossRef]
- Mohammadinejad, P.; Mileto, A.; Yu, L.; Leng, S.; Guimaraes, L.S.; Missert, A.D.; Jensen, C.T.; Gong, H.; McCollough, C.H.; Fletcher, J.G. Ct noise-reduction methods for lower-dose scanning: Strengths and weaknesses of iterative reconstruction algorithms and new techniques. Radiographics 2021, 41, 1493–1508. [Google Scholar] [CrossRef]
- Dunet, V.; Bernasconi, M.; Hajdu, S.D.; Meuli, R.A.; Daniel, R.T.; Zerlauth, J.-B. Impact of metal artifact reduction software on image quality of gemstone spectral imaging dual-energy cerebral CT angiography after intracranial aneurysm clipping. Neuroradiology 2017, 59, 845–852. [Google Scholar] [CrossRef]






| Research | Protocol | Dose Reduction (%) | |
|---|---|---|---|
| Mono-Phasic (mSv) | Three-Phasic (mSv) | ||
| Chandarana et al., 2008 [47] | 11.1 | 27.8 | 61 |
| Flors et al., 2013 [48] | 9.8 | 22.4 | 64.1 |
| Stolzman et al., 2008 [49] | 10.9 | 27.4 | 61 |
| Buffa et al., 2014 [50] | 10.5 | 27.4 | 61.7 |
| Kazimierczak et al., 2023 [51] | 10.69 | 27.96 | 61.37 |
| Reconstruction Technique | Advantage | Application |
|---|---|---|
| Low-energy VMI | Higher sensitivity for iodine. | Improved endoleak detection. Contrast dose reduction. Salvage of suboptimal contrast examination. |
| High-energy VMI | Reduction in calcium blooming artifacts. Metal artifact and beam-hardening reduction. | Reduction in artifacts from stentgraft structures and embolization materials. Better visualization of stent lumen. Improved visualization of calcified vessels. |
| Virtual noncontrast images | Reduction in number of phases of examination. | Reduction in radiation dose. Characteristic of incidental findings in abbreviated examination protocols (without true noncontrast phase). |
| Material decomposition | Identification of elemental composition of tissues. | Plaque characterization. Improved separation of calcium from iodine. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierczak, W.; Kazimierczak, N.; Serafin, Z. Review of Clinical Applications of Dual-Energy CT in Patients after Endovascular Aortic Repair. J. Clin. Med. 2023, 12, 7766. https://doi.org/10.3390/jcm12247766
Kazimierczak W, Kazimierczak N, Serafin Z. Review of Clinical Applications of Dual-Energy CT in Patients after Endovascular Aortic Repair. Journal of Clinical Medicine. 2023; 12(24):7766. https://doi.org/10.3390/jcm12247766
Chicago/Turabian StyleKazimierczak, Wojciech, Natalia Kazimierczak, and Zbigniew Serafin. 2023. "Review of Clinical Applications of Dual-Energy CT in Patients after Endovascular Aortic Repair" Journal of Clinical Medicine 12, no. 24: 7766. https://doi.org/10.3390/jcm12247766
APA StyleKazimierczak, W., Kazimierczak, N., & Serafin, Z. (2023). Review of Clinical Applications of Dual-Energy CT in Patients after Endovascular Aortic Repair. Journal of Clinical Medicine, 12(24), 7766. https://doi.org/10.3390/jcm12247766









