Associations between Elemental Metabolic Dynamics and Default Mode Network Functional Connectivity Are Altered in Autism
Abstract
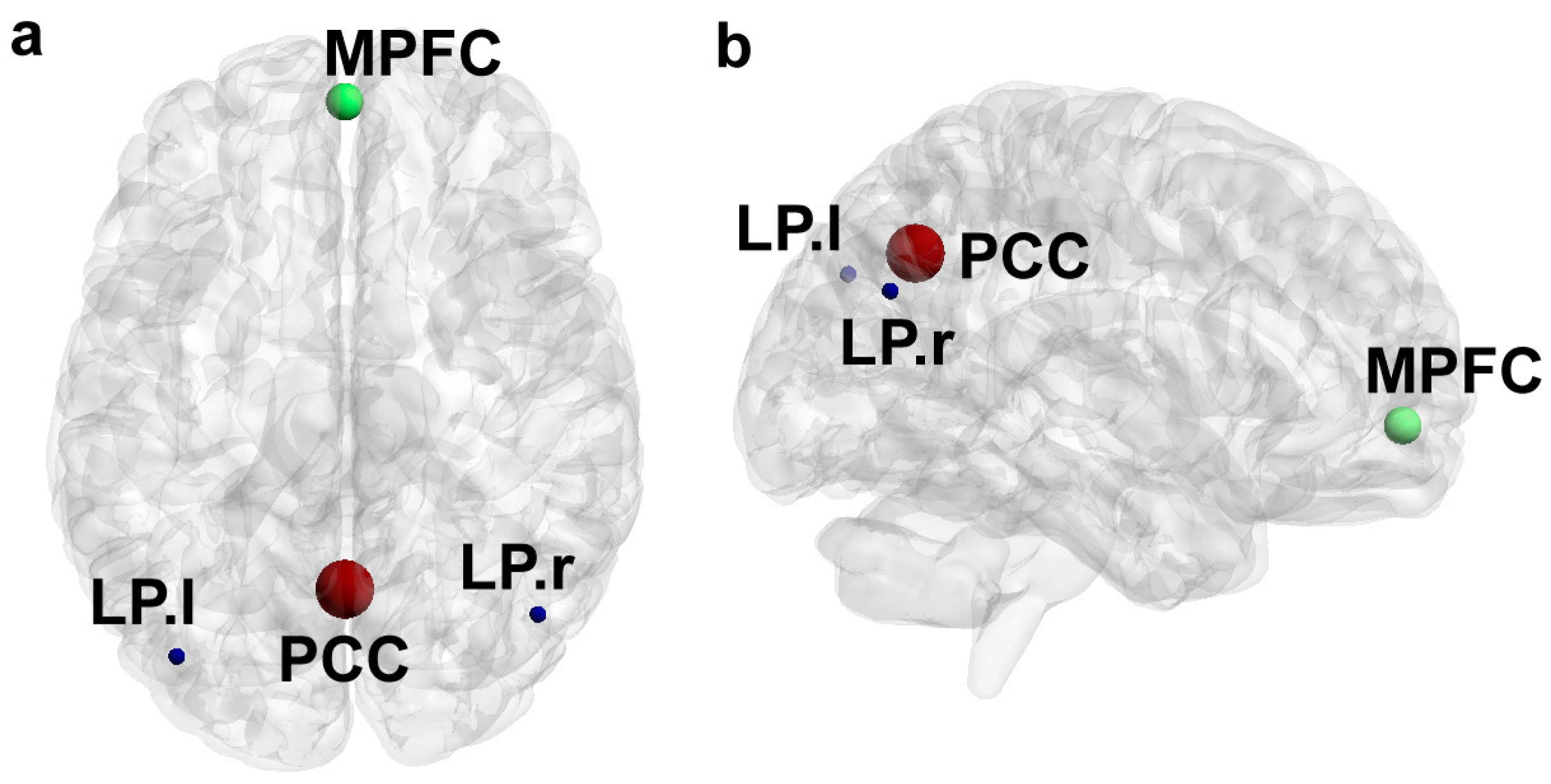
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuroimaging
2.3. Hair Analysis
2.4. Feature Engineering
2.5. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Associations between Elemental and Functional Dynamics
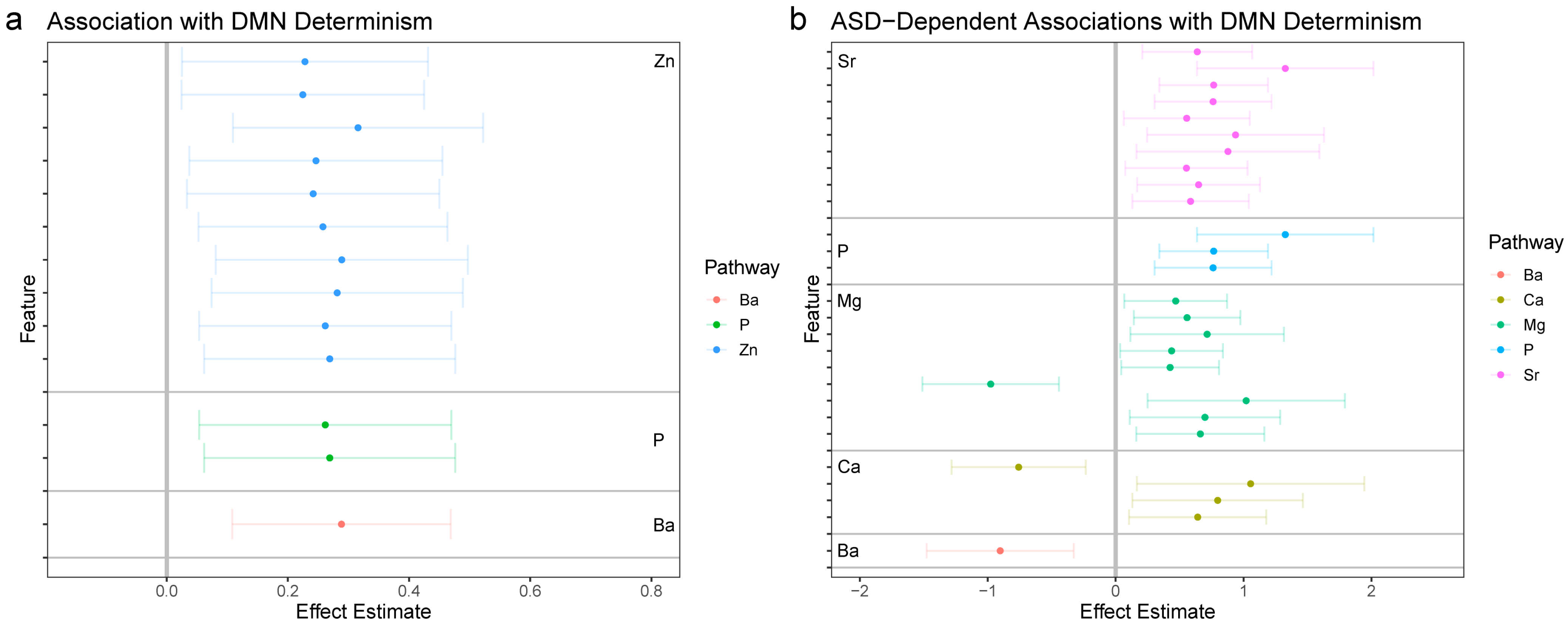
3.2.1. DMN-Wide Associations
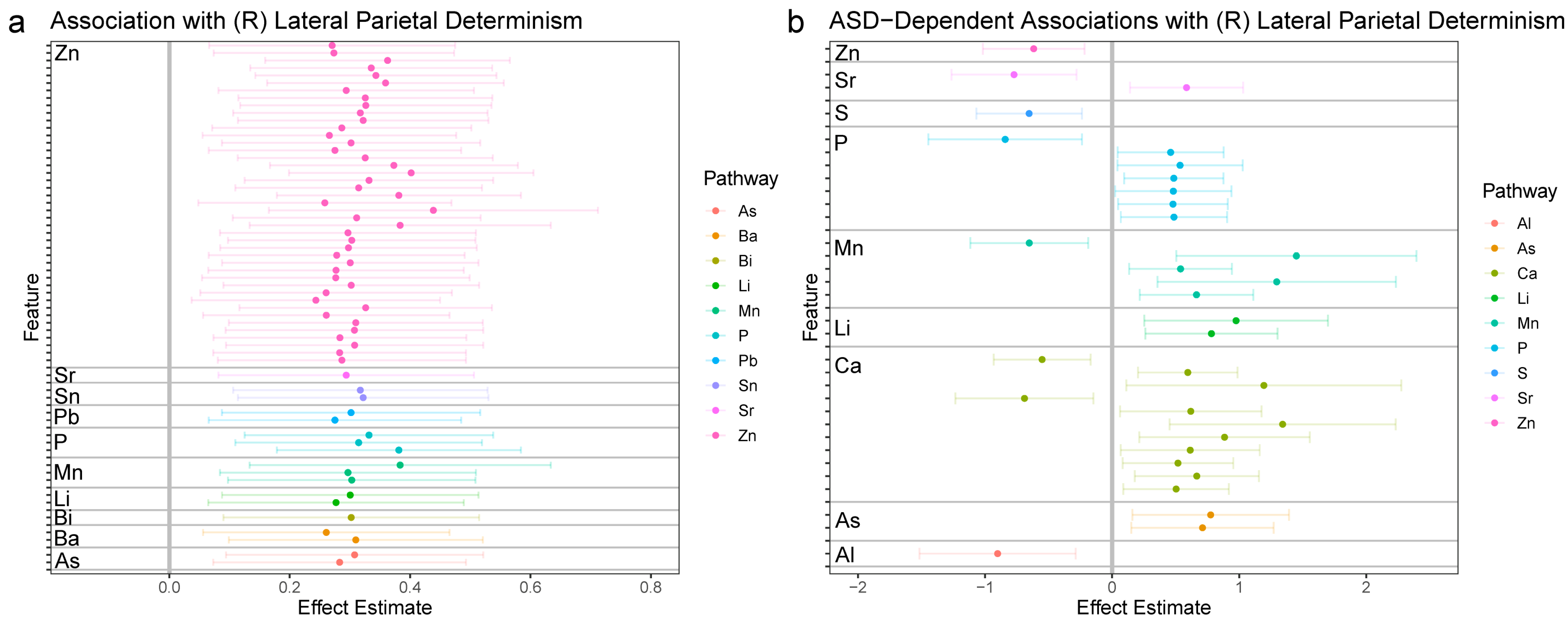
3.2.2. Right Lateral Parietal (LPR)
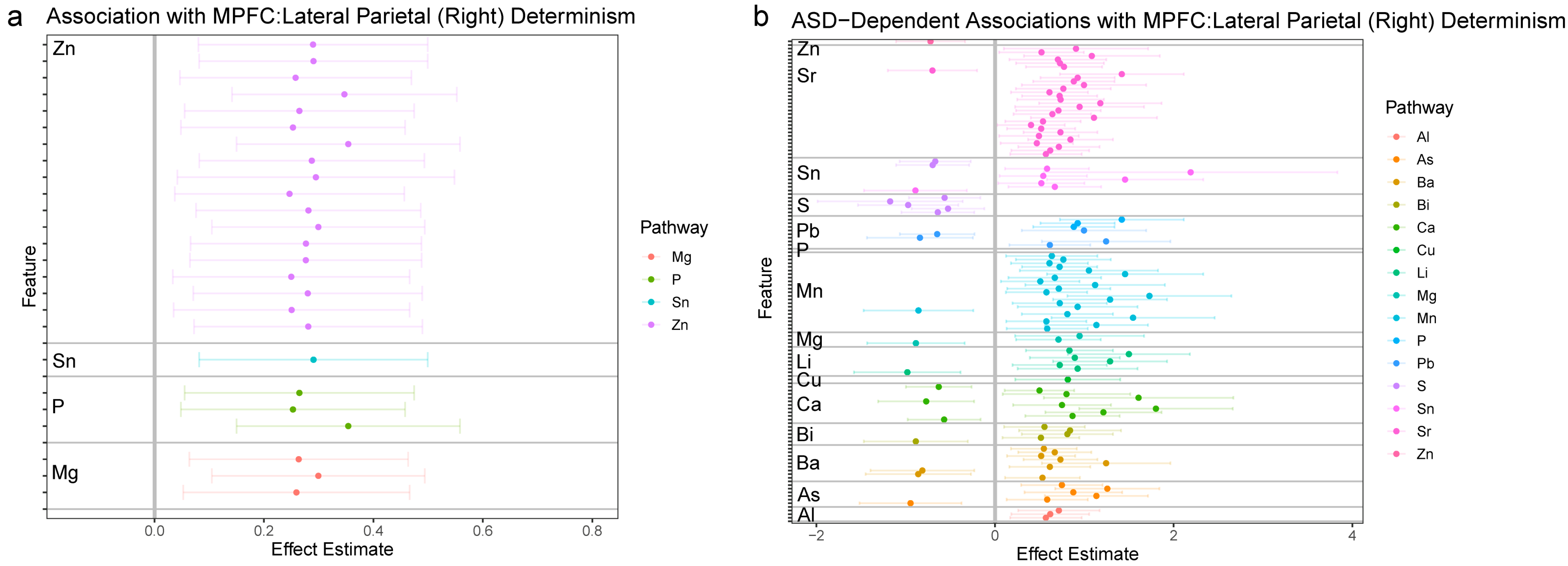
3.2.3. MPFC: LPR Synchronization
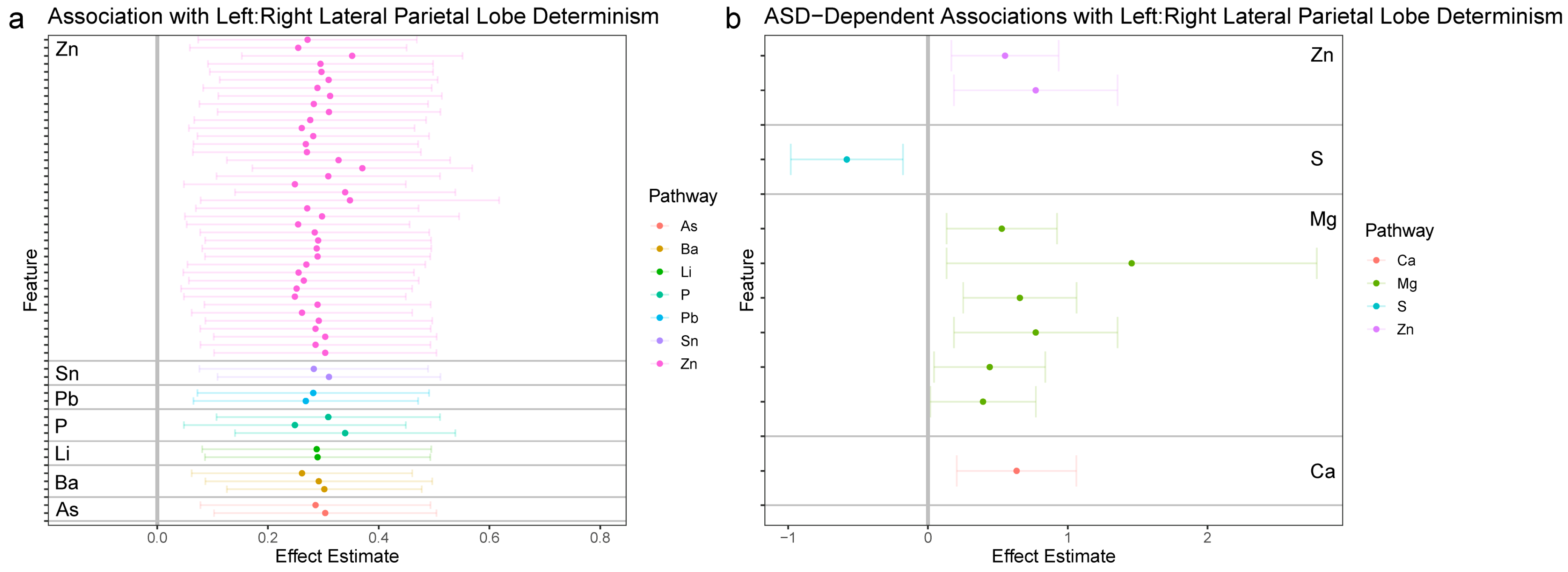
3.2.4. LPL and LPR Synchronization
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bölte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef] [Green Version]
- Volk, H.E.; Ames, J.L.; Chen, A.; Fallin, M.D.; Hertz-Picciotto, I.; Halladay, A.; Hirtz, D.; Lavin, A.; Ritz, B.; Zoeller, T.; et al. Considering Toxic Chemicals in the Etiology of Autism. Pediatrics 2022, 149, e2021053012. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Choi, J.; Lee, W.J.; Do, J.T. Genetic and Epigenetic Etiology Underlying Autism Spectrum Disorder. J. Clin. Med. 2020, 9, 966. [Google Scholar] [CrossRef] [Green Version]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loth, E.; Spooren, W.; Ham, L.M.; Isaac, M.B.; Auriche-Benichou, C.; Banaschewski, T.; Baron-Cohen, S.; Broich, K.; Bölte, S.; Bourgeron, T.; et al. Identification and validation of biomarkers for autism spectrum disorders. Nat. Rev. Drug Discov. 2016, 15, 70–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtin, P.; Austin, C.; Curtin, A.; Gennings, C.; Arora, M.; Tammimies, K.; Willfors, C.; Berggren, S.; Siper, P.; Rai, D.; et al. Dynamical features in fetal and postnatal zinc-copper metabolic cycles predict the emergence of autism spectrum disorder. Sci. Adv. 2018, 4, eaat1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, C.; Curtin, P.; Curtin, A.; Gennings, C.; Arora, M.; Tammimies, K.; Isaksson, J.; Willfors, C.; Bölte, S. Dynamical properties of elemental metabolism distinguish attention deficit hyperactivity disorder from autism spectrum disorder. Transl. Psychiatry 2019, 9, 238. [Google Scholar] [CrossRef] [Green Version]
- Austin, C.; Curtin, P.; Arora, M.; Reichenberg, A.; Curtin, A.; Iwai-Shimada, M.; Wright, R.O.; Wright, R.J.; Remnelius, K.L.; Isaksson, J.; et al. Elemental Dynamics in Hair Accurately Predict Future Autism Spectrum Disorder Diagnosis: An International Multi-Center Study. J. Clin. Med. 2022, 11, 7154. [Google Scholar] [CrossRef]
- Curtin, P.; Curtin, A.; Austin, C.; Gennings, C.; Tammimies, K.; Bölte, S.; Arora, M. Recurrence quantification analysis to characterize cyclical components of environmental elemental exposures during fetal and postnatal development. PLoS ONE 2017, 12, e0187049. [Google Scholar] [CrossRef] [Green Version]
- Curtin, P.; Neufield, J.; Curtin, A.; Arora, M.; Bölte, S. Altered Periodic Dynamics in the Default Mode Network in Autism and Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2022, 91, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Merced-Nieves, F.M.; Arora, M.; Wright, R.O.; Curtin, P. Metal mixtures and neurodevelopment: Recent findings and emerging principles. Curr. Opin. Toxicol. 2021, 26, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Von Stackelberg, K.; Guzy, E.; Chu, T.; Henn, B.C. Exposure to Mixtures of Metals and Neurodevelopmental Outcomes: A Multidisciplinary Review Using an Adverse Outcome Pathway Framework. Risk Anal. 2015, 35, 971–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, A.P.; Henn, B.C.; Wright, R.O. Perinatal and Childhood Exposure to Cadmium, Manganese, and Metal Mixtures and Effects on Cognition and Behavior: A Review of Recent Literature. Curr. Environ. Heal. Rep. 2015, 2, 284–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals: A silent pandemic. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef]
- Dorea, J.G. Neurotoxic Metal Coexposures and Neurodevelopment. Environ. Health Perspect. 2012, 120, a226. [Google Scholar] [CrossRef] [Green Version]
- Schildroth, S.; Kordas, K.; Bauer, J.A.; Wright, R.O.; Henn, B.C. Environmental Metal Exposure, Neurodevelopment, and the Role of Iron Status: A Review. Curr. Environ. Heal. Rep. 2022, 9, 758–787. [Google Scholar] [CrossRef]
- Arora, M.; Reichenberg, A.; Willfors, C.; Austin, C.; Gennings, C.; Berggren, S.; Lichtenstein, P.; Anckarsäter, H.; Tammimies, K.; Bölte, S. Fetal and postnatal metal dysregulation in autism. Nat. Commun. 2017, 8, 15493. [Google Scholar] [CrossRef] [Green Version]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef]
- Anderson, J.S.; Nielsen, J.A.; Froehlich, A.L.; DuBray, M.B.; Druzgal, T.J.; Cariello, A.N.; Cooperrider, J.R.; Zielinski, B.A.; Ravichandran, C.; Fletcher, P.T.; et al. Functional connectivity magnetic resonance imaging classification of autism. Brain 2011, 134, 3742–3754. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, J.A.; Zielinski, B.A.; Fletcher, P.T.; Alexander, A.L.; Lange, N.; Bigler, E.D.; Lainhart, J.E.; Anderson, J.S. Multisite functional connectivity MRI classification of autism: ABIDE results. Front. Hum. Neurosci. 2013, 7, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plitt, M.; Barnes, K.A.; Martin, A. Functional connectivity classification of autism identifies highly predictive brain features but falls short of biomarker standards. NeuroImage Clin. 2015, 7, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Murdaugh, D.L.; Shinkareva, S.V.; Deshpande, H.R.; Wang, J.; Pennick, M.R.; Kana, R.K. Differential Deactivation during Mentalizing and Classification of Autism Based on Default Mode Network Connectivity. PLoS ONE 2012, 7, e50064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neufeld, J.; Kuja-Halkola, R.; Mevel, K.; Cauvet, E.; Fransson, P.; Bölte, S. Alterations in resting state connectivity along the autism trait continuum: A twin study. Mol. Psychiatry 2018, 23, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, A.; Lynch, C.J.; Schaer, M.; Menon, V. The Default Mode Network in Autism. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2017, 2, 476–486. [Google Scholar] [CrossRef]
- Nair, A.; Jolliffe, M.; Lograsso, Y.S.S.; Bearden, C.E. A Review of Default Mode Network Connectivity and Its Association with Social Cognition in Adolescents with Autism Spectrum Disorder and Early-Onset Psychosis. Front. Psychiatry 2020, 11, 614. [Google Scholar] [CrossRef]
- Harikumar, A.; Evans, D.W.; Dougherty, C.C.; Carpenter, K.L.; Michael, A.M. A Review of the Default Mode Network in Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorder. Brain Connect. 2021, 11, 253–263. [Google Scholar] [CrossRef]
- Kaboodvand, N.; Iravani, B.; Fransson, P. Dynamic synergetic configurations of resting-state networks in ADHD. Neuroimage 2020, 207, 116347. [Google Scholar] [CrossRef]
- Bölte, S.; Willfors, C.; Berggren, S.; Norberg, J.; Poltrago, L.; Mevel, K.; Coco, C.; Fransson, P.; Borg, J.; Sitnikov, R.; et al. The Roots of Autism and ADHD Twin Study in Sweden (RATSS). Twin Res. Hum. Genet. 2014, 17, 164–176. [Google Scholar] [CrossRef]
- Tzoutio-Mazoyera, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Tzourio-Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Makris, N.; Goldstein, J.M.; Kennedy, D.; Hodge, S.M.; Caviness, V.S.; Faraone, S.V.; Tsuang, M.T.; Seidman, L.J. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr. Res. 2006, 83, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.M.; Seidman, L.J.; Makris, N.; Ahern, T.; O’Brien, L.M.; Caviness, V.S.; Kennedy, D.N.; Faraone, S.V.; Tsuang, M.T. Hypothalamic Abnormalities in Schizophrenia: Sex Effects and Genetic Vulnerability. Biol. Psychiatry 2007, 61, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Webber, C.L., Jr.; Zbilut, J.P. Dynamical assessment of physiological systems and states using recurrence plot strategies. J. Appl. Physiol. 1994, 76, 965–973. [Google Scholar] [CrossRef]
- Marwan, N.; Romano, M.C.; Thiel, M.; Kurths, J. Recurrence plots for the analysis of complex systems. Phys. Rep. 2007, 438, 237–329. [Google Scholar] [CrossRef]
- Curtin, P.; Austin, C.; Curtin, A.; Gennings, C.; Figueroa-Romero, C.; Mikhail, K.A.; Botero, T.M.; Goutman, S.A.; Feldman, E.L.; Arora, M. Dysregulated biodynamics in metabolic attractor systems precede the emergence of amyotrophic lateral sclerosis. PLoS Comput. Biol. 2020, 16, e1007773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datseris, G. DynamicalSystems.jl: A Julia software library for chaos and nonlinear dynamics. J. Open Source Softw. 2018, 3, 598. [Google Scholar] [CrossRef]
- Aschner, J.L.; Anderson, A.; Slaughter, J.C.; Aschner, M.; Steele, S.; Beller, A.; Mouvery, A.; Furlong, H.M.; Maitre, N.L. Neuroimaging identifies increased manganese deposition in infants receiving parenteral nutrition. Am. J. Clin. Nutr. 2015, 102, 1482–1489. [Google Scholar] [CrossRef] [Green Version]
- Dion, L.-A.; Bouchard, M.F.; Sauvé, S.; Barbeau, B.; Tucholka, A.; Major, P.; Gilbert, G.; Mergler, D.; Saint-Amour, D. MRI pallidal signal in children exposed to manganese in drinking water. Neurotoxicology 2016, 53, 124–131. [Google Scholar] [CrossRef]
- De Water, E.; Papazaharias, D.M.; Ambrosi, C.; Mascaro, L.; Iannilli, E.; Gasparotti, R.; Lucchini, R.G.; Austin, C.; Arora, M.; Tang, C.Y.; et al. Early-life dentine manganese concentrations and intrinsic functional brain connectivity in adolescents: A pilot study. PLoS ONE 2019, 14, e0220790. [Google Scholar] [CrossRef] [Green Version]
- De Water, E.; Proal, E.; Wang, V.; Medina, S.M.; Schnaas, L.; Téllez-Rojo, M.M.; Wright, R.O.; Tang, C.Y.; Horton, M.K. Prenatal manganese exposure and intrinsic functional connectivity of emotional brain areas in children. Neurotoxicology 2018, 64, 85–93. [Google Scholar] [CrossRef]
- Thomason, M.E.; Hect, J.L.; Rauh, V.A.; Trentacosta, C.; Wheelock, M.D.; Eggebrecht, A.T.; Espinoza-Heredia, C.; Burt, S.A. Prenatal lead exposure impacts cross-hemispheric and long-range connectivity in the human fetal brain. Neuroimage 2019, 191, 186–192. [Google Scholar] [CrossRef]
- Alshaikh, B.; Zeed, M.A.; Yusuf, K.; Guin, M.; Fenton, T. Effect of enteral zinc supplementation on growth and neurodevelopment of preterm infants: A systematic review and meta-analysis. J. Perinatol. 2022, 42, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Adamo, A.M.; Oteiza, P.I. Zinc deficiency and neurodevelopment: The case of neurons. Biofactors 2010, 36, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boscarino, G.; Gasparini, C.; Conti, M.G.; Di Chiara, M.; Terrin, G. Zinc levels in neonatal life influence long-term neurodevelopment. J. Perinatol. 2021, 41, 1196–1197. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.; Zavaleta, N.; Kannass, K.N.; Lazarte, F.; Albornoz, C.; Kapa, L.L.; Caulfield, L.E. Zinc Supplementation Sustained Normative Neurodevelopment in a Randomized, Controlled Trial of Peruvian Infants Aged 6–18 Months. J. Nutr. 2014, 144, 1298–1305. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, H.; Taki, Y.; Nouchi, R.; Yokoyama, R.; Kotozaki, Y.; Nakagawa, S.; Sekiguchi, A.; Iizuka, K.; Hanawa, S.; Araki, T.; et al. Succeeding in deactivating: Associations of hair zinc levels with functional and structural neural mechanisms. Sci. Rep. 2020, 10, 12364. [Google Scholar] [CrossRef] [PubMed]
- Pourtavakoli, A.; Ghafouri-Fard, S. Calcium signaling in neurodevelopment and pathophysiology of autism spectrum disorders. Mol. Biol. Rep. 2022, 49, 10811–10823. [Google Scholar] [CrossRef]
- Sun, M.; Wu, X.; Yu, Y.; Wang, L.; Xie, D.; Zhang, Z.; Chen, L.; Lu, A.; Zhang, G.; Li, F. Disorders of Calcium and Phosphorus Metabolism and the Proteomics/Metabolomics-Based Research. Front. Cell Dev. Biol. 2020, 8, 576110. [Google Scholar] [CrossRef]
- Henn, B.C.; Ettinger, A.S.; Schwartz, J.; Téllez-Rojo, M.M.; Lamadrid-Figueroa, H.; Hernández-Avila, M.; Schnaas, L.; Amarasiriwardena, C.; Bellinger, D.C.; Hu, H.; et al. Early Postnatal Blood Manganese Levels and Children’s Neurodevelopment. Epidemiology 2010, 21, 433–439. [Google Scholar] [CrossRef]
- Yamamoto, M.; Eguchi, A.; Sakurai, K.; Nakayama, S.F.; Sekiyama, M.; Mori, C.; Kamijima, M. Longitudinal analyses of maternal and cord blood manganese levels and neurodevelopment in children up to 3 years of age: The Japan Environment and Children’s Study (JECS). Environ. Int. 2022, 161, 107126. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [Green Version]
- McLean, F.C. Physiology of calcium and phosphorus metabolism. J. Am. Med. Women’s Assoc. 1969, 24, 21–30. Available online: https://www.ncbi.nlm.nih.gov/pubmed/4237212 (accessed on 4 December 2022).
- Irizar, A.; Molinuevo, A.; Andiarena, A.; Jimeno-Romero, A.; Román, A.S.; Broberg, K.; Llop, S.; Soler-Blasco, R.; Murcia, M.; Ballester, F.; et al. Prenatal manganese serum levels and neurodevelopment at 4 years of age. Environ. Res. 2021, 197, 111172. [Google Scholar] [CrossRef] [PubMed]
- Solís-Chagoyán, H.; Flores-Soto, E.; Reyes-García, J.; Valdés-Tovar, M.; Calixto, E.; Montaño, L.M.; Benítez-King, G. Voltage-Activated Calcium Channels as Functional Markers of Mature Neurons in Human Olfactory Neuroepithelial Cells: Implications for the Study of Neurodevelopment in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2016, 17, 941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiore, M.; Barone, R.; Copat, C.; Grasso, A.; Cristaldi, A.; Rizzo, R.; Ferrante, M. Metal and essential element levels in hair and association with autism severity. J. Trace Elements Med. Biol. 2020, 57, 126409. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Curtin, P.; Austin, C. Systems and Methods for Diagnostics for Biological Disorders Associated with Periodic Variations in Metal Metabolism. U.S. Patent 2022/0236236, 28 July 2022. [Google Scholar]
| Total | Autism | Control | Zygosity (MZ, DZ) 1 | Age (Mean ± SD) | |
|---|---|---|---|---|---|
| Overall | 124 | 30 | 94 | 81 MZ, 43 DZ | 15.32 ± 4.02 |
| Male | 66 | 17 | 49 | 44 MZ, 22 DZ | 14.59 ± 2.89 |
| Female | 58 | 13 | 45 | 37 MZ, 21 DZ | 16.15 ± 4.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curtin, P.; Neufeld, J.; Curtin, A.; Austin, C.; Isaksson, J.; Remnelius, K.L.; Norrman, H.N.; Arora, M.; Bölte, S. Associations between Elemental Metabolic Dynamics and Default Mode Network Functional Connectivity Are Altered in Autism. J. Clin. Med. 2023, 12, 1022. https://doi.org/10.3390/jcm12031022
Curtin P, Neufeld J, Curtin A, Austin C, Isaksson J, Remnelius KL, Norrman HN, Arora M, Bölte S. Associations between Elemental Metabolic Dynamics and Default Mode Network Functional Connectivity Are Altered in Autism. Journal of Clinical Medicine. 2023; 12(3):1022. https://doi.org/10.3390/jcm12031022
Chicago/Turabian StyleCurtin, Paul, Janina Neufeld, Austen Curtin, Christine Austin, Johan Isaksson, Karl Lundin Remnelius, Hjalmar Nobel Norrman, Manish Arora, and Sven Bölte. 2023. "Associations between Elemental Metabolic Dynamics and Default Mode Network Functional Connectivity Are Altered in Autism" Journal of Clinical Medicine 12, no. 3: 1022. https://doi.org/10.3390/jcm12031022






