Ultrasound during the COVID-19 Pandemic: A Global Approach
Abstract
:1. Introduction
2. Lung Ultrasound
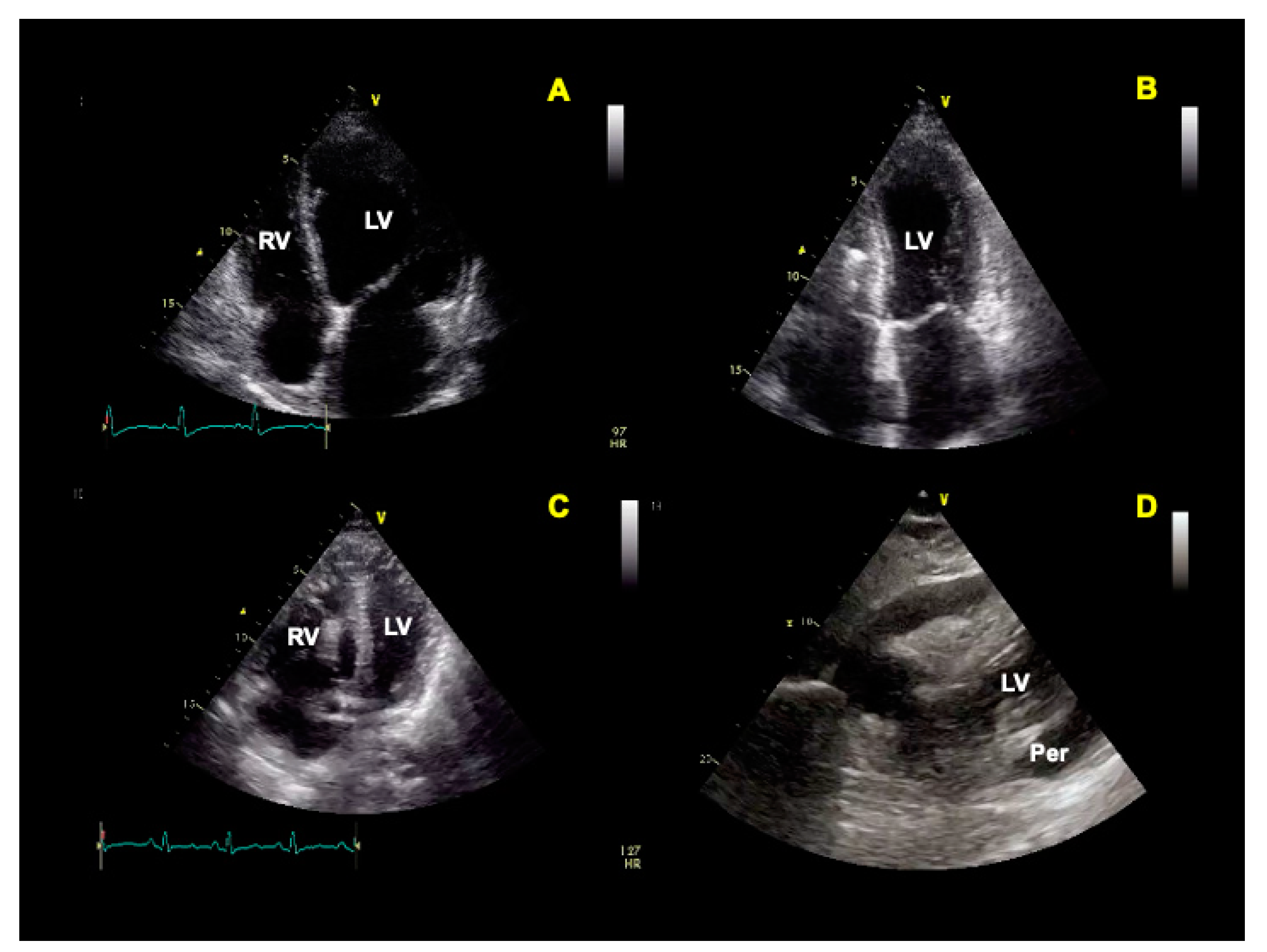
3. Echocardiography
4. Vascular Ultrasound
5. Ultrasound in ICU
5.1. Lung Ultrasound
5.2. Cardiovascular Ultrasound
5.3. Ultrasound in Shock and Fluid Administration
5.4. POCUS Protocols and Other Applications
6. Vaccination-Associated Reactive Lymphadenopathy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, A.; Mele, D.; Palermi, S.; Lombardi, A.; Di Giannuario, G.; Rizzo, M.; Campana, M.; Marrazzo, G.; Scarafile, R.; Gimelli, A.; et al. Ecografia del polmone: Quello che il cardiologo dovrebbe conoscere. G. Ital. di Cardiol. 2021, 22, 638–647. [Google Scholar]
- Elrobaa, I.H.; New, K.J. COVID-19: Pulmonary and Extra Pulmonary Manifestations. Front. Public Health 2021, 9, 711616. [Google Scholar] [CrossRef]
- Bajema, K.L.; Oster, A.M.; McGovern, O.L.; Lindstrom, S.; Stenger, M.R.; Anderson, T.C.; Isenhour, C.; Clarke, K.R.; Evans, M.E.; Chu, V.T.; et al. Persons Evaluated for 2019 Novel Coronavirus-United States, January 2020. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 166–170. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; Yu, T.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Teo, J. Early Detection of Silent Hypoxia in COVID-19 Pneumonia Using Smartphone Pulse Oximetry. J. Med Syst. 2020, 44, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Fox, B.A.; Urba, W.J.; Anderson, A.C.; Atkins, M.B.; Borden, E.C.; Brahmer, J.R.; Butterfield, L.H.; Cesano, A.; Chen, D.S.; et al. Insights from immuno-oncology: The Society for Immunotherapy of Cancer Statement on access to IL-6-targeting therapies for COVID-19. J. Immunother. Cancer 2020, 8, e000878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, S.; Xiong, Y.; Liu, H.; Niu, L.; Guo, J.; Liao, M.; Xiao, S.-Y. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020, 33, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S.Y. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J. Thorac. Oncol. 2020, 15, 700–704. [Google Scholar] [CrossRef]
- Kameda, T.; Mizuma, Y.; Taniguchi, H.; Fujita, M.; Taniguchi, N. Point-of-care lung ultrasound for the assessment of pneumonia: A narrative review in the COVID-19 era. J. Med. Ultrason. 2001, 48, 31–43. [Google Scholar] [CrossRef]
- Reissig, A.; Copetti, R.; Kroegel, C. Current role of emergency ultrasound of the chest. Crit. Care Med. 2011, 39, 839–845. [Google Scholar] [CrossRef] [PubMed]
- A Chavez, M.; Shams, N.; E Ellington, L.; Naithani, N.; Gilman, R.H.; Steinhoff, M.C.; Santosham, M.; E Black, R.; Price, C.; Gross, M.; et al. Lung ultrasound for the diagnosis of pneumonia in adults: A systematic review and meta-analysis. Respir. Res. 2014, 15, 50. [Google Scholar] [CrossRef] [Green Version]
- Volpicelli, G.; Gargani, L. Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J. 2020, 12, 22. [Google Scholar] [CrossRef]
- Lichter, Y.; Topilsky, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Gal Oz, A.; Vine, J.; Goren, O.; Cohen, B.; et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020, 46, 1873. [Google Scholar] [CrossRef] [PubMed]
- Bonadia, N.; Carnicelli, A.; Piano, A.; Buonsenso, D.; Gilardi, E.; Kadhim, C.; Torelli, E.; Petrucci, M.; Di Maurizio, L.; Biasucci, D.G.; et al. Lung Ultrasound Findings Are Associated with Mortality and Need for Intensive Care Admission in COVID-19 Patients Evaluated in the Emergency Department. Ultrasound Med. Biol. 2020, 46, 2927–2937. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Via, G.; Melniker, L.; Goffi, A.; Tavazzi, G.; Neri, L.; Villen, T.; Hoppmann, R.; Mojoli, F.; Noble, V.; et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): International expert consensus. Crit. Care 2020, 24, 702. [Google Scholar] [CrossRef]
- Shokoohi, H.; Duggan, N.M.; García-de-Casasola Sánchez, G.; Torres-Arrese, M.; Tung-Chen, Y. Lung ultrasound monitoring in patients with COVID-19 on home isolation. Am. J. Emerg. Med. 2020, 38, 2759.e5–2759.e8. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [Green Version]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermi, S.; Serio, A.; Vecchiato, M.; Sirico, F.; Gambardella, F.; Ricci, F.; Iodice, F.; Radmilovic, J.; Russo, V.; D’Andrea, A. Potential role of an athlete-focused echocardiogram in sports eligibility. World J. Cardiol. 2021, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Windecker, S.; Andreini, D.; Arbelo, E.; Barbato, E.; Bartorelli, A.L.; Baumbach, A.; Behr, E.R.; Berti, S.; Bueno, H.; et al. European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 1-epidemiology, pathophysiology, and diagnosis. Eur. Heart J. 2020, 43, 1033–1058. [Google Scholar]
- Kirkpatrick, J.N.; Mitchell, C.; Taub, C.; Kort, S.; Hung, J.; Swaminathan, M. ASE Statement on Protection of Patients and Echocardiography Service Providers During the 2019 Novel Coronavirus Outbreak: Endorsed by the American College of Cardiology. J. Am. Soc. Echocardiogr. 2020, 33, 648. [Google Scholar] [CrossRef] [PubMed]
- Carrizales-Sepúlveda, E.F.; Vera-Pineda, R.; Flores-Ramírez, R.; Hernández-Guajardo, D.A.; Pérez-Contreras, E.; Lozano-Ibarra, M.M.; Ordaz-Farías, A. Echocardiographic Manifestations in COVID-19: A Review. Heart Lung Circ. 2021, 30, 1117–1129. [Google Scholar] [CrossRef]
- Cau, R.; Bassareo, P.; Saba, L. Cardiac Involvement in COVID-19—Assessment with Echocardiography and Cardiac Magnetic Resonance Imaging. SN Compr. Clin. Med. 2020, 2, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Citro, R.; Pontone, G.; Bellino, M.; Silverio, A.; Iuliano, G.; Baggiano, A.; Manka, R.; Iesu, S.; Vecchione, C.; Asch, F.M.; et al. Role of multimodality imaging in evaluation of cardiovascular involvement in COVID-19. Trends Cardiovasc. Med. 2020, 31, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, L.; D’Angelo, E.C.; Paolisso, P.; Toniolo, S.; Fabrizio, M.; Angeli, F.; Donati, F.; Magnani, I.; Rinaldi, A.; Bartoli, L.; et al. The value of ECG changes in risk stratification of COVID-19 patients. Ann. Noninvasive Electrocardiol. 2021, 26, e12815. [Google Scholar] [CrossRef]
- Rathore, S.S.; Rojas, G.A.; Sondhi, M.; Pothuru, S.; Pydi, R.; Kancherla, N.; Singh, R.; Ahmed, N.K.; Shah, J.; Tousif, S.; et al. Myocarditis associated with Covid-19 disease: A systematic review of published case reports and case series. Int. J. Clin. Pr. 2021, 75, e14470. [Google Scholar]
- Goerlich, E.; Minhas, A.S.; Mukherjee, M.; Sheikh, F.H.; Gilotra, N.A.; Sharma, G.; Michos, E.D.; Hays, A.G. Multimodality Imaging for Cardiac Evaluation in Patients with COVID-19. Curr. Cardiol. Rep. 2021, 23, 44. [Google Scholar] [CrossRef]
- Escher, F.; Westermann, D.; Gaub, R.; Pronk, J.; Bock, T.; Al-Saadi, N.; Kühl, U.; Schultheiss, H.-P.; Tschöpe, C. Development of diastolic heart failure in a 6-year follow-up study in patients after acute myocarditis. Heart 2010, 97, 709–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olszewski, R.; Timperley, J.; Cezary, S.; Monaghan, M.; Nihoyannopoulis, P.; Senior, R.; Becher, H. The clinical applications of contrast echocardiography. Eur. J. Echocardiogr. 2007, 8, s13–s23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeboye, A.; Alkhatib, D.; Butt, A.; Yedlapati, N.; Garg, N. A Review of the Role of Imaging Modalities in the Evaluation of Viral Myocarditis with a Special Focus on COVID-19-Related Myocarditis. Diagnostics 2022, 12, 549. [Google Scholar] [CrossRef] [PubMed]
- Başar, E.Z.; Usta, E.; Akgün, G.; Güngör, H.S.; Sönmez, H.E.; Babaoglu, K. Is strain echocardiography a more sensitive indicator of myocardial involvement in patients with multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2? Cardiol. Young 2022, 32, 1657–1667. [Google Scholar] [CrossRef]
- Gherbesi, E.; Bergamaschi, L.; Cusmano, I.; Tien, T.T.; Paolisso, P.; Foà, A.; Pizzi, C.; Barosi, A. The usefulness of speckle tracking echocardiography in identifying subclinical myocardial dysfunction in young adults recovered from mild COVID-19. Echocardiography 2022, 39, 1190–1197. [Google Scholar] [CrossRef]
- D’Andrea, A.; Cante, L.; Palermi, S.; Carbone, A.; Ilardi, F.; Sabatella, F.; Crescibene, F.; Di Maio, M.; Giallauria, F.; Messalli, G.; et al. COVID-19 Myocarditis: Prognostic Role of Bedside Speckle-Tracking Echocardiography and Association with Total Scar Burden. Int. J. Environ. Res. Public Health 2022, 19, 5898. [Google Scholar] [CrossRef]
- Baigent, C.; Windecker, S.; Andreini, D.; Arbelo, E.; Barbato, E.; Bartorelli, A.L.; Baumbach, A.; Behr, E.; Berti, S.; Bueno, H.; et al. ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 2-care pathways, treatment, and follow-up. Eur. Heart J. 2022, 43, 1059–1103. [Google Scholar]
- Palmisano, A.; Gambardella, M.; D’Angelo, T.; Vignale, D.; Ascione, R.; Gatti, M.; Peretto, G.; Federico, F.; Shah, A.; Esposito, A. Advanced cardiac imaging in the spectrum of COVID-19 related cardiovascular involvement. Clin. Imaging 2022, 90, 78–89. [Google Scholar] [CrossRef]
- Bryndza, M.; Legutko, J.; Kleczynski, P.; Kapelak, B.; Bartus, K.; Litwinowicz, R. Impact of COVID-19 on the incidence of post-acute myocardial infarction mechanical complications. Ann. Cardiothorac. Surg. 2021, 11, 319–321. [Google Scholar] [CrossRef]
- De Rosa, S.; Spaccarotella, C.; Basso, C.; Calabro, M.P.; Curcio, A.; Filardi, P.P.; Mancone, M.; Mercuro, G.; Muscoli, S.; Nodari, S.; et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur. Heart J. 2020, 41, 2083–2088. [Google Scholar] [CrossRef]
- Bryndza, M.A.; Litwinowicz, R.; Bartuś, S.; Nosal, M.; Godlewski, J.; Orzechowska, A.; Wiśniewski, A.; Korpak-Wysocka, R.; Rzeszutko, Ł.; Kocik, P.; et al. Incidence of mechanical complications following myocardial infarction during the first two months of the COVID-19 pandemic in the Southern Poland region: A multicenter study. Kardiol. Pol. 2021, 79, 66–68. [Google Scholar] [CrossRef] [PubMed]
- French, J.K.; Hellkamp, A.S.; Armstrong, P.W.; Cohen, E.; Kleiman, N.S.; O’Connor, C.M.; Holmes, D.R.; Hochman, J.S.; Granger, C.B.; Mahaffey, K.W. Mechanical Complications After Percutaneous Coronary Intervention in ST-Elevation Myocardial Infarction (from APEX-AMI). Am. J. Cardiol. 2010, 105, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.P.; Cremer, J.; Falk, V.; Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.-P.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2014, 35, 2541–2619. [Google Scholar]
- Vincent, J.L.; Levi, M.; Hunt, B.J. Prevention and management of thrombosis in hospitalised patients with COVID-19 pneumonia. Lancet Respir. Med. 2022, 10, 214–220. [Google Scholar] [CrossRef]
- Cheruiyot, I.; Kipkorir, V.; Ngure, B.; Misiani, M.; Munguti, J.; Ogeng’o, J. Arterial Thrombosis in Coronavirus Disease 2019 Patients: A Rapid Systematic Review. Ann. Vasc. Surg. 2021, 70, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Benigni, A.; Casiraghi, F.; Ng, L.F.P.; Renia, L.; Remuzzi, G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2020, 17, 46–64. [Google Scholar] [CrossRef]
- Shaw, R.J.; Bradbury, C.; Abrams, S.T.; Wang, G.; Toh, C.H. COVID-19 and immunothrombosis: Emerging understanding and clinical management. Br. J. Haematol. 2021, 194, 518–529. [Google Scholar] [CrossRef]
- Ward, S.E.; Fogarty, H.; Karampini, E.; Lavin, M.; Schneppenheim, S.; Dittmer, R.; Morrin, H.; Glavey, S.; Ni Cheallaigh, C.; Bergin, C.; et al. ADAMTS13 regulation of VWF multimer distribution in severe COVID-19. J. Thromb. Haemost. 2021, 19, 1914–1921. [Google Scholar] [CrossRef]
- FitzGerald, E.S.; Chen, Y.; Fitzgerald, K.A.; Jamieson, A.M. Lung Epithelial Cell Transcriptional Regulation as a Factor in COVID-19–associated Coagulopathies. Am. J. Respir. Cell Mol. Biol. 2021, 64, 687–697. [Google Scholar] [CrossRef]
- Jiménez, D.; García-Sanchez, A.; Rali, P.; Muriel, A.; Bikdeli, B.; Ruiz-Artacho, P.; Le Mao, R.; Rodríguez, C.; Hunt, B.J.; Monreal, M. Incidence of VTE and Bleeding Among Hospitalized Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Chest 2021, 159, 1182–1196. [Google Scholar] [CrossRef]
- Khan, F.; Tritschler, T.; Kahn, S.R.; Rodger, M.A. Venous thromboembolism. Lancet 2021, 398, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Needleman, L.; Cronan, J.; Lilly, M.; Merli, G.; Adhikari, S.; Hertzberg, B. Ultrasound for Lower Extremity Deep Venous Thrombosis: Multidisciplinary Recommendations From the Society of Radiologists in Ultrasound Consensus Conference. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 282. [Google Scholar] [CrossRef]
- Di Vilio, A.; Vergara, A.; Desiderio, A.; Iodice, F.; Serio, A.; Palermi, S.; Gambardella, F.; Sperlongano, S.; Gioia, R.; Acitorio, M.; et al. Incremental value of compression ultrasound sonography in the emergency department. World J. Crit. Care Med. 2021, 10, 194. [Google Scholar] [CrossRef]
- Santoliquido, A.; Porfidia, A.; Nesci, A.; De Matteis, G.; Marrone, G.; Porceddu, E.; Cammà, G.; Giarretta, I.; Fantoni, M.; Landi, F.; et al. Incidence of Deep Vein Thrombosis among non-ICU Patients Hospitalized for COVID-19 Despite Pharmacological Thromboprophylaxis. J. Thromb. Haemost. 2020, 18, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Llitjos, J.-F.; Leclerc, M.; Chochois, C.; Monsallier, J.-M.; Ramakers, M.; Auvray, M.; Merouani, K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020, 18, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, D.; Capodaglio, C.; Maino, C.; Corso, R.; Leni, D.; Fior, D.; Giandola, T.; Ragusi, M.; Franzesi, C.T.; Gandola, D.; et al. Compressive ultrasound can predict early pulmonary embolism onset in COVID patients. J. Ultrasound 2022, 25, 571. [Google Scholar] [CrossRef]
- Voicu, S.; Bonnin, P.; Malissin, I.; Mohamedi, N.; M’Rad, A.; Ekhérian, J.M.; Sutterlin, L.; Naim, G.; Lacoste-Palasset, T.; Deye, N.; et al. Characteristics of deep vein thrombosis in the critically ill COVID-19 patient-An observational cohort study with Doppler ultrasound measurements. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 686–694. [Google Scholar]
- Tung-Chen, Y.; Calderón, R.; Marcelo, C.; Deodati, F.; Mateos, M.; Castellano, A.; Álvarez, B.; Marco, J.; Ordieres, L. Duplex Ultrasound Screening for Deep and Superficial Vein Thrombosis in COVID-19 Patients. J. Ultrasound Med. 2022, 41, 1095–1100. [Google Scholar] [CrossRef]
- Rabbia, C.; Matricardi, L. Eco-Color-Doppler Vascolare; Minerva Medica: Torino, Italy, 2005; pp. 767–777. [Google Scholar]
- Ziai, W.C.; Hopkins, J.; Cho, S.-M.; Johansen, M.C.; Ergin, B.; Bahouth, M.N. Transcranial Doppler in Acute COVID-19 Infection Unexpected Associations. Stroke 2021, 52, 2422–2426. [Google Scholar]
- Alexandrov, A.V.; Sloan, M.A.; Tegeler, C.H.; Newell, D.N.; Lumsden, A.; Garami, Z.; Levy, C.R.; Wong, L.K.; Douville, C.; Kaps, M.; et al. Practice standards for transcranial Doppler (TCD) ultrasound. Part II. Clinical indications and expected outcomes. J Neuroimaging 2012, 22, 215–224. [Google Scholar]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [PubMed]
- Marcic, M.; Marcic, L.; Kojundzic, S.L.; Guic, M.M.; Marcic, B.; Caljkusic, K. Chronic Endothelial Dysfunction after COVID-19 Infection Shown by Transcranial Color-Coded Doppler: A Cross-Sectional Study. Biomedicines 2022, 10, 2550. [Google Scholar] [PubMed]
- Yau, O.; Gin, K.; Luong, C.; Jue, J.; Abolmaesumi, P.; Tsang, M.; Nair, P.; Tsang, T.S. Point-of-care ultrasound in the COVID-19 era: A scoping review. Echocardiography 2021, 38, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Perez Herrero, M.A.; Sci, B.J.; de la Torre, M.; Fajardo Perez, M.; Galluccio, F. Pocus in COVID-19 Patients with Vascular Complications: What Have We Learnt from the Pandemic? Biomed. J. Sci. Tech. Res. 2022, 43, 34754–34762. [Google Scholar]
- Peng, Q.-Y.; Wang, X.-T.; Zhang, L.-N.; Chinese Critical Care Ultrasound Study Group (CCUSG). Using echocardiography to guide the treatment of novel coronavirus pneumonia. Crit. Care 2020, 24, 143. [Google Scholar] [CrossRef] [Green Version]
- Buonsenso, D.; Pata, D.; Chiaretti, A. COVID-19 outbreak: Less stethoscope, more ultrasound. Lancet Respir. Med. 2020, 8, e27. [Google Scholar]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Zetlaoui, P. Ecografia e gestione delle vie aeree. EMC Anest. Rianim. 2020, 25, 1–8. [Google Scholar] [CrossRef]
- Chowdhury, A.; Punj, J.; Pandey, R.; Darlong, V.; Sinha, R.; Bhoi, D. Ultrasound is a reliable and faster tool for confirmation of endotracheal intubation compared to chest auscultation and capnography when performed by novice anaesthesia residents-A prospective controlled clinical trial. Saudi J. Anaesth. 2020, 14, 15. [Google Scholar]
- Soldati, G.; Copetti, R. Ecografia Toracica. 2019. Available online: https://www.libreriauniversitaria.it/ecografia-toracica-cd-rom-soldati/libro/9788871104621 (accessed on 18 December 2022).
- Dres, M.; Dubé, B.-P.; Goligher, E.; Vorona, S.; Demiri, S.; Morawiec, E.; Mayaux, J.; Brochard, L.; Similowski, T.; Demoule, A. Usefulness of Parasternal Intercostal Muscle Ultrasound during Weaning from Mechanical Ventilation. Anesthesiology 2020, 132, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Chiles, C.; Ravin, C.E. Radiographic recognition of pneumothorax in the intensive care unit. Crit. Care Med. 1986, 14, 677–680. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Mezière, G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: The comet-tail artifact. Intensive Care Med. 1988, 24, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G. Sonographic diagnosis of pneumothorax. Intensive Care Med. 2011, 37, 224–232. [Google Scholar] [CrossRef]
- Mongodi, S.; Via, G.; Girard, M.; Rouquette, I.; Misset, B.; Braschi, A.; Mojoli, F.; Bouhemad, B. Lung Ultrasound for Early Diagnosis of Ventilator-Associated Pneumonia. Chest 2016, 149, 969–980. [Google Scholar] [CrossRef]
- Lung Ultrasound before and after Fiberbronchoscopy-Modifications May Improve Ventilator-Associated Pneumonia Diagnosis-The GRIP. Available online: http://www.the-grip.org/lung-ultrasound/lung-ultrasound-before-and-after-fiberbronchoscopy-modifications-may-improve-ventilator-associated-pneumonia-diagnosis-2/ (accessed on 27 December 2022).
- Zagli, G.; Cozzolino, M.; Terreni, A.; Biagioli, T.; Caldini, A.L.; Peris, A. Diagnosis of ventilator-associated pneumonia: A pilot, exploratory analysis of a new score based on procalcitonin and chest echography. Chest 2014, 146, 1578–1585. [Google Scholar] [CrossRef]
- Pastore, M.C.; Ilardi, F.; Stefanini, A.; Mandoli, G.E.; Palermi, S.; Bandera, F.; Benfari, G.; Esposito, R.; Lisi, M.; Pasquini, A.; et al. Bedside Ultrasound for Hemodynamic Monitoring in Cardiac Intensive Care Unit. J. Clin. Med. 2022, 11, 7538. [Google Scholar] [CrossRef] [PubMed]
- Teran, F.; Burns, K.M.; Narasimhan, M.; Goffi, A.; Mohabir, P.; Horowitz, J.M.; Yuriditsky, E.; Nagdev, A.; Panebianco, N.; Chin, E.J.; et al. Critical Care Transesophageal Echocardiography in Patients during the COVID-19 Pandemic. J. Am. Soc. Echocardiogr. 2020, 33, 1040–1047. [Google Scholar] [CrossRef]
- Gong, X.; Yuan, B.; Yuan, Y. Incidence and prognostic value of pulmonary embolism in COVID-19: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0263580. [Google Scholar] [CrossRef]
- Klasnja, S.; Manojlovic, A.; Popadic, V.; Ivankovic, T.; Ninkovic, N.; Rajovic, N.; Popovic, M.; Nikolic, N.; Brajkovic, M.; Radojevic, A.; et al. Point-of-Care Echocardiographic Characteristics of COVID-19 Patients with Pulmonary Embolism. Diagnostics 2022, 12, 2380. [Google Scholar] [CrossRef]
- Satoskar, M.A.; Metkus, T.; Soleimanifard, A.; Shade, J.K.; Trayanova, N.A.; Michos, E.D.; Mukherjee, M.; Schiminger, M.; Post, W.S.; Hays, A.G. Improving risk prediction for pulmonary embolism in COVID-19 patients using echocardiography. Pulm. Circ. 2022, 12, e12036. [Google Scholar] [CrossRef]
- Polito, M.V.; Silverio, A.; Di Maio, M.; Bellino, M.; Scudiero, F.; Russo, V.; Rasile, B.; Alfano, C.; Citro, R.; Parodi, G.; et al. Prognostic Implications of Right Ventricular Function and Pulmonary Pressures Assessed by Echocardiography in Hospitalized Patients with COVID-19. J. Pers. Med. 2021, 11, 1245. [Google Scholar] [CrossRef]
- Anile, A.; Castiglione, G.; Zangara, C.; Calabrò, C.; Vaccaro, M.; Sorbello, M. COVID-19: The New Ultrasound Alphabet in SARS-CoV-2 Era. Anesth. Analg. 2020, 131, E232–E234. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.; Gambardella, M.; Palermi, S.; Frecentese, F.; Serio, A.; Sperlongano, S.; Tavarozzi, R.; D’Andrea, A.; De Luca, M.; Politi, C. Liver and heart failure: An ultrasound relationship. J. Basic Clin. Physiol. Pharmacol. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Linde-Zwirble, W.T.; Bittner, E.A.; Sahatjian, J.; Hansell, D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: An analysis of a large national database. Intensive Care Med. 2017, 43, 625–632. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit. Care Med. 2020, 48, E440–E469. [Google Scholar] [CrossRef]
- Piliego, C.; Strumia, A.; Stone, M.B.; Pascarella, G. The Ultrasound-Guided Triage: A New Tool for Prehospital Management of COVID-19 Pandemic. Anesth. Analg. 2020, 131, E93–E94. [Google Scholar] [CrossRef]
- Via, G.; Tavazzi, G.; Price, S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: A physiologically based point of view. Intensive Care Med. 2016, 42, 1164–1167. [Google Scholar] [CrossRef]
- Boyd, J.H.; Sirounis, D.; Maizel, J.; Slama, M. Echocardiography as a guide for fluid management. Crit. Care 2016, 20, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Monnet, X.; Marik, P.E.; Teboul, J.L. Prediction of fluid responsiveness: An update. Ann. Intensive Care 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, S.; Sauthoff, H. Assessing Extravascular Lung Water With Ultrasound: A Tool to Individualize Fluid Management? J. Intensive Care Med. 2020, 35, 1356–1362. [Google Scholar] [CrossRef]
- Koratala, A.; Ronco, C.; Kazory, A. Need for Objective Assessment of Volume Status in Critically Ill Patients with COVID-19: The Tri-POCUS Approach. Cardiorenal. Med. 2020, 10, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.L.S.; Pazeli Júnior, J.M.; Bastos, M.G. Role of point-of-care ultrasound during the COVID-19 pandemic: Our recommendations in the management of dialytic patients. Ultrasound J. 2020, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Trezzi, M.; Torzillo, D.; Ceriani, E.; Costantino, G.; Caruso, S.; Damavandi, P.T.; Genderini, A.; Cicardi, M.; Montano, N.; Cogliati, C. Lung ultrasonography for the assessment of rapid extravascular water variation: Evidence from hemodialysis patients. Intern. Emerg. Med. 2011, 8, 409–415. [Google Scholar] [CrossRef]
- Gültekin, H.; Güven, M. Optic Nerve Sheath Diameter, Intensive Care Unit admission & COVID-19-Related-In-hospital Mortality. QJM 2022, hcac242. [Google Scholar]
- Magoon, R.; Suresh, V.; Manohar Lohia Hospital, R.; Kharak Singh Marg, B. Optic Nerve Sheath Diameter in COVID-19: There’s More to it than Meets the Eye. Available online: https://academic.oup.com/qjmed/advance-article/doi/10.1093/qjmed/hcad002/6972782 (accessed on 27 December 2022).
- Pansell, J.; Rudberg, P.C.; Bell, M.; Friman, O.; Cooray, C. Optic nerve sheath diameter is associated with outcome in severe COVID-19. Sci. Rep. 2022, 12, 17255. [Google Scholar] [CrossRef] [PubMed]
- Ertl, M.; Knüppel, C.; Veitweber, M.; Wagner, A.; Pfister, K.; Wendl, C.; Baldaranov, D.; Beck, J.; Linker, R.A.; Schlachetzki, F. Normal Age-and Sex-Related Values of the Optic Nerve Sheath Diameter and Its Dependency on Position and Positive End-Expiratory Pressure. Ultrasound Med. Biol. 2020, 46, 3279–3285. [Google Scholar] [CrossRef]
- Caruso, D.; Zerunian, M.; Pucciarelli, F.; Lucertini, E.; Bracci, B.; Polidori, T.; Guido, G.; Polici, M.; Rucci, C.; Iannicelli, E.; et al. Imaging of abdominal complications of COVID-19 infection. BJR Open 2021, 3, 20200052. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e3. [Google Scholar] [CrossRef]
- Ignat, M.; Philouze, G.; Aussenac-Belle, L.; Faucher, V.; Collange, O.; Mutter, D.; Pessaux, P. Small bowel ischemia and SARS-CoV-2 infection: An underdiagnosed distinct clinical entity. Surgery 2020, 168, 14–16. [Google Scholar] [CrossRef]
- Rha, S.E.; Ha, H.K.; Lee, S.-H.; Kim, J.-H.; Kim, J.-K.; Kim, J.H.; Kim, P.N.; Lee, M.-G.; Auh, Y. CT and MR Imaging Findings of Bowel Ischemia from Various Primary Causes. Radiographics 2000, 20, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Coneybeare, D.; Das, D.; Lema, P.; Chang, B.; Ng, L. COVUS: An Algorithm to Maximize the Use of Point-of-Care Ultrasound in the Emergency Management of COVID-19. J. Emerg. Med. 2021, 61, 61. [Google Scholar] [CrossRef] [PubMed]
- Toraskar, K.; Zore, R.R.; Gupta, G.A.; Gondse, B.; Pundpal, G.; Kadam, S.; Pawaskar, S.; Setia, M.S. Utility and diagnostic test properties of pulmonary and cardiovascular point of care ultra-sonography (POCUS) in COVID-19 patients admitted to critical care unit. Eur. J. Radiol. Open 2022, 9, 100451. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Mezière, G.A. The BLUE-points: Three standardized points used in the BLUE-protocol for ultrasound assessment of the lung in acute respiratory failure. Crit. Ultrasound J. 2011, 3, 109–110. [Google Scholar] [CrossRef] [Green Version]
- Oveland, N.P.; Bogale, N.; Waldron, B.; Bech, K.; Sloth, E. Focus assessed transthoracic echocardiography (FATE) to diagnose pleural effusions causing haemodynamic compromise. Case Rep. Clin. Med. 2013, 2, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Sharifian-Dorche, M.; Bahmanyar, M.; Sharifian-Dorche, A.; Mohammadi, P.; Nomovi, M.; Mowla, A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J. Neurol. Sci. 2021, 428, 117607. [Google Scholar] [CrossRef]
- Ha, S.M.; Chu, A.J.; Lee, J.; Kim, S.-Y.; Lee, S.H.; Yoen, H.; Cho, N.; Moon, W.K.; Chang, J.M. US Evaluation of Axillary Lymphadenopathy Following COVID-19 Vaccination: A Prospective Longitudinal Study. Radiology 2022, 305, 46–53. [Google Scholar] [CrossRef]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef]
- Lederer, K.; Castaño, D.; Atria, D.G.; Oguin, T.H.; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 2020, 53, 1281–1295.e5. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, J.Y.; Yi, S.Y. Axillary Lymphadenopathy on Ultrasound after COVID-19 Vaccination and Its Influencing Factors: A Single-Center Study. J. Clin. Med. 2022, 11, 238. [Google Scholar] [CrossRef]
- Moderna COVID-19 Vaccine’s Reactions and Adverse Events | CDC. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html (accessed on 27 December 2022).
- Adin, M.E.; Isufi, E.; Kulon, M.; Pucar, D. Association of COVID-19 mRNA Vaccine With Ipsilateral Axillary Lymph Node Reactivity on Imaging. JAMA Oncol. 2021, 7, 1241–1242. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, S.; Kim, E.; Plaunova, A.; Bukhman, R.; Sarmiento, R.D.; Samreen, N.; Awal, D.; Sheth, M.M.; Toth, H.B.; Moy, L.; et al. Axillary Adenopathy after COVID-19 Vaccine: No Reason to Delay Screening Mammogram. Radiology 2022, 303, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Tavarozzi, R.; Manzato, E.; Lombardi, A.; Tavarozzi, R.; Manzato, E.; Lombardi, A. Lymph Node Ultrasound in Lymphoproliferative Disorders: Where Are We Now? J. Clin. Imaging Sci. 2021, 11, 22. [Google Scholar] [CrossRef] [PubMed]

| Lung | Heart | Veins |
|---|---|---|
| Bilateral multiple or confluent B lines. | Left or right ventricle increased endocavitary dimensions | Thrombosis ultrasound findings: non-compressible venous segment; loss of phasic flow on Valsalva maneuver; absent color flow; lack of flow augmentation with calf squeeze; increased flow in superficial veins |
| Peripheral parenchymal consolidations with distribution reflect interstitial involvement. | Normal or increased wall thickness | Larger diameter of common femoral vein and lower peak blood-flow velocities |
| Irregular pleural line with thickenings and discontinuity | Global or regional ventricular systolic dysfunction | |
| Bilateral pleural effusion | Diastolic dysfunction | |
| Left ventricle thrombus | ||
| Pericardial involvement with effusion |
| First Author, Year of Publication (Ref.) | Article Type | Setting | POCUS Protocols |
|---|---|---|---|
| Koratala A, 2020 [90] | Editorial | Critically ill Patients with COVID-19 | Tri-POCUS approach:
|
| Anile A, 2020 [81] | Commentary | Critically ill Patients with COVID-19 | COVID-US approach: C: cardiac evaluation O: outputs → renal resistive index, velocity-time integral V: ventilation → B-line patterns, hyperinflation and recruitment response, lung score, pneumothorax/effusion. I: intubation → prediction of difficult laryngoscopy/intubation, endotracheal intubation confirmation D: Doppler and deep venous thromboembolism/pulmonary embolism |
| Coneybeare, 2021 [101] | Review | Patients under investigation for COVID-19 | COVUS approach:
|
| Toraskar K, 2022 [102] | Cross-sectional study | Critically ill Patients with COVID-19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, A.; De Luca, M.; Fabiani, D.; Sabatella, F.; Del Giudice, C.; Caputo, A.; Cante, L.; Gambardella, M.; Palermi, S.; Tavarozzi, R.; et al. Ultrasound during the COVID-19 Pandemic: A Global Approach. J. Clin. Med. 2023, 12, 1057. https://doi.org/10.3390/jcm12031057
Lombardi A, De Luca M, Fabiani D, Sabatella F, Del Giudice C, Caputo A, Cante L, Gambardella M, Palermi S, Tavarozzi R, et al. Ultrasound during the COVID-19 Pandemic: A Global Approach. Journal of Clinical Medicine. 2023; 12(3):1057. https://doi.org/10.3390/jcm12031057
Chicago/Turabian StyleLombardi, Anna, Mariarosaria De Luca, Dario Fabiani, Francesco Sabatella, Carmen Del Giudice, Adriano Caputo, Luigi Cante, Michele Gambardella, Stefano Palermi, Rita Tavarozzi, and et al. 2023. "Ultrasound during the COVID-19 Pandemic: A Global Approach" Journal of Clinical Medicine 12, no. 3: 1057. https://doi.org/10.3390/jcm12031057
APA StyleLombardi, A., De Luca, M., Fabiani, D., Sabatella, F., Del Giudice, C., Caputo, A., Cante, L., Gambardella, M., Palermi, S., Tavarozzi, R., Russo, V., & D’Andrea, A. (2023). Ultrasound during the COVID-19 Pandemic: A Global Approach. Journal of Clinical Medicine, 12(3), 1057. https://doi.org/10.3390/jcm12031057








