Repetitive Bleomycin-Based Electrochemotherapy Improves Antitumor Effectiveness in 3D Tumor Models of Conjunctival Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Culturing of CM Cell Lines
2.2. Treatment of Spheroids
2.3. Determination of Spheroid Growth
2.4. Determination of Spheroid Viability Using ATP Measurement
2.5. Immunocytochemistry
2.6. Outgrowth Capacity of Treated Spheroids
2.7. Statistical Analysis
3. Results
3.1. Spheroid Growth Is Significantly Reduced in CM Cells after Repetitive ECT Treatment
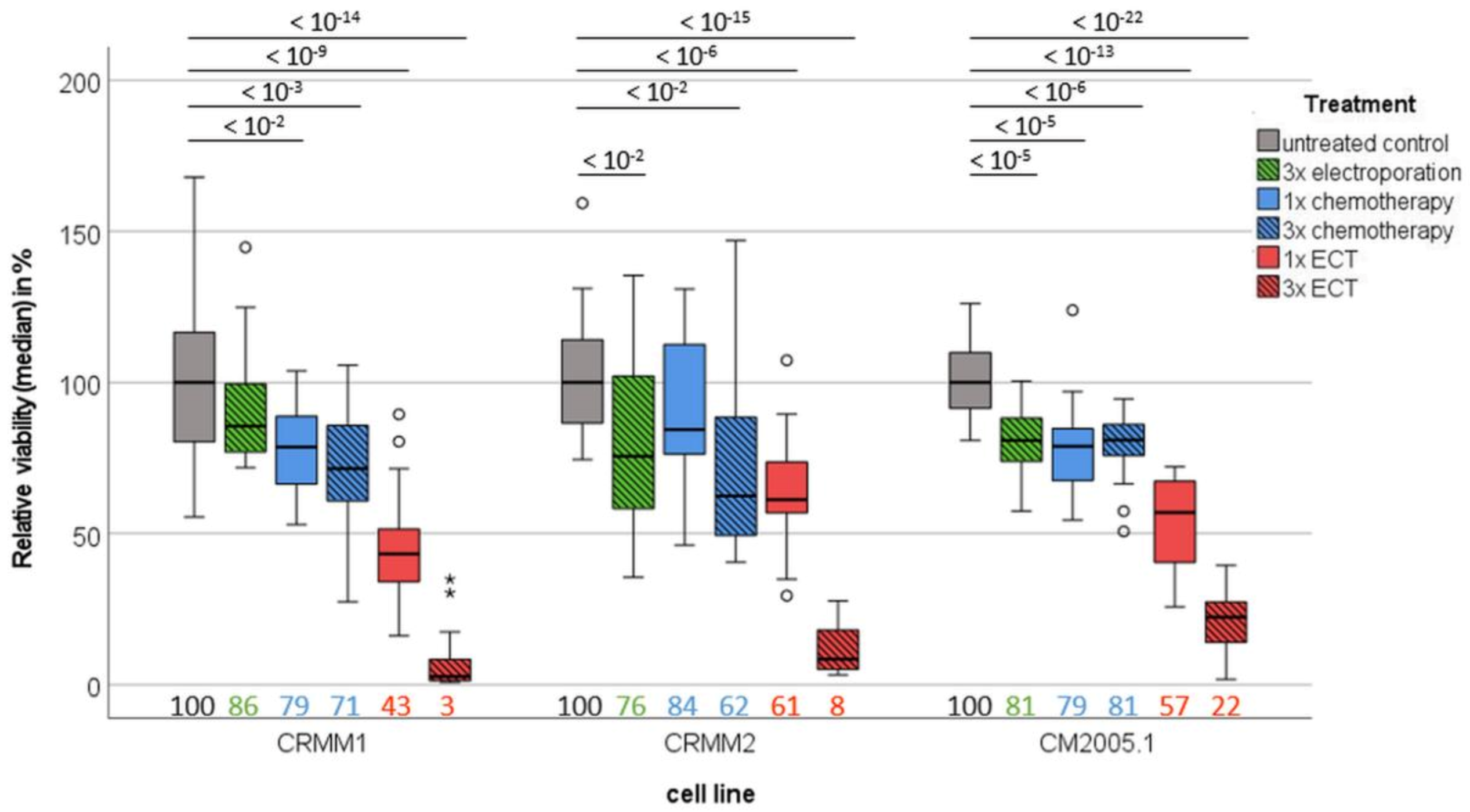
3.2. Spheroid Viability Is Significantly Decreased in CM Cell Lines after Repeated ECT Treatment
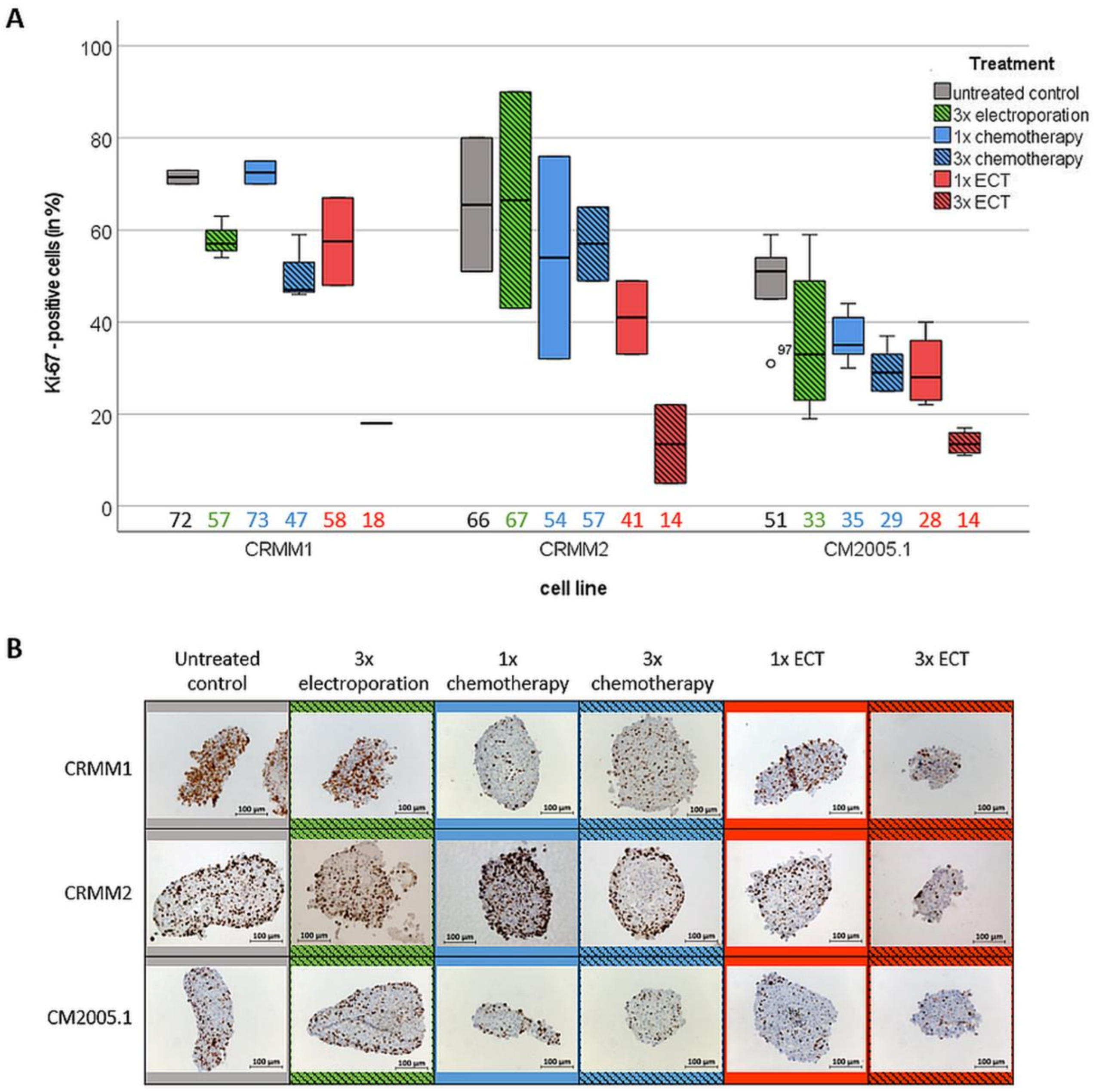
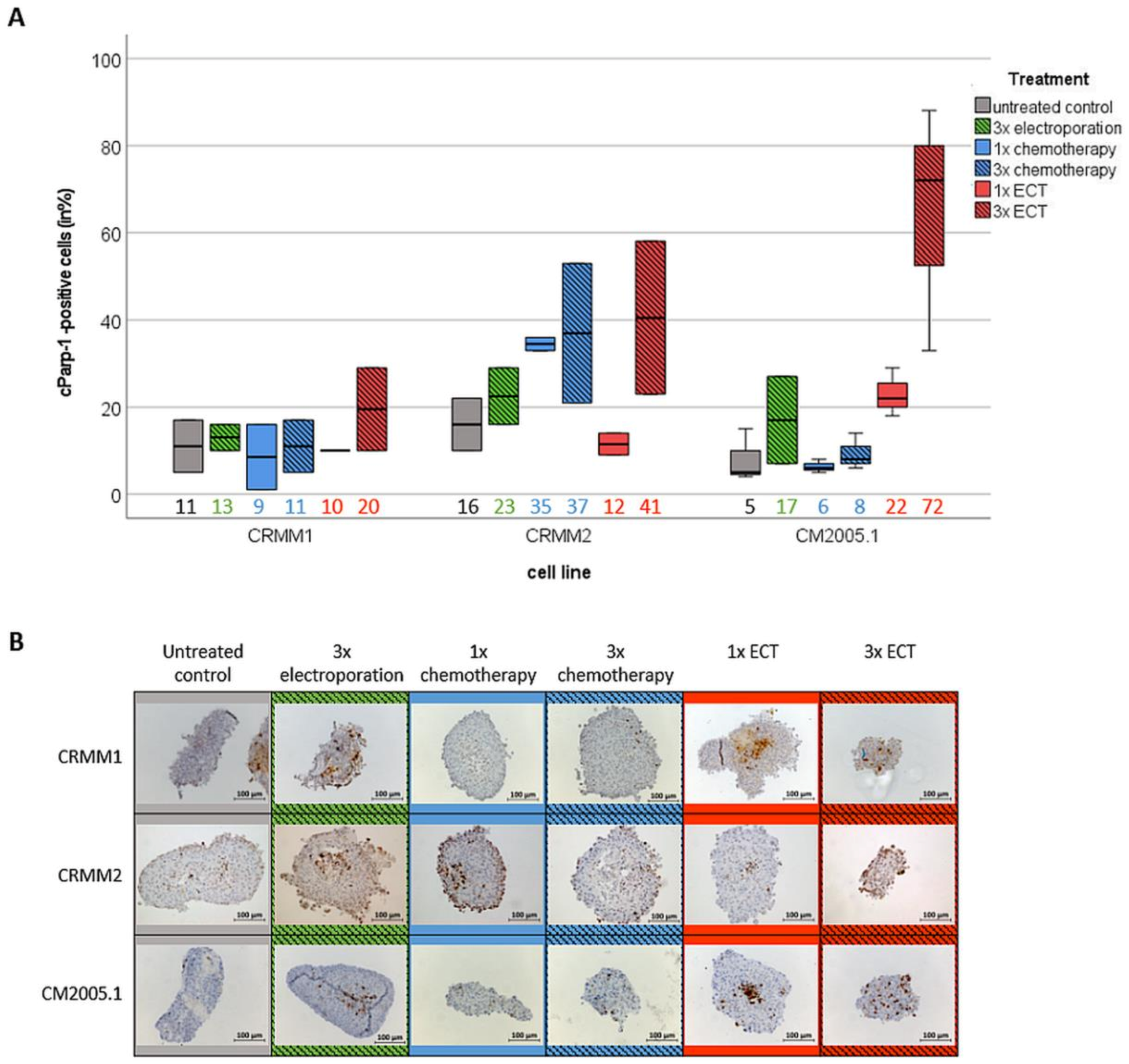
3.3. Repetitive ECT Treatment of CM Spheroids Is Associated with Decreased Proliferation Capacity and an Increase of Apoptotic Cells
3.4. Repetitive ECT-Treated Spheroids Have a Worse Outgrowth Capacity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaštelan, S.; Gverović Antunica, A.; Beketić Orešković, L.; Salopek Rabatić, J.; Kasun, B.; Bakija, I. Conjunctival melanoma—Epidemiological trends and features. Pathol. Oncol. Res. 2018, 24, 787–796. [Google Scholar] [CrossRef]
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The national cancer data base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The american college of surgeons commission on cancer and the american cancer society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- Isager, P.; Engholm, G.; Overgaard, J.; Storm, H. Uveal and conjunctival malignant melanoma in denmark 1943-97: Observed and relative survival of patients followed through 2002. Ophthalmic Epidemiol. 2006, 13, 85–96. [Google Scholar] [CrossRef]
- Wong, J.R.; Nanji, A.A.; Galor, A.; Karp, C.L. Management of conjunctival malignant melanoma: A review and update. Expert Rev. Ophthalmol. 2014, 9, 185–204. [Google Scholar] [CrossRef]
- Rossi, E.; Schinzari, G.; Maiorano, B.A.; Pagliara, M.M.; Di Stefani, A.; Bria, E.; Peris, K.; Blasi, M.A.; Tortora, G. Conjunctival melanoma: Genetic and epigenetic insights of a distinct type of melanoma. Int. J. Mol. Sci. 2019, 20, 5447. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; Westekemper, H.; Murali, R.; Mach, M.; Schilling, B.; Wiesner, T.; Schimming, T.; Livingstone, E.; Sucker, A.; Grabellus, F.; et al. Conjunctival melanomas harbor braf and nras mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin. Cancer Res. 2013, 19, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Rivolta, C.; Royer-Bertrand, B.; Rimoldi, D.; Schalenbourg, A.; Zografos, L.; Leyvraz, S.; Moulin, A. Uv light signature in conjunctival melanoma; not only skin should be protected from solar radiation. J. Hum. Genet. 2016, 61, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.L.; Cosgarea, I.; Süßkind, D.; Murali, R.; Möller, I.; Reis, H.; Leonardelli, S.; Schilling, B.; Schimming, T.; Hadaschik, E.; et al. Nf1 mutations in conjunctival melanoma. Br. J. Cancer 2018, 118, 1243–1247. [Google Scholar] [CrossRef]
- Van Raamsdonk, C.D.; Bezrookove, V.; Green, G.; Bauer, J.; Gaugler, L.; O’Brien, J.M.; Simpson, E.M.; Barsh, G.S.; Bastian, B.C. Frequent somatic mutations of gnaq in uveal melanoma and blue naevi. Nature 2009, 457, 599–602. [Google Scholar] [CrossRef]
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations in gna11 in uveal melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef]
- Swaminathan, S.S.; Field, M.G.; Sant, D.; Wang, G.; Galor, A.; Dubovy, S.R.; Harbour, J.W.; Karp, C.L. Molecular characteristics of conjunctival melanoma using whole-exome sequencing. JAMA Ophthalmol. 2017, 135, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Missotten, G.S.; Keijser, S.; De Keizer, R.J.W.; De Wolff-Rouendaal, D. Conjunctival melanoma in the netherlands: A nationwide study. Investig. Ophthalmol. Vis. Sci. 2005, 46, 75–82. [Google Scholar] [CrossRef] [PubMed]
- van Poppelen, N.M.; van Ipenburg, J.A.; van den Bosch, Q.; Vaarwater, J.; Brands, T.; Eussen, B.; Magielsen, F.; Dubbink, H.J.; Paridaens, D.; Brosens, E.; et al. Molecular genetics of conjunctival melanoma and prognostic value of tert promoter mutation analysis. Int. J. Mol. Sci. 2021, 22, 5784. [Google Scholar] [CrossRef]
- Castiello, M.; Dughiero, F.; Scandola, F.; Sieni, E.; Campana, L.; Rossi, C.; Mattei, M.D.; Pellati, A.; Ongaro, A. A new grid electrode for electrochemotherapy treatment of large skin tumors. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1424–1432. [Google Scholar] [CrossRef]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of esope (european standard operating procedures of electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Jaroszeski, M.J.; Dang, V.; Pottinger, C.; Hickey, J.; Gilbert, R.; Heller, R. Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer Drugs 2000, 11, 201–208. [Google Scholar] [CrossRef]
- Probst, U.; Fuhrmann, I.; Beyer, L.; Wiggermann, P. Electrochemotherapy as a new modality in interventional oncology: A review. Technol. Cancer Res. Treat. 2018, 17, 1533033818785329. [Google Scholar] [CrossRef] [PubMed]
- Gothelf, A.; Mir, L.M.; Gehl, J. Electrochemotherapy: Results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat. Rev. 2003, 29, 371–387. [Google Scholar] [CrossRef]
- Edhemovic, I.; Brecelj, E.; Gasljevic, G.; Marolt Music, M.; Gorjup, V.; Mali, B.; Jarm, T.; Kos, B.; Pavliha, D.; Grcar Kuzmanov, B.; et al. Intraoperative electrochemotherapy of colorectal liver metastases. J. Surg. Oncol. 2014, 110, 320–327. [Google Scholar] [CrossRef]
- Kis, E.G.; Baltas, E.; Ocsai, H.; Vass, A.; Nemeth, I.B.; Varga, E.; Olah, J.; Kemeny, L.; Toth-Molnar, E. Electrochemotherapy in the treatment of locally advanced or recurrent eyelid-periocular basal cell carcinomas. Sci. Rep. 2019, 9, 4285. [Google Scholar] [CrossRef]
- Sersa, G.; Cufer, T.; Paulin, S.M.; Cemazar, M.; Snoj, M. Electrochemotherapy of chest wall breast cancer recurrence. Cancer Treat. Rev. 2012, 38, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Domanico, R.; Trapasso, S.; Santoro, M.; Pingitore, D.; Allegra, E. Electrochemotherapy in combination with chemoradiotherapy in the treatment of oral carcinomas in advanced stages of disease: Efficacy, safety, and clinical outcomes in a small number of selected cases. Drug Des. Devel. Ther. 2015, 9, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Sersa, G. The state-of-the-art of electrochemotherapy before the esope study; advantages and clinical uses. Eur. J. Cancer Suppl. 2006, 4, 52–59. [Google Scholar] [CrossRef]
- Snoj, M.; Rudolf, Z.; Cemazar, M.; Jancar, B.; Sersa, G. Successful sphincter-saving treatment of anorectal malignant melanoma with electrochemotherapy, local excision and adjuvant brachytherapy. Anticancer Drugs 2005, 16, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Heller, R.; Jaroszeski, M.J.; Reintgen, D.S.; Puleo, C.A.; DeConti, R.C.; Gilbert, R.A.; Glass, L.F. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer 1998, 83, 148–157. [Google Scholar] [CrossRef]
- Shimizu, T.; Nikaido, T.; Gomyo, H.; Yoshimura, Y.; Horiuchi, A.; Isobe, K.; Ebara, S.; Takaoka, K. Electrochemotherapy for digital chondrosarcoma. J. Orthop. Sci. 2003, 8, 248–251. [Google Scholar] [CrossRef]
- Fiorentzis, M.; Katopodis, P.; Kalirai, H.; Seitz, B.; Viestenz, A.; Coupland, S.E. Conjunctival melanoma and electrochemotherapy: Preliminary results using 2d and 3d cell culture models in vitro. Acta Ophthalmol. 2019, 97, e632–e640. [Google Scholar] [CrossRef] [PubMed]
- Fiorentzis, M.; Viestenz, A.; Seitz, B.; Coupland, S.E.; Heinzelmann, J. Electrochemotherapy in 3d ocular melanoma spheroids using a customized electrode. J. Vis. Exp. 2020, 158, e60611. [Google Scholar] [CrossRef] [PubMed]
- Kimlin, L.C.; Casagrande, G.; Virador, V.M. In vitro three-dimensional (3d) models in cancer research: An update. Mol. Carcinog. 2013, 52, 167–182. [Google Scholar] [CrossRef]
- Jacques, C.; Marchesi, I.; Fiorentino, F.P.; Chatelais, M.; Lilli, N.L.; Appel, K.; Lejeune, B.; Floris, I. A micro-immunotherapy sequential medicine mim-seq displays immunomodulatory effects on human macrophages and anti-tumor properties towards in vitro 2d and 3d models of colon carcinoma and in an in vivo subcutaneous xenograft colon carcinoma model. Int. J. Mol. Sci. 2022, 23, 6059. [Google Scholar] [CrossRef]
- Nii, T.; Makino, K.; Tabata, Y. Three-dimensional culture system of cancer cells combined with biomaterials for drug screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef]
- Yip, D.; Cho, C.H. A multicellular 3d heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem. Biophys. Res. Commun. 2013, 433, 327–332. [Google Scholar] [CrossRef]
- Pampaloni, F.; Stelzer, E.H.; Masotti, A. Three-dimensional tissue models for drug discovery and toxicology. Recent Pat. Biotechnol. 2009, 3, 103–117. [Google Scholar] [CrossRef]
- Nareyeck, G.; Wuestemeyer, H.; von der Haar, D.; Anastassiou, G. Establishment of two cell lines derived from conjunctival melanomas. Exp. Eye Res. 2005, 81, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Keijser, S.; Maat, W.; Missotten, G.S.; de Keizer, R.J. A new cell line from a recurrent conjunctival melanoma. Br. J. Ophthalmol. 2007, 91, 1566–1567. [Google Scholar] [CrossRef][Green Version]
- Robin, T.; Capes-Davis, A.; Bairoch, A. Clastr: The cellosaurus str similarity search tool—A precious help for cell line authentication. Int. J. Cancer 2020, 146, 1299–1306. [Google Scholar] [CrossRef]
- Uphoff, C.C.; Drexler, H.G. Detecting mycoplasma contamination in cell cultures by polymerase chain reaction. In Cancer Cell Culture: Methods and Protocols; Cree, I.A., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 93–103. [Google Scholar]
- Fiorentzis, M.; Viestenz, A.; Siebolts, U.; Seitz, B.; Coupland, S.E.; Heinzelmann, J. The potential use of electrochemotherapy in the treatment of uveal melanoma: In vitro results in 3d tumor cultures and in vivo results in a chick embryo model. Cancers 2019, 11, 1344. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. Imagej2: Imagej for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Esmaeili, N.; Friebe, M. Electrochemotherapy: A review of current status, alternative igp approaches, and future perspectives. J. Healthc. Eng. 2019, 2019, 2784516. [Google Scholar] [CrossRef] [PubMed]
- Fiorentzis, M.; Katopodis, P.; Kalirai, H.; Seitz, B.; Viestenz, A.; Coupland, S.E. Image analysis of 3d conjunctival melanoma cell cultures following electrochemotherapy. Biomedicines 2020, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Jaroszeski, M.J.; Gilbert, R.; Perrott, R.; Heller, R. Enhanced effects of multiple treatment electrochemotherapy. Melanoma Res. 1996, 6, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Shankayi, Z.; Firoozabadi, S.M. Antitumor efficiency of electrochemotherapy by high and low frequencies and repetitive therapy in the treatment of invasive ductal carcinoma in balb/c mice. Cell J. 2012, 14, 110–115. [Google Scholar] [PubMed]
- Snoj, M.; Cemazar, M.; Slekovec Kolar, B.; Sersa, G. Effective treatment of multiple unresectable skin melanoma metastases by electrochemotherapy. Croat. Med. J. 2007, 48, 391–395. [Google Scholar] [PubMed]
- Garbay, J.-R.; Billard, V.; Bernat, C.; Mir, L.M.; Morsli, N.; Robert, C. Successful repetitive treatments by electrochemotherapy of multiple unresectable kaposi sarcoma nodules. Eur. J. Cancer Suppl. 2006, 4, 29–31. [Google Scholar] [CrossRef]
- Quaglino, P.; Mortera, C.; Osella-Abate, S.; Barberis, M.; Illengo, M.; Rissone, M.; Savoia, P.; Bernengo, M.G. Electrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastases. Ann. Surg. Oncol. 2008, 15, 2215. [Google Scholar] [CrossRef] [PubMed]
- Heller, R.; Coppola, D.; Pottinger, C.; Gilbert, R.; Jaroszeski, M.J. Effect of electrochemotherapy on muscle and skin. Technol. Cancer Res. Treat. 2002, 1, 385–392. [Google Scholar] [CrossRef]
- Sersa, G.; Miklavcic, D.; Cemazar, M.; Rudolf, Z.; Pucihar, G.; Snoj, M. Electrochemotherapy in treatment of tumours. Eur. J. Surg. Oncol. 2008, 34, 232–240. [Google Scholar] [CrossRef]
- Sersa, G.; Stabuc, B.; Cemazar, M.; Jancar, B.; Miklavcic, D.; Rudolf, Z. Electrochemotherapy with cisplatin: Potentiation of local cisplatin antitumour effectiveness by application of electric pulses in cancer patients. Eur. J. Cancer 1998, 34, 1213–1218. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinzelmann, J.; Hecht, S.; Vogt, A.R.; Siebolts, U.; Kaatzsch, P.; Viestenz, A. Repetitive Bleomycin-Based Electrochemotherapy Improves Antitumor Effectiveness in 3D Tumor Models of Conjunctival Melanoma. J. Clin. Med. 2023, 12, 1087. https://doi.org/10.3390/jcm12031087
Heinzelmann J, Hecht S, Vogt AR, Siebolts U, Kaatzsch P, Viestenz A. Repetitive Bleomycin-Based Electrochemotherapy Improves Antitumor Effectiveness in 3D Tumor Models of Conjunctival Melanoma. Journal of Clinical Medicine. 2023; 12(3):1087. https://doi.org/10.3390/jcm12031087
Chicago/Turabian StyleHeinzelmann, Joana, Sabine Hecht, Alexander Ruben Vogt, Udo Siebolts, Peter Kaatzsch, and Arne Viestenz. 2023. "Repetitive Bleomycin-Based Electrochemotherapy Improves Antitumor Effectiveness in 3D Tumor Models of Conjunctival Melanoma" Journal of Clinical Medicine 12, no. 3: 1087. https://doi.org/10.3390/jcm12031087
APA StyleHeinzelmann, J., Hecht, S., Vogt, A. R., Siebolts, U., Kaatzsch, P., & Viestenz, A. (2023). Repetitive Bleomycin-Based Electrochemotherapy Improves Antitumor Effectiveness in 3D Tumor Models of Conjunctival Melanoma. Journal of Clinical Medicine, 12(3), 1087. https://doi.org/10.3390/jcm12031087





