In Achilles Tendinopathy the Symptomatic Tendon Differs from the Asymptomatic Tendon While Exercise Therapy Has Little Effect on Asymmetries—An Ancillary Analysis of Data from a Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Characteristics and Experimental Design
2.2. Intervention
2.3. Plantar Flexor Muscle Characteristics
2.3.1. Maximum Ankle Joint Moment
2.3.2. Plantar Flexor Muscle Architecture
2.4. Achilles Tendon Relative and Absolute Properties
2.5. Jump Performance
2.6. VISA-A Score
2.7. Leg Dominance
2.8. Statistics
3. Results
3.1. Baseline Comparison between the Symptomatic and the Asymptomatic Side
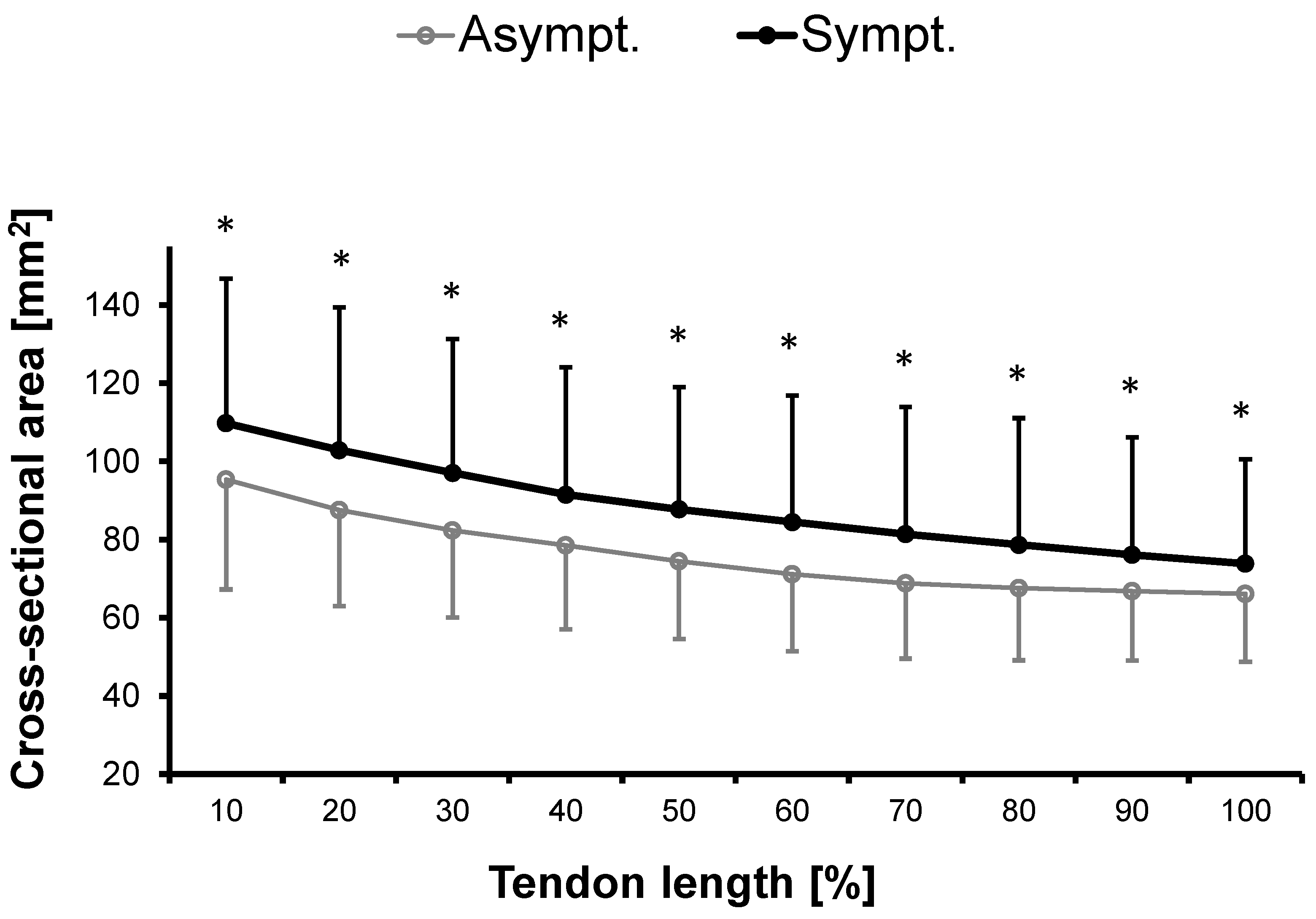
3.1.1. Cross-Sectional Area
3.1.2. Laterality
3.2. Absolute ASYMMETRY INdex
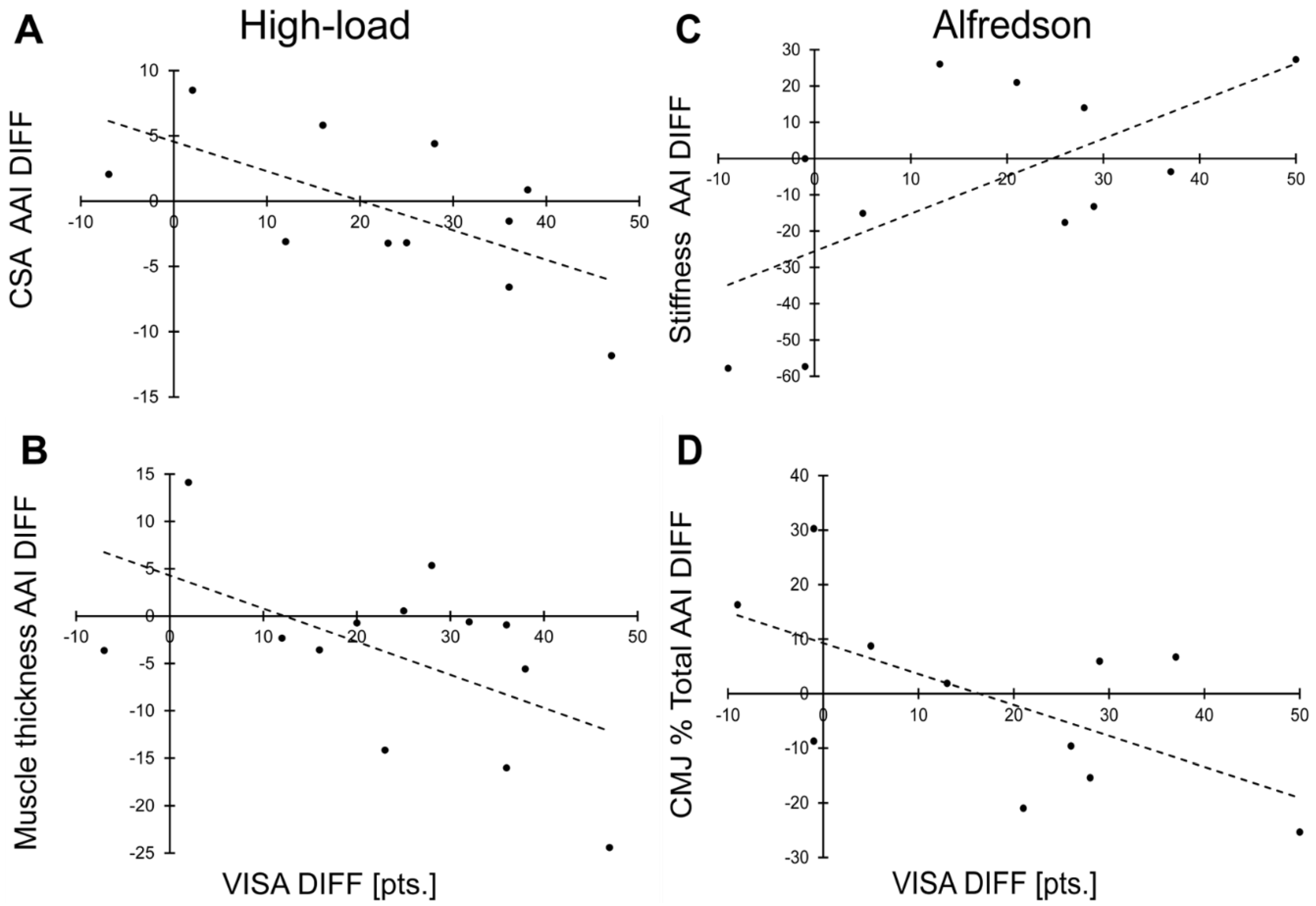
3.3. Correlation to VISA-A Score
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Prim. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; Barry, S.; Watson, P. Plantarflexor strength and endurance deficits associated with mid-portion Achilles tendinopathy: The role of soleus. Phys. Ther. Sport 2019, 37, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kujala, U.M.; Sarna, S.; Kaprio, J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin. J. Sports Med. 2005, 15, 133–135. [Google Scholar] [CrossRef]
- Kader, D. Achilles tendinopathy: Some aspects of basic science and clinical management. Br. J. Sports Med. 2002, 36, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Abate, M.; Silbernagel, K.G.; Siljeholm, C.; Di Iorio, A.; de Amicis, D.; Salini, V.; Werner, S.; Paganelli, R. Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res. Ther. 2009, 11, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar]
- Hewett, T.E.; Ford, K.R.; Hoogenboom, B.J.; Myer, G.D. Understanding and preventing acl injuries: Current biomechanical and epidemiologic considerations—Update 2010. N. Am. J. Sports Phys. Ther. 2010, 5, 234–251. [Google Scholar]
- Paterno, M.V.; Ford, K.R.; Myer, G.D.; Heyl, R.; Hewett, T.E. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin. J. Sports Med. 2007, 17, 258–262. [Google Scholar] [CrossRef]
- Schmitt, L.C.; Paterno, M.V.; Hewett, T.E. The Impact of Quadriceps Femoris Strength Asymmetry on Functional Performance at Return to Sport Following Anterior Cruciate Ligament Reconstruction. J. Orthop. Sports Phys. Ther. 2012, 42, 750–759. [Google Scholar] [CrossRef] [Green Version]
- Fendri, T.; Rebai, H.; Harrabi, M.A.; Chaari, F.; Boyas, S.; Beaune, B.; Sahli, S. Athletes with unilateral patellar tendinopathy have increased subsequent lower extremity musculoskeletal injury risk. Eur. J. Sports Sci. 2021, 22, 1908–1915. [Google Scholar] [CrossRef]
- Ford, K.R.; Myer, G.D.; Hewett, T.E. Valgus knee motion during landing in high school female and male basketball players. Med. Sci. Sports Exerc. 2003, 35, 1745–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterno, M.V.; Schmitt, L.C.; Ford, K.R.; Rauh, M.J.; Myer, G.D.; Huang, B.; Hewett, T.E. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am. J. Sports Med. 2010, 38, 1968–1978. [Google Scholar] [CrossRef]
- Helme, M.; Tee, J.; Emmonds, S.; Low, C. Does lower-limb asymmetry increase injury risk in sport? A systematic review. Phys. Ther. Sport 2021, 49, 204–213. [Google Scholar] [CrossRef]
- Bains, B.S.; Porter, K. Lower limb tendinopathy in athletes. Trauma 2006, 8, 213–224. [Google Scholar] [CrossRef]
- McAuliffe, S.; Tabuena, A.; McCreesh, K.; O’Keeffe, M.; Hurley, J.; Comyns, T.; Purtill, H.; O’Neill, S.; O’Sullivan, K. Altered Strength Profile in Achilles Tendinopathy: A Systematic Review and Meta-Analysis. J. Athl. Train. 2019, 54, 889–900. [Google Scholar] [CrossRef] [Green Version]
- Hasani, F.; Vallance, P.; Haines, T.; Munteanu, S.E.; Malliaras, P. Are Plantarflexor Muscle Impairments Present Among Individuals with Achilles Tendinopathy and Do They Change with Exercise? A Systematic Review with Meta-analysis. Sports Med. Open 2021, 7, 18. [Google Scholar] [CrossRef]
- Chimenti, R.L.; Flemister, A.S.; Tome, J.; McMahon, J.M.; Flannery, M.A.; Xue, Y.; Houck, J.R. Altered tendon characteristics and mechanical properties associated with insertional achilles tendinopathy. J. Orthop. Sports Phys. Ther. 2014, 44, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Tillander, B.; Gauffin, H.; Lyth, J.; Knutsson, A.; Timpka, T. Symptomatic Achilles Tendons are Thicker than Asymptomatic Tendons on Ultrasound Examination in Recreational Long-Distance Runners. Sports 2019, 7, 245. [Google Scholar] [CrossRef] [Green Version]
- Hirschmüller, A.; Frey, V.; Deibert, P.; Konstantinidis, L.; Mayer, F.; Sudkamp, N.; Helwig, P. Achilles tendon power Doppler sonography in 953 long distance runners—A cross sectional study. Ultraschall Med. 2010, 31, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.K.; Thelen, D.; Yakey, J.M.; Heiderscheit, B.C.; Wilson, J.J.; Lee, K.S. Regional shear wave elastography of Achilles tendinopathy in symptomatic versus contralateral Achilles tendons. Eur. Radiol. 2022, 33, 720–729. [Google Scholar] [CrossRef]
- Risch, L.; Stoll, J.; Schomöller, A.; Engel, T.; Mayer, F.; Cassel, M. Intraindividual Doppler Flow Response to Exercise Differs Between Symptomatic and Asymptomatic Achilles Tendons. Front. Physiol. 2021, 12, 617497. [Google Scholar] [CrossRef] [PubMed]
- Agres, A.N.; Duda, G.N.; Gehlen, T.J.; Arampatzis, A.; Taylor, W.R.; Manegold, S. Increased unilateral tendon stiffness and its effect on gait 2-6 years after Achilles tendon rupture. Scand. J. Med. Sci. Sports 2015, 25, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Sánchez, A.; Abián, P.; Jimenez, F.; Abián-Vicén, J. Structural and mechanical properties of the Achilles tendon in senior badminton players: Operated vs. non-injured tendons. Clin. Biomech. 2021, 85, 105366. [Google Scholar] [CrossRef]
- Hoeffner, R.; Svensson, R.B.; Bjerregaard, N.; Kjær, M.; Magnusson, S.P. Persistent Deficits after an Achilles Tendon Rupture: A Narrative Review. Transl. Sports Med. 2022, 2022, 7445398. [Google Scholar] [CrossRef]
- Bohm, S.; Mersmann, F.; Marzilger, R.; Schroll, A.; Arampatzis, A. Asymmetry of Achilles tendon mechanical and morphological properties between both legs. Scand. J. Med. Sci. Sports 2015, 25, e124–e132. [Google Scholar] [CrossRef]
- Bravo-Sánchez, A.; Abián, P.; Jiménez, F.; Abián-Vicén, J. Myotendinous asymmetries derived from the prolonged practice of badminton in professional players. PLoS ONE 2019, 14, e0222190. [Google Scholar] [CrossRef] [Green Version]
- Bayliss, A.J.; Weatherholt, A.M.; Crandall, T.T.; Farmer, D.L.; McConnell, J.C.; Crossley, K.M.; Warden, S.J. Achilles tendon material properties are greater in the jump leg of jumping athletes. J. Musculoskelet. Neuronal Interact. 2016, 16, 105–112. [Google Scholar]
- Couppé, C.; Kongsgaard, M.; Aagaard, P.; Hansen, P.; Bojsen-Moller, J.; Kjaer, M.; Magnusson, S.P. Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J. Appl. Physiol. 2008, 105, 805–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sichting, F.; Kram, N.C.; Legerlotz, K. An Identical Twin Study on Human Achilles Tendon Adaptation: Regular Recreational Exercise at Comparatively Low Intensities Can Increase Tendon Stiffness. Front. Physiol. 2022, 12, 777403. [Google Scholar] [CrossRef]
- Rosager, S.; Aagaard, P.; Dyhre-Poulsen, P.; Neergaard, K.; Kjaer, M.; Magnusson, S.P. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand. J. Med. Sci. Sports 2002, 12, 90–98. [Google Scholar] [CrossRef]
- Scott, A.; Docking, S.; Vicenzino, B.; Alfredson, H.; Murphy, R.J.; Carr, A.J.; Zwerver, J.; Lundgreen, K.; Finlay, O.; Pollock, N.; et al. Sports and exercise-related tendinopathies: A review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. Br. J. Sports Med. 2013, 47, 536–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silbernagel, K.G. Does one size fit all when it comes to exercise treatment for Achilles tendinopathy? J. Orthop. Sports Phys. Ther. 2014, 44, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Andres, B.M.; Murrell, G.A.C. Treatment of tendinopathy: What works, what does not, and what is on the horizon. Clin. Orthop. Relat. Res. 2008, 466, 1539–1554. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, P.; Barton, C.J.; Reeves, N.D.; Langberg, H. Achilles and patellar tendinopathy loading programmes: A systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med. 2013, 43, 267–286. [Google Scholar] [CrossRef]
- Alfredson, H.; Pietila, T.; Jonsson, P.; Lorentzon, R. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am. J. Sports Med. 1998, 26, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Rowe, V.; Hemmings, S.; Barton, C.; Malliaras, P.; Maffulli, N.; Morrissey, D. Conservative management of midportion Achilles tendinopathy: A mixed methods study, integrating systematic review and clinical reasoning. Sports Med. 2012, 42, 941–967. [Google Scholar] [CrossRef]
- Arampatzis, A.; Karamanidis, K.; Albracht, K. Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. J. Exp. Biol. 2007, 210, 2743–2753. [Google Scholar] [CrossRef] [Green Version]
- Arampatzis, A.; Peper, A.; Bierbaum, S.; Albracht, K. Plasticity of human Achilles tendon mechanical and morphological properties in response to cyclic strain. J Biomech. 2010, 43, 3073–3079. [Google Scholar] [CrossRef]
- Bohm, S.; Mersmann, F.; Tettke, M.; Kraft, M.; Arampatzis, A. Human Achilles tendon plasticity in response to cyclic strain: Effect of rate and duration. J Exp Biol. 2014, 217, 4010–4017. [Google Scholar] [CrossRef] [Green Version]
- Beyer, R.; Kongsgaard, M.; Hougs Kjaer, B.; Ohlenschlaeger, T.; Kjaer, M.; Magnusson, S.P. Heavy Slow Resistance Versus Eccentric Training as Treatment for Achilles Tendinopathy: A Randomized Controlled Trial. Am. J. Sports Med. 2015, 43, 1704–1711. [Google Scholar] [CrossRef]
- Krogh, T.P.; Jensen, T.T.; Madsen, M.N.; Fredberg, U. An Isometric and Functionally Based 4-Stage Progressive Loading Program in Achilles Tendinopathy: A 12-Month Pilot Study. Transl. Sports Med. 2022, 2022, 6268590. [Google Scholar] [CrossRef]
- Radovanović, G.; Bohm, S.; Peper, K.K.; Arampatzis, A.; Legerlotz, K. Evidence-based high-loading tendon exercise for 12 weeks leads to increased tendon stiffness and cross-sectional area in Achilles tendinopathy: A controlled clinical trial. Sports Med.–Open 2022, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Radovanović, G.; Kunz, J.; Bohm, S.; Arampatzis, A.; Legerlotz, K. Reliable and effective novel home-based training set-up for application of an evidence-based high-loading stimulus to improve triceps surae function. J. Sports Sci. 2021, 39, 2786–2795. [Google Scholar] [CrossRef] [PubMed]
- Silbernagel, K.G.; Thomeé, R.; Eriksson, B.I.; Karlsson, J. Continued sports activity, using a pain-monitoring model, during rehabilitation in patients with Achilles tendinopathy: A randomized controlled study. Am. J. Sports Med. 2007, 35, 897–906. [Google Scholar] [CrossRef]
- Silbernagel, K.G.; Crossley, K.M. A Proposed Return-to-Sport Program for Patients With Midportion Achilles Tendinopathy: Rationale and Implementation. J. Orthop. Sports Phys. Ther. 2015, 45, 876–886. [Google Scholar] [CrossRef] [Green Version]
- Arampatzis, A.; Morey-Klapsing, G.; Karamanidis, K.; DeMonte, G.; Stafilidis, S.; Brüggemann, G.-P. Differences between measured and resultant joint moments during isometric contractions at the ankle joint. J Biomech. 2005, 38, 885–892. [Google Scholar] [CrossRef]
- Mademli, L.; Arampatzis, A.; Morey-Klapsing, G.; Bruggemann, G.-P. Effect of ankle joint position and electrode placement on the estimation of the antagonistic moment during maximal plantarflexion. J. Electromyogr. Kinesiol. 2004, 14, 591–597. [Google Scholar] [CrossRef]
- Marzilger, R.; Legerlotz, K.; Panteli, C.; Bohm, S.; Arampatzis, A. Reliability of a semi-automated algorithm for the vastus lateralis muscle architecture measurement based on ultrasound images. Eur. J. Appl. Physiol. 2018, 118, 291–301. [Google Scholar] [CrossRef]
- Pentidis, N.; Mersmann, F.; Bohm, S.; Schroll, A.; Giannakou, E.; Aggelousis, N.; Arampatzis, A. Development of Muscle-Tendon Adaptation in Preadolescent Gymnasts and Untrained Peers: A 12-Month Longitudinal Study. Med. Sci. Sports Exerc. 2021, 53, 2565–2576. [Google Scholar] [CrossRef]
- An, K.N.; Takahashi, K.; Harrigan, T.P.; Chao, E.Y. Determination of muscle orientations and moment arms. J. Biomech. Eng. 1984, 106, 280–282. [Google Scholar] [CrossRef]
- Arampatzis, A.; de Monte, G.; Karamanidis, K. Effect of joint rotation correction when measuring elongation of the gastrocnemius medialis tendon and aponeurosis. J. Electromyogr. Kinesiol. 2008, 18, 503–508. [Google Scholar] [CrossRef] [PubMed]
- de Monte, G.; Arampatzis, A.; Stogiannari, C.; Karamanidis, K. In vivo motion transmission in the inactive gastrocnemius medialis muscle-tendon unit during ankle and knee joint rotation. J. Electromyogr. Kinesiol. 2006, 16, 413–422. [Google Scholar] [CrossRef]
- Fortin, M.; Battié, M.C. Quantitative paraspinal muscle measurements: Inter-software reliability and agreement using OsiriX and ImageJ. Phys. Ther. 2012, 92, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Linthorne, N.P. Analysis of standing vertical jumps using a force platform. Am. J. Phys. 2001, 69, 1198. [Google Scholar] [CrossRef] [Green Version]
- Moir, G.L. Three Different Methods of Calculating Vertical Jump Height from Force Platform Data in Men and Women. Meas Phys. Educ. Exerc. Sci. 2008, 12, 207–218. [Google Scholar] [CrossRef]
- Lohrer, H.; Nauck, T. Cross-cultural adaptation and validation of the VISA-A questionnaire for German-speaking achilles tendinopathy patients. BMC Musculoskelet. Disord. 2009, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.M.; Cook, J.L.; Purdam, C.; Visentini, P.J.; JRoss, J.; Mavulli, N.; Taunton, J.E.; Khan, K.M. The VISA-A questionnaire: A valid and reliable index of the clinical severity of Achilles tendinopathy. Br. J. Sports Med. 2001, 35, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Karamanidis, K.; Arampatzis, A.; Brüggemann, G.-P. Symmetry and reproducibility of kinematic parameters during various running techniques. Med. Sci. Sports Exerc. 2003, 35, 1009–1016. [Google Scholar] [CrossRef] [Green Version]
- Arya, S.; Kulig, K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J. Appl. Physiol. 2010, 108, 670–675. [Google Scholar] [CrossRef] [Green Version]
- Syha, R.; Springer, F.; Würslin, C.; Ipach, I.; Ketelsen, D.; Grözinger, G.; Notohamiprodjo, M.; Nikolaou, K.; Claussen, C.D.; Schick, F.; et al. Tendinopathy of the achilles tendon: Volume assessed by automated contour detection in submillimeter isotropic 3-dimensional magnetic resonance imaging data sets recorded at a field strength of 3 T. J. Comput. Assist. Tomogr. 2015, 39, 250–256. [Google Scholar] [CrossRef]
- Szaro, P.; Ghali Gataa, K. The correlations between dimensions of the normal tendon and tendinopathy changed Achilles tendon in routine magnetic resonance imaging. Sci. Rep. 2021, 11, 6131. [Google Scholar] [CrossRef]
- Wang, J.H.-C.; Komatsu, I. Tendon Stem Cells: Mechanobiology and Development of Tendinopathy. Adv. Exp. Med. Biol. 2016, 920, 53–62. [Google Scholar] [CrossRef]
- De Vos, R.-J.; Weir, A.; Cobben, L.P.J.; Tol, J.L. The value of power Doppler ultrasonography in Achilles tendinopathy: A prospective study. Am. J. Sports Med. 2007, 35, 1696–1701. [Google Scholar] [CrossRef]
- Knobloch, K. The use of a neovascularization score to predict clinical severity in Achilles tendinopathy. Am. J. Sports Med. 2008, 36, 395. [Google Scholar] [CrossRef] [PubMed]
- Wren, T.A.L.; Lindsey, D.P.; Beaupré, G.S.; Carter, D.R. Effects of creep and cyclic loading on the mechanical properties and failure of human Achilles tendons. Ann. Biomed. Eng. 2003, 31, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Nuri, L.; Obst, S.J.; Newsham-West, R.; Barrett, R.S. The tendinopathic Achilles tendon does not remain iso-volumetric upon repeated loading: Insights from 3D ultrasound. J. Exp. Biol. 2017, 220, 3053–3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.H.-C.; Guo, Q.; Li, B. Tendon biomechanics and mechanobiology—A minireview of basic concepts and recent advancements. J. Hand Ther. 2012, 25, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-J.; Kulig, K. The neuromechanical adaptations to Achilles tendinosis. J. Physiol. 2015, 593, 3373–3387. [Google Scholar] [CrossRef] [Green Version]
- Kulig, K.; Chang, Y.-J.; Winiarski, S.; Bashford, G.R. Ultrasound-Based Tendon Micromorphology Predicts Mechanical Characteristics of Degenerated Tendons. Ultrasound Med. Biol. 2016, 42, 664–673. [Google Scholar] [CrossRef]
- Obst, S.J.; Heales, L.J.; Schrader, B.L.; Davis, S.A.; Dodd, K.A.; Holzberger, C.J.; Beavis, L.B.; Barrett, R.S. Are the Mechanical or Material Properties of the Achilles and Patellar Tendons Altered in Tendinopathy? A Systematic Review with Meta-analysis. Sports Med. 2018, 48, 2179–2198. [Google Scholar] [CrossRef]
- Coombes, B.K.; Tucker, K.; Vicenzino, B.; Vuvan, V.; Mellor, R.; Heales, L.; Nordez, A.; Hug, F. Achilles and patellar tendinopathy display opposite changes in elastic properties: A shear wave elastography study. Scand. J. Med. Sci. Sports 2018, 28, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.P.; Langberg, H.; Kjaer, M. The pathogenesis of tendinopathy: Balancing the response to loading. Nat. Rev. Rheumatol. 2010, 6, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Lazarczuk, S.L.; Maniar, N.; Opar, D.A.; Duhig, S.J.; Shield, A.; Barrett, R.S.; Bourne, M.N. Mechanical, Material and Morphological Adaptations of Healthy Lower Limb Tendons to Mechanical Loading: A Systematic Review and Meta-Analysis. Sports Med. 2022, 52, 2405–2429. [Google Scholar] [CrossRef] [PubMed]
- Heales, L.J.; Lim, E.C.W.; Hodges, P.W.; Vicenzino, B. Sensory and motor deficits exist on the non-injured side of patients with unilateral tendon pain and disability--implications for central nervous system involvement: A systematic review with meta-analysis. Br. J. Sports Med. 2014, 48, 1400–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callow, J.H.; Cresswell, M.; Damji, F.; Seto, J.; Hodgson, A.J.; Scott, A. The Distal Free Achilles Tendon Is Longer in People with Tendinopathy than in Controls: A Retrospective Case-Control Study. Transl. Sports Med. 2022, 2022, 6585980. [Google Scholar] [CrossRef]
- Yu, C.; Deng, L.; Li, L.; Zhang, X.; Fu, W. Exercise Effects on the Biomechanical Properties of the Achilles Tendon-A Narrative Review. Biology 2022, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.P.; Hansen, M.; Langberg, H.; Miller, B.; Haraldsson, B.; Westh, E.K.; Koskinen, S.; Aagaard, P.; Kjaer, M. The adaptability of tendon to loading differs in men and women. Int. J. Exp. Pathol. 2007, 88, 237–240. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | All Patients (n = 41) | Alfredson (n = 11) | High-Load (n = 13) |
|---|---|---|---|
| Age [years] | 40.2 ± 9.2 (24–55) | 38.7 ± 8.8 (24–52) | 40.1 ± 8.4 (27–52) |
| Body height [cm] | 181.9 ± 5.8 (173–198) | 180.0 ± 4.8 (175–186) | 184.1 ± 6.4 (177–198) |
| Body mass [kg] | 81.4 ± 10.6 (64–110) | 77.5 ± 7.4 (65–88) | 86.1 ± 11.7 (70–110) * |
| Body mass index [kg/m2] | 24.6 ± 3.0 (20–37) | 23.9 ± 2.0 (21–28) | 25.3 ± 2.9 (22–32) |
| Activity level [hours/week] A | 6.7 ± 4.6 (0–22.5) | 5.9 ± 4.0 (1–12) | 8.2 ± 5.4 (2–22.5) |
| Laterality (left/right) | 6/32 A | 1/9 B | 2/11 |
| Symptomatic AT (left/right) | 24/17 | 4/7 | 7/6 |
| Symptom localization (Ins./mid-portion) | 17/24 | 6/5 | 6/7 |
| Symptom duration [months] | 25.9 ± 45.7 (3–264) | 44.5 ± 77.6 (3–264) | 21.2 ± 31.2 (3–96) |
| VISA-A PRE [points] | 57.4 ± 13.9 (19–78) | 41.1 ± 14.8 (29–73) | 58.4 ± 11.7 (36–73) |
| Alfredson | High-Load | |
|---|---|---|
| Procedure | Upright standing position, exercises were carried out both with extended knee and bended knee. Start position: Only the forefoot of the injured leg was placed on the edge of a stair at maximum plantar flexion, lowering the heel under full body weight with an eccentric phase of 3 s. We ensured eccentric-only contractions of the plantar flexors by using the healthy leg to return to the start position. The use of a full ankle range of motion in the eccentric phase was encouraged. | Seated position on the floor with extended knees and a feedback-fitted sling fixed around the pelvis that was equipped with a foot plate [43]. The individual training load was calculated based on 90% of the mean of five executed MVCs. Start position: The forefoot (with shoes) was placed in the foot plate. The sling was adjusted as tightly as possible, to execute 90% isometric plantar flexor contractions at a standardized ankle angle position (90°) (i.e., foot sole 90° perpendicular to the tibia). |
| Warm-up | No warm-up. | Three sets of five isometric sub-maximal plantar flexor contractions with a rest of1 min in between. |
| Repetitions per session | Three sets of 15 reps. with extended knee and three sets of 15 reps. with bended knee. | Five sets of four repetitions, each contraction lasting 3 s at 90% isometric plantar flexor MVC, followed by 3 s rest. |
| Resting time | One min after each set. | One min after each set. |
| Frequency | Two sessions per day. | Four times per week. |
| Pain | Up to 5/10 (NRS scale for pain) was allowed for exercise treatment, up to 3/10 (NRS scale for pain) for other activities [44]. | Up to 5/10 (NRS scale for pain) was allowed for exercise treatment, up to 3/10 (NRS scale for pain) for other activities [44]. |
| Optional progression | Up to 5% load increase per week; progression was allowed after 2 weeks and only if pain level was < 6/10 (NRS scale) [44] and individual rating of perceived exertion was <3/10 (NRS scale) [45]. | Up to 5% load increase per week; progression was allowed after 2 weeks and only if pain level was <6/10 (NRS scale) [44] and individual rating of perceived exertion was <3/10 (NRS scale) [45]. |
| Parameter | Asymptomatic PRE (n = 41) | Symptomatic PRE (n = 41) | r | AAI PRE (n = 41) |
|---|---|---|---|---|
| Moment (MVC) [Nm] | 239.61 ± 44.30 | 239.41 ± 41.65 | 0.729 # | 9.71 ± 8.77 |
| Force [N] | 3642.90 ± 824.51 | 3411.42 ± 759.79 * | 0.715 # | 14.91 ± 11.99 |
| Muscle fiber length | 73.66 ± 11.51 | 74.45 ± 11.44 | 0.769 # | 9.07 ± 5.64 |
| Pennation angle | 16.41 ± 2.45 | 16.15 ± 2.00 | 0.488 # | 11.86 ± 8.66 |
| Muscle thickness | 18.84 ± 2.91 | 18.90 ± 3.18 | 0.542 # | 11.55 ± 11.52 |
| Elongation [mm] | 12.29 ± 2.72 | 12.04 ± 3.39 | 0.454 # | 21.29 ± 20.22 |
| Stiffness [N/mm] | 358.46 ± 123.05 | 378.50 ± 112.59 | 0.074 | 32.35 ± 27.22 |
| Strain [%] | 6.22 ± 1.42 | 6.07 ± 1.75 | 0.425 # | 22.60 ± 19.24 |
| CSA (mean) [mm2] | 76.44 ± 19.38 A | 87.42 ± 28.62 A,* | 0.798 # | 14.89 ± 14.19 A |
| Youngs modulus [GPa] | 0.99 ± 0.38 A | 0.92 ± 0.34 A | 0.358 # | 34.58 ± 26.34 A |
| Stress [MPa] | 50.10 ± 16.94 A | 42.17 ± 16.18 A,* | 0.826 # | 23.83 ± 17.25 A |
| Vasc. (intratend.) [mm2] | 1.51 ± 3.26 | 5.15 ± 11.15 * | 0.213 | 90.70 ± 94.86 |
| Vasc. (overall) [mm2] | 10.75 ± 8.80 | 16.74 ± 16.21 * | 0.292 | 84.94 ± 60.35 |
| CMJ (Impulse %) [%] | 52.33 ±7.68 B | 47.66 ± 7.68 B | n. a. | 27.41 ± 16.19 B |
| DJ (Impulse %) [%] | 52.13 ± 11.50 B | 47.86 ± 11.50 B | n. a. | 33.24 ± 32.53 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radovanović, G.; Bohm, S.; Arampatzis, A.; Legerlotz, K. In Achilles Tendinopathy the Symptomatic Tendon Differs from the Asymptomatic Tendon While Exercise Therapy Has Little Effect on Asymmetries—An Ancillary Analysis of Data from a Controlled Clinical Trial. J. Clin. Med. 2023, 12, 1102. https://doi.org/10.3390/jcm12031102
Radovanović G, Bohm S, Arampatzis A, Legerlotz K. In Achilles Tendinopathy the Symptomatic Tendon Differs from the Asymptomatic Tendon While Exercise Therapy Has Little Effect on Asymmetries—An Ancillary Analysis of Data from a Controlled Clinical Trial. Journal of Clinical Medicine. 2023; 12(3):1102. https://doi.org/10.3390/jcm12031102
Chicago/Turabian StyleRadovanović, Goran, Sebastian Bohm, Adamantios Arampatzis, and Kirsten Legerlotz. 2023. "In Achilles Tendinopathy the Symptomatic Tendon Differs from the Asymptomatic Tendon While Exercise Therapy Has Little Effect on Asymmetries—An Ancillary Analysis of Data from a Controlled Clinical Trial" Journal of Clinical Medicine 12, no. 3: 1102. https://doi.org/10.3390/jcm12031102








