Comparative Survival Outcomes of Hyperthermic Intraperitoneal Chemotherapy, Intraperitoneal Chemotherapy and Intravenous Chemotherapy for Primary Advanced Ovarian Cancer: A Network Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Evaluation
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
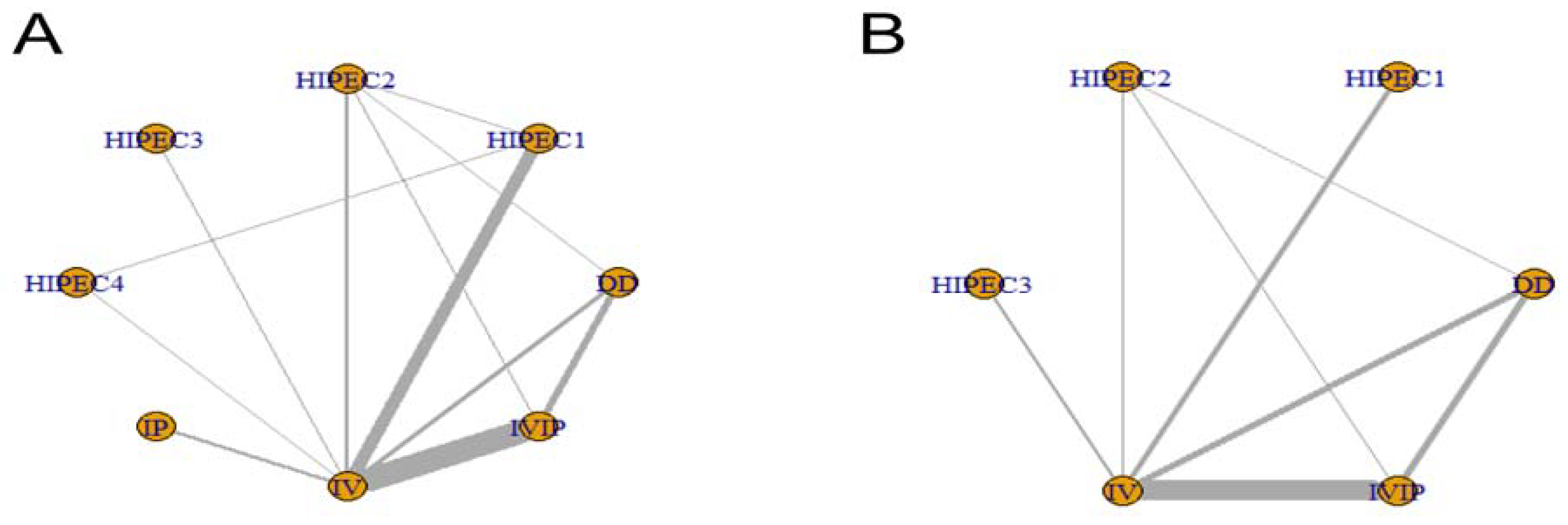
3.2. Network Map
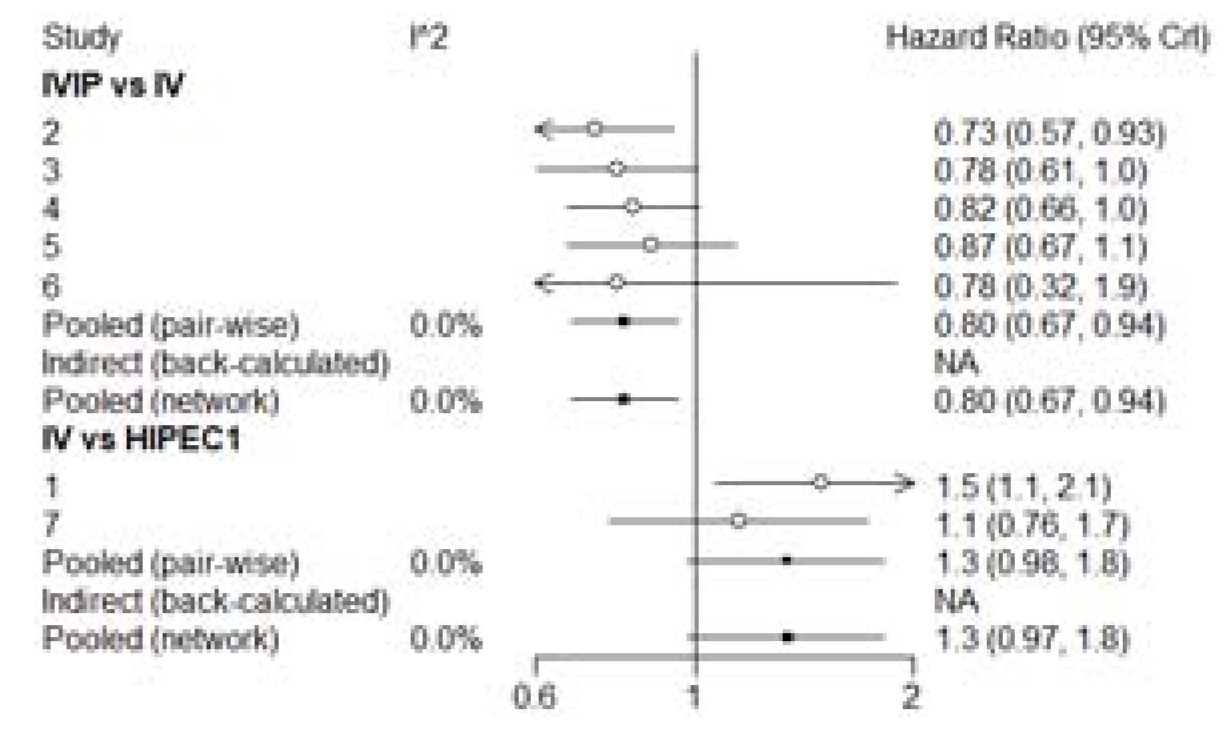
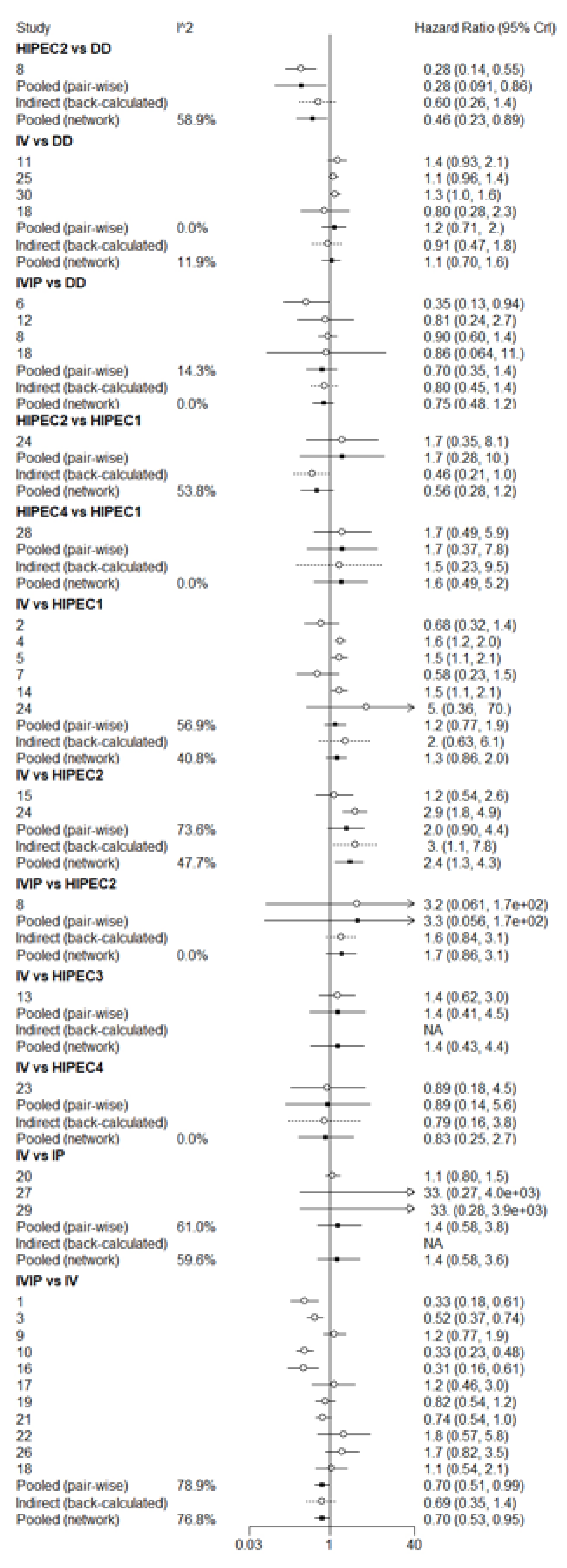
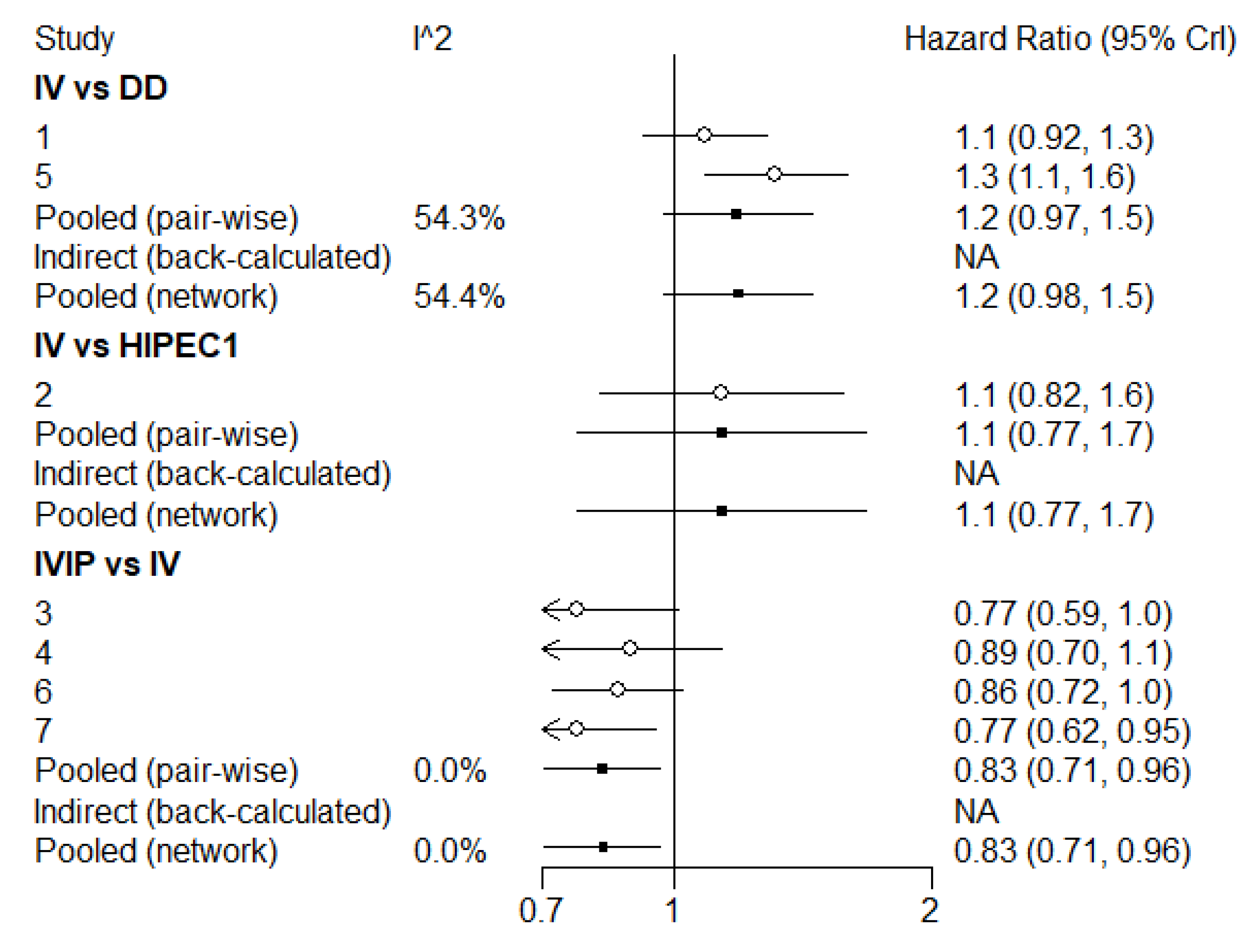
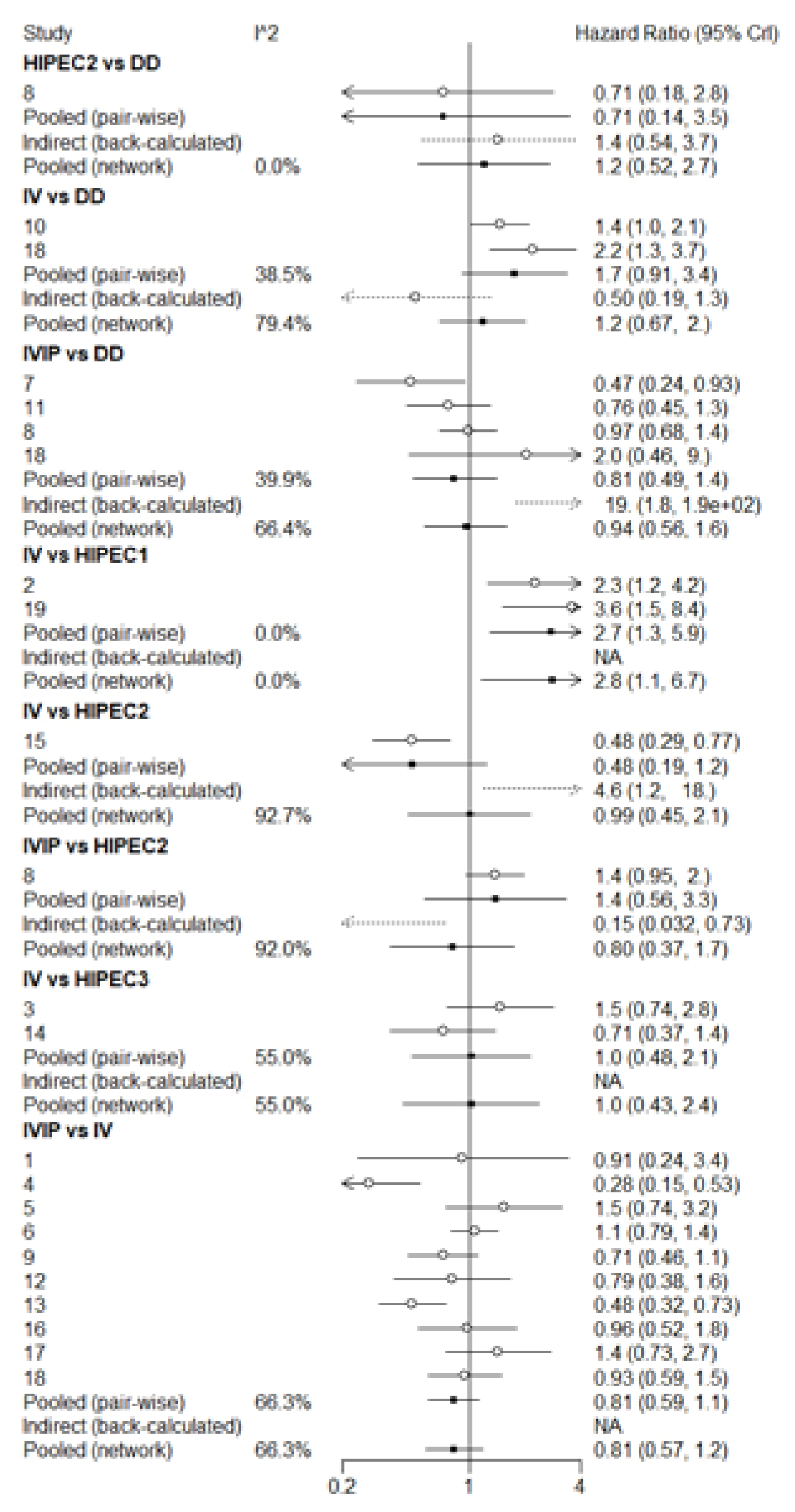
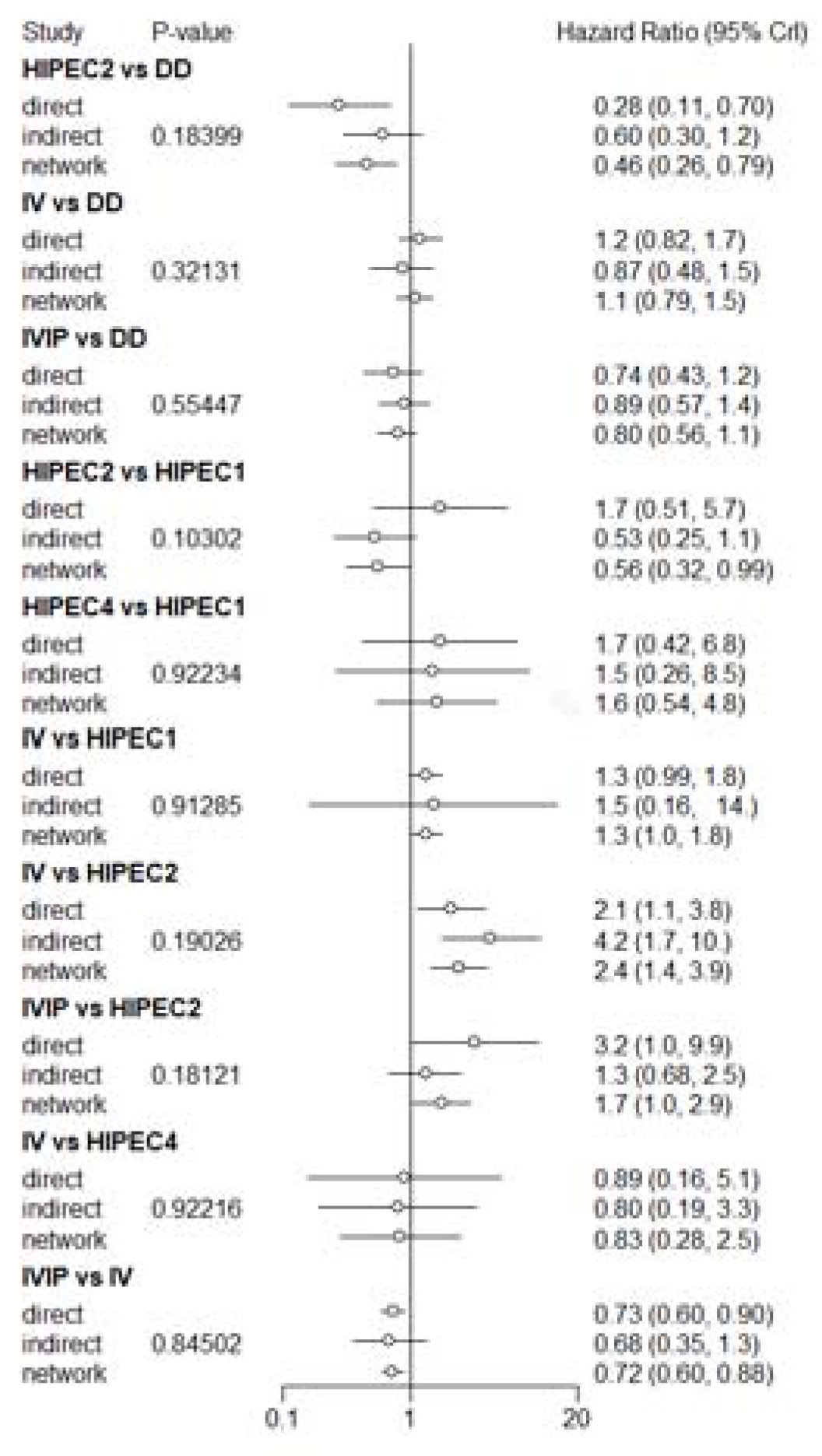
3.3. Overall Survival (OS)
3.4. Progression-Free Survival (PFS)
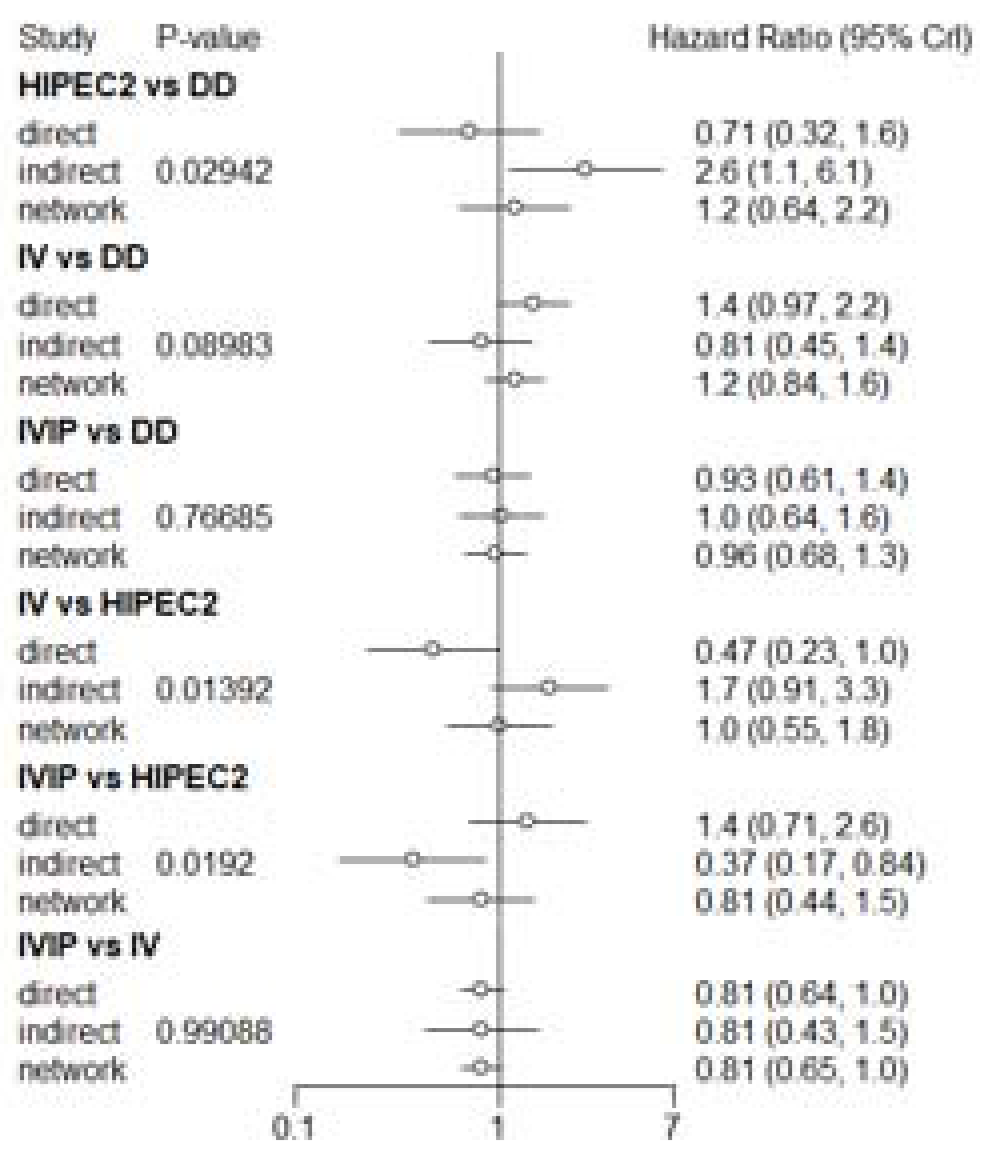
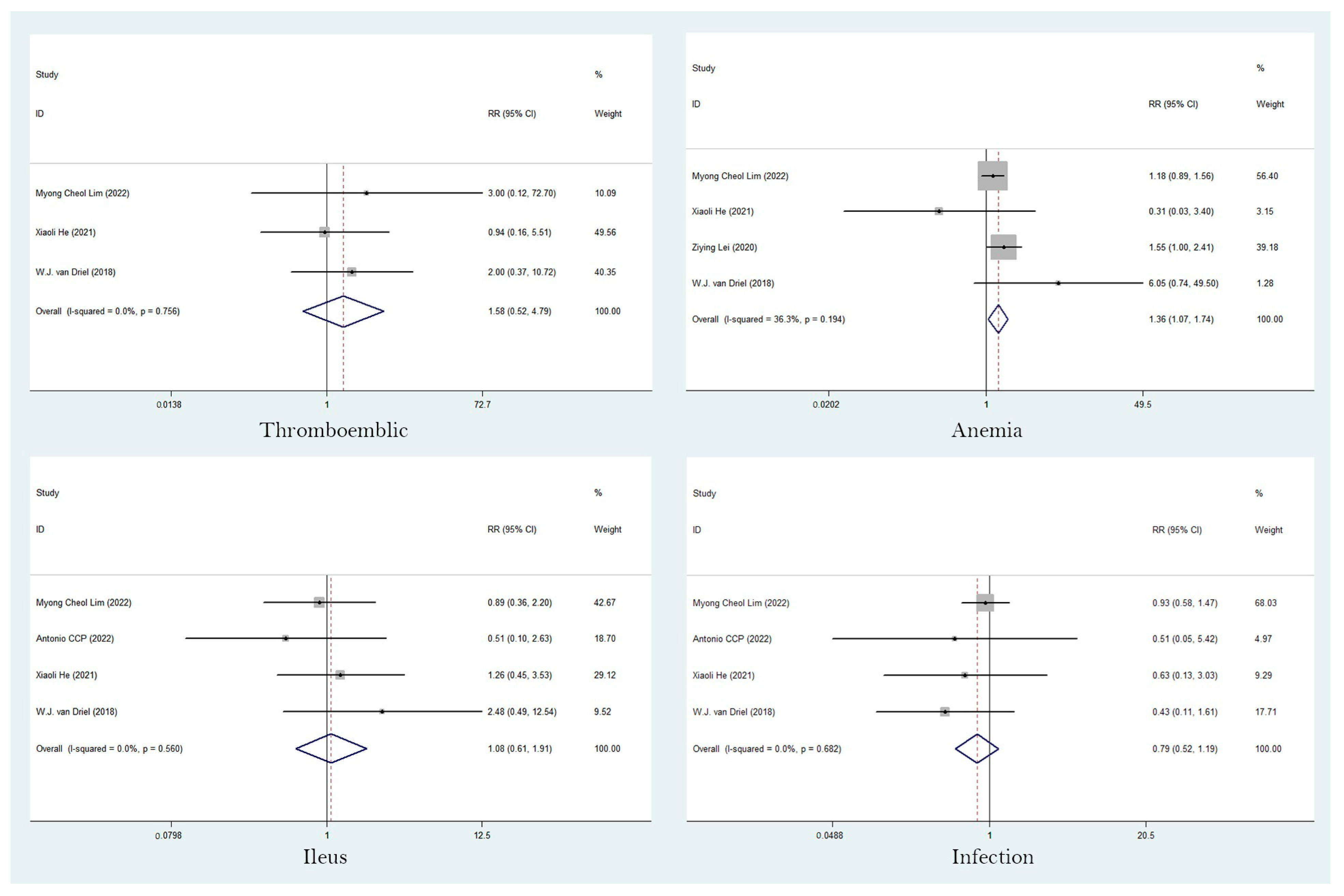
3.5. Adverse Events (AEs)
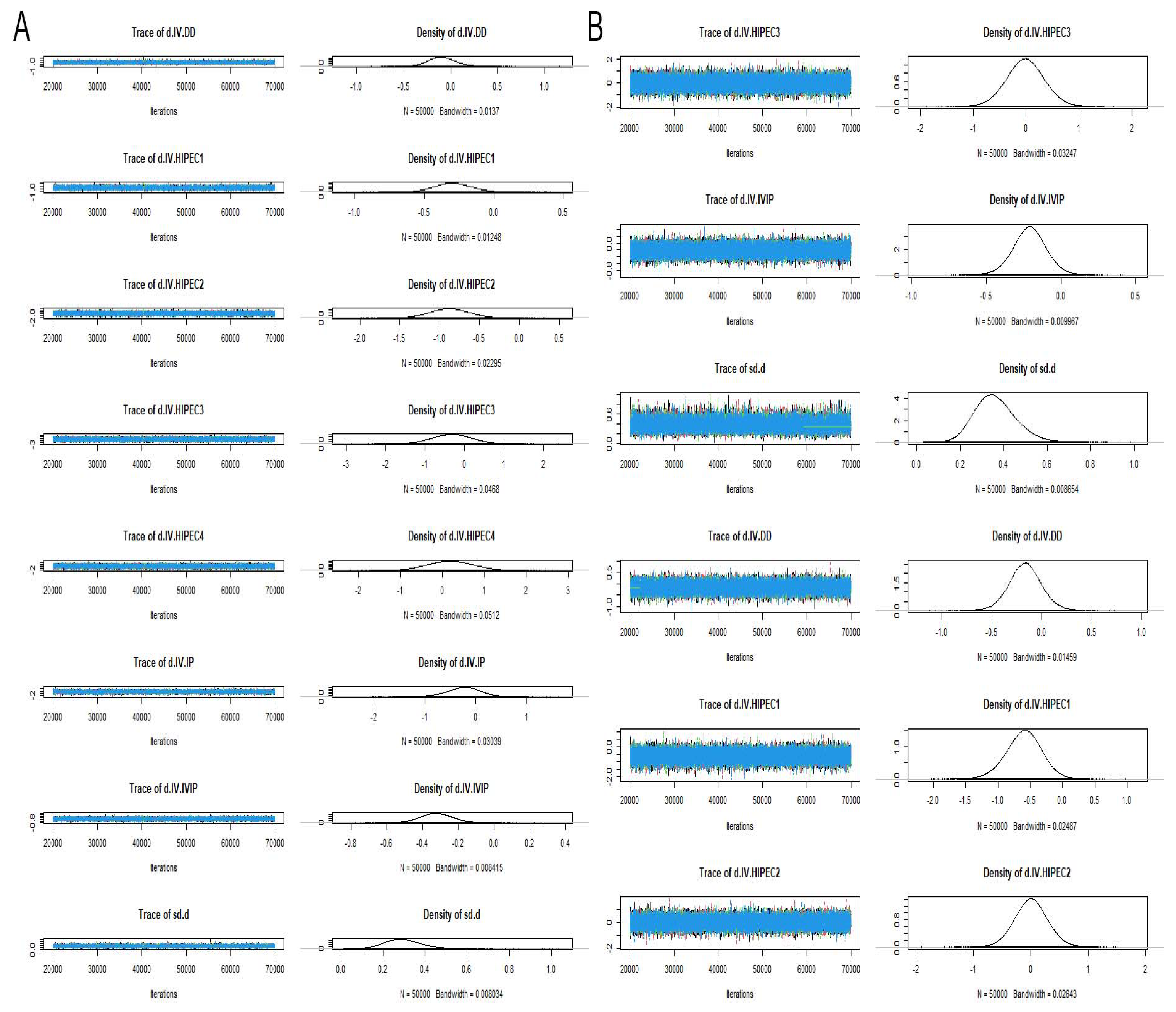
3.6. Risk of Heterogeneity, Inconsistency and Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wethington, S.; Armstrong, D.; Johnston, F. Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Epithelial Ovarian Cancer. JAMA Surg. 2022, 157, 383. [Google Scholar] [CrossRef]
- Armstrong, D.; Alvarez, R.; Backes, F.; Bakkum-Gamez, J.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.; Chitiyo, V.; Cristea, M.; et al. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022. J. Natl. Compr. Cancer Netw. JNCCN 2022, 20, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J. Role of surgery and hyperthermic intraperitoneal chemotherapy in ovarian cancer. ESMO Open 2021, 6, 100149. [Google Scholar] [CrossRef] [PubMed]
- Bonnot, P.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, P.; Good, M.; Hughes, M. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) with cisplatin in pediatric patients with peritoneal mesothelioma: A single institution experience and long term follow up. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. North Am. Hyperth. Group 2021, 38, 326–331. [Google Scholar] [CrossRef]
- Kusamura, S.; Barretta, F.; Yonemura, Y.; Sugarbaker, P.; Moran, B.; Levine, E.; Goere, D.; Baratti, D.; Nizri, E.; Morris, D.; et al. The Role of Hyperthermic Intraperitoneal Chemotherapy in Pseudomyxoma Peritonei After Cytoreductive Surgery. JAMA Surg. 2021, 156, e206363. [Google Scholar] [CrossRef]
- Armstrong, D.; Bundy, B.; Wenzel, L.; Huang, H.; Baergen, R.; Lele, S.; Copeland, L.; Walker, J.; Burger, R. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef]
- Clamp, A.; James, E.; McNeish, I.; Dean, A.; Kim, J.; O’Donnell, D.; Hook, J.; Coyle, C.; Blagden, S.; Brenton, J.; et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): Primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet 2019, 394, 2084–2095. [Google Scholar] [CrossRef]
- Filis, P.; Mauri, D.; Markozannes, G.; Tolia, M.; Filis, N.; Tsilidis, K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: A systematic review and meta-analysis of randomized trials. ESMO Open 2022, 7, 100586. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.; Chaimani, A.; Schmid, C.; Cameron, C.; Ioannidis, J.; Straus, S.; Thorlund, K.; Jansen, J.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.; Stewart, L.; Ghersi, D.; Burdett, S.; Sydes, M. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Altman, D.; Gøtzsche, P.; Jüni, P.; Moher, D.; Oxman, A.; Savovic, J.; Schulz, K.; Weeks, L.; Sterne, J. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Clamp, A.R.; James, E.C.; McNeish, I.A.; Dean, A.; Kim, J.W.; O’Donnell, D.M.; Hook, J.; Coyle, C.; Blagden, S.; Brenton, J.D.; et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal cancer treatment (ICON8): Overall survival results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2022, 23, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Chang, S.; Park, B.; Yoo, H.; Yoo, C.; Nam, B.; Park, S. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Antonio, C.; Alida, G.; Elena, G.; Rocío, G.; Jerónimo, M.; Luis, A.; Aníbal, N.; Francisco, B.; Jesús, G.; Pablo, R.; et al. Cytoreductive Surgery With or Without HIPEC After Neoadjuvant Chemotherapy in Ovarian Cancer: A Phase 3 Clinical Trial. Ann. Surg. Oncol. 2022, 29, 2617–2625. [Google Scholar] [CrossRef]
- He, X.; Wei, L.; Li, R.; Jing, S.; Jia, L.; Ji, D.; Li, Y.; Wang, Y.; Zhu, Y. Dense hyperthermic intraperitoneal chemotherapy with cisplatin in patients with stage III serous epithelial ovarian cancer: A retrospective study. BMC Cancer 2021, 21, 738. [Google Scholar] [CrossRef]
- Gruner, M.; Chambers, L.M.; Yao, M.; Chichura, A.; Morton, M.; Costales, A.B.; Horowitz, M.; Rose, P.G.; Debernardo, R.; Michener, C.M. Anastomotic leak following interval debulking surgery with or without hyperthermic intraperitoneal chemotherapy in women with advanced epithelial ovarian Cancer. Gynecol. Oncol. 2021, 162, 645–651. [Google Scholar] [CrossRef]
- Manning-Geist, B.L.; Sullivan, M.W.; Sarda, V.; Gockley, A.A.; Del Carmen, M.G.; Matulonis, U.; Growdon, W.B.; Horowitz, N.S.; Berkowitz, R.S.; Clark, R.M.; et al. Disease Distribution at Presentation Impacts Benefit of IP Chemotherapy Among Patients with Advanced-Stage Ovarian Cancer. Ann. Surg. Oncol. 2021, 28, 6705–6713. [Google Scholar] [CrossRef]
- Kim, S.; Maganti, M.; Bernardini, M.; Laframboise, S.; Ferguson, S.; May, T. Efficacy and toxicity of intraperitoneal chemotherapy as compared to intravenous chemotherapy in the treatment of patients with advanced ovarian cancer. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2022, 157, 59–66. [Google Scholar] [CrossRef]
- Lei, Z.Y.; Wang, Y.; Wang, J.H.; Wang, K.; Tian, J.; Zhao, Y.; Chen, L.P.; Wang, J.; Luo, J.L.; Jia, M.M.; et al. Evaluation of Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Stage III Epithelial Ovarian Cancer. JAMA Netw. Open 2020, 3, e2013940. [Google Scholar] [CrossRef] [PubMed]
- Ting, W.H.; Hsiao, C.H.; Chen, H.H.; Wei, M.C.; Lin, H.H.; Hsiao, S.M. Comparisons of Clinical Outcomes in Women with Advanced Ovarian Cancer Treated with Frontline Intraperitoneal versus Dose-Dense Platinum/Paclitaxel Chemotherapy without Bevacizumab. Int. J. Environ. Res. Public Health 2020, 17, 3603. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, T.; Nagao, S.; Suzuki, K.; Kaneda, M.; Yamamoto, K.; Jimi, T.; Yano, H.; Kitai, M.; Shiozaki, T.; Matsuoka, K.; et al. Dose-dense paclitaxel and carboplatin vs. conventional paclitaxel and carboplatin as neoadjuvant chemotherapy for advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer: A retrospective study. Int. J. Clin. Oncol. 2020, 25, 502–507. [Google Scholar] [CrossRef]
- Murphy, M.; Martin, G.; Mahmoudjafari, Z.; Bivona, C.; Grauer, D.; Henry, D. Intraperitoneal paclitaxel and cisplatin compared with dose-dense paclitaxel and carboplatin for patients with stage III ovarian cancer. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2020, 26, 1566–1574. [Google Scholar] [CrossRef]
- Rettenmaier, M.; Micha, J.; Bohart, R.; Goldstein, B. A retrospective study comparing the efficacy of dose-dense chemotherapy, intraperitoneal chemotherapy and dose-dense chemotherapy with hyperthermic intraperitoneal chemotherapy in the treatment of advanced stage ovarian carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 244, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Bixel, K.; Vetter, M.; Davidson, B.; Berchuck, A.; Cohn, D.; Copeland, L.; Fowler, J.; Havrilesky, L.; Lee, P.; O’Malley, D.; et al. Intraperitoneal chemotherapy following neoadjuvant chemotherapy and optimal interval tumor reductive surgery for advanced ovarian cancer. Gynecol. Oncol. 2020, 156, 530–534. [Google Scholar] [CrossRef]
- Lee, J.; Curtin, J.P.; Muggia, F.M.; Pothuri, B.; Boyd, L.R.; Blank, S.V. Timing is everything: Intraperitoneal chemotherapy after primary or interval debulking surgery for advanced ovarian cancer. Cancer Chemother. Pharm. 2018, 82, 55–63. [Google Scholar] [CrossRef]
- Ceresoli, M.; Verrengia, A.; Montori, G.; Busci, L.; Coccolini, F.; Ansaloni, L.; Frigerio, L. Effect of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on relapse pattern in primary epithelial ovarian cancer: A propensity score based case-control study. J. Gynecol. Oncol. 2018, 29, e53. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.; van der Velden, J.; Arts, H.J.; Massuger, L.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Mendivil, A.; Abaid, L.N.; Brown, J.V.; Mori, K.; Lopez, K.; Goldstein, B. Consolidation hyperthermic intraperitoneal chemotherapy for the treatment of advanced stage ovarian carcinoma: A 3-year experience. J. Clin. Oncol. 2017, 35, 405–410. [Google Scholar] [CrossRef]
- Miller, E.M.; Tymon-Rosario, J.; Xie, X.; Xue, X.; Gressel, G.M.; Miller, D.T.; Kuo, D.Y.; Nevadunsky, N.S. Utilization of intraperitoneal chemotherapy for optimally cytoreduced advanced stage epithelial ovarian cancer: A 10-year single institution experience with a racially diverse urban population. Gynecol. Oncol. 2017, 147, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Eoh, K.J.; Lee, J.Y.; Nam, E.J.; Kim, S.; Kim, Y.T.; Kim, S.W. Long-Term Survival Analysis of Intraperitoneal versus Intravenous Chemotherapy for Primary Ovarian Cancer and Comparison between Carboplatin- and Cisplatin-based Intraperitoneal Chemotherapy. J. Korean Med. Sci. 2017, 32, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Provencher, D.; Gallagher, C.; Parulekar, W.; Ledermann, J.; Armstrong, D.; Brundage, M.; Gourley, C.; Romero, I.; Gonzalez-Martin, A.; Feeney, M.; et al. OV21/PETROC: A randomized Gynecologic Cancer Intergroup phase II study of intraperitoneal versus intravenous chemotherapy following neoadjuvant chemotherapy and optimal debulking surgery in epithelial ovarian cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Sioulas, V.D.; Schiavone, M.B.; Kadouri, D.; Zivanovic, O.; Roche, K.L.; O’Cearbhaill, R.; Abu-Rustum, N.R.; Levine, D.A.; Sonoda, Y.; Gardner, G.J.; et al. Optimal primary management of bulky stage IIIC ovarian, fallopian tube and peritoneal carcinoma: Are the only options complete gross resection at primary debulking surgery or neoadjuvant chemotherapy? Gynecol. Oncol. 2017, 145, 15–20. [Google Scholar] [CrossRef]
- Nicoletto, M.; Casarin, A.; Baldoni, A.; Rulli, E.; Tasca, G.; Baretta, Z.; Artioli, G.; Lombardi, G.; Cappetta, A.; Floriani, I.; et al. Intraperitoneal Chemotherapy in Patients Pretreated for Ovarian Cancer Matched with Patients Treated with Parenteral Chemotherapy. Anticancer Res. 2016, 36, 6541–6546. [Google Scholar] [CrossRef]
- Mueller, J.J.; Kelly, A.; Zhou, Q.; Iasonos, A.; Long Roche, K.; Sonoda, Y.; O’Cearbhaill, R.E.; Zivanovic, O.; Chi, D.S.; Gardner, G.J. Intraperitoneal chemotherapy after interval debulking surgery for advanced-stage ovarian cancer: Feasibility and outcomes at a comprehensive cancer center. Gynecol. Oncol. 2016, 143, 496–503. [Google Scholar] [CrossRef]
- Cascales-Campos, P.; López-López, V.; Gil, J.; Arévalo-Pérez, J.; Nieto, A.; Barceló, F.; Gil, E.; Parrilla, P. Hyperthermic intraperitoneal chemotherapy with paclitaxel or cisplatin in patients with stage III-C/IV ovarian cancer. Is there any difference? Surg. Oncol. 2016, 25, 164–170. [Google Scholar] [CrossRef]
- Al Mutairi, N.J.; Le, T. Does modality of adjuvant chemotherapy after interval surgical debulking matter in epithelial ovarian cancer?: An exploratory analysis. Int. J. Gynecol. Cancer 2014, 24, 461–467. [Google Scholar] [CrossRef]
- Pignata, S.; Scambia, G.; Katsaros, D.; Gallo, C.; Pujade-Lauraine, E.; De Placido, S.; Bologna, A.; Weber, B.; Raspagliesi, F.; Panici, P.B.; et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 396–405. [Google Scholar] [CrossRef]
- Yoon, J.; Koo, Y.; Kim, M.; Kim, T.; Lim, K.; Lee, K. Survival outcomes and toxicity of intraoperative intraperitoneal chemotherapy in advanced epithelial ovarian cancer. Obstet. Gynecol. Sci. 2014, 57, 484–491. [Google Scholar] [CrossRef]
- Katsumata, N.; Yasuda, M.; Isonishi, S.; Takahashi, F.; Michimae, H.; Kimura, E.; Aoki, D.; Jobo, T.; Kodama, S.; Terauchi, F.; et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol. 2013, 14, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Paek, J.; Nam, E.J.; Kim, S.H.; Kim, J.H.; Kim, Y.T. The feasibility of carboplatin-based intraperitoneal chemotherapy in ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 152, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Casares, F.; Rufián, S.; Rubio, M.; Díaz, C.; Díaz, R.; Casado, A.; Arjona, A.; Muñoz-Villanueva, M.; Muntané, J. The role of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) in the treatment of peritoneal carcinomatosis in recurrent ovarian cancer. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2009, 11, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Lee, J.M.; Ryu, K.S.; Lee, Y.S.; Park, Y.G.; Hur, S.Y.; Ahn, W.S.; Namkoong, S.E. Treatment of ovarian cancer with paclitaxel- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol. Oncol. 2007, 106, 193–200. [Google Scholar] [CrossRef]
- Markman, M.; Kennedy, A.; Webster, K.; Peterson, G.; Kulp, B.; Belinson, J. Combination chemotherapy with carboplatin and docetaxel in the treatment of cancers of the ovary and fallopian tube and primary carcinoma of the peritoneum. J. Clin. Oncol. 2001, 19, 1901–1905. [Google Scholar] [CrossRef]
- Somashekhar, S.; Yethadka, R.; Kumar, C.R.; Ashwin, K.; Zaveri, S.; Rauthan, A. Toxicity profile of chemotherapy agents used in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2020, 46, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, D.; Lee, Y.; Ha, H.; Chang, Y.; Chang, S.; Park, S.; Lim, M. Quality of life outcomes from the randomized trial of hyperthermic intraperitoneal chemotherapy following cytoreductive surgery for primary ovarian cancer (KOV-HIPEC-01). J. Gynecol. Oncol. 2022, 33, e54. [Google Scholar] [CrossRef]





| Study | Study Year | Stage | Study Design | Group | Number | Age, Years | OS HR (95% CI) | PFS HR (95% CI) | Follow-Up (Months) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2022 | Clamp AR | 2011–2014 | IC–IV EOC | RCT | IV | 522 | 63 (55–68) | 0.87(0.73–1.05) | 0.92 (0.78–1.09) | 84 |
| [14] | DD | 523 | 61 (54–67) | ||||||||
| 2 | 2022 | Lim MC | 2010–2016 | III/IV EOC | RCT | HIPEC1 | 92 | 52.0 (46–59.5) | 0.87 (0.58–1.32) | 0.88 (0.63–1.21) | 69.4 |
| [15] | IV | 92 | 53.5 (47.5–61) | ||||||||
| 3 | 2022 | Antonio CCP | 2018–2020 | III/IV EOC | RCT | HIPEC1 | 35 | 56 (29–75) | 0.78 (0.32–1.88) | NA | 60 |
| [16] | IV | 36 | 65.5 (40–75) | ||||||||
| 4 | 2021 | He X | 2012–2017 | III EOC | retrospective study | HIPEC1 | 121 | 58.38 | 0.52 (0.35–0.78) | NA | 90 |
| [17] | IV | 76 | 55.2 | ||||||||
| 5 | 2021 | Gruner M | 2017–2020 | III/IV HGSOC | cohort study | HIPEC3 | 21 | 63.1 ± 9.2 | NA | 0.69 (0.36, 1.38) | 36 |
| [18] | IV | 40 | 66.3 ± 9.5 | ||||||||
| 6 | 2021 | Manning-Geist BL | 2010–2014 | IIIC EOC | retrospective study | IVIP (miliary) | 41 | 0.33 (0.18–0.61) | 0.28 (0.15–0.53) | >80 | |
| [19] | IV (miliary) | 49 | |||||||||
| IVIP (nonmiliary) | 23 | 1.47 (0.7–3.09) | 1.53 (0.74–3.19) | ||||||||
| IV (nonmiliary) | 55 | ||||||||||
| 7 | 2021 | Kim SR | 2001–2015 | IIIC/IV HGSOC | retrospective study | IVIP | 91 | 55.1 ± 10.02 | 0.52 (0,36–0.73) | 1.05 (0,79–1.4) | >120 |
| [20] | IV | 180 | 59.8 ± 11.3 | ||||||||
| 8 | 2020 | Lei Z | 2010–2017 | III EOC | retrospective study | HIPEC1 | 425 | 55.1 | 0.64(0.50–0.82) | NA | 60 |
| [21] | IV | 159 | 54.6 | ||||||||
| 9 | 2020 | Ting WH | 2006–2019 | II–IV OC | retrospective study | IVIP | 22 | 52.6 ± 7.4 | 0.35 (0.13–0.93) | 0.47 (0.24–0.94) | >50 |
| [22] | DD | 28 | 54.2 ± 8.7 | ||||||||
| 10 | 2020 | Shibutani T | 2006–2015 | advanced EOC | retrospective study | DD | 101 | 61 (35–79) | 0.72(0.48–1.06) | 0.69 (0.46–0.96) | >80 |
| [23] | IV | 70 | |||||||||
| 11 | 2020 | Murphy M | 2010–2018 | III OC | retrospective study | IVIP | 44 | 63 (35–81) | 0.81 (0.24–2.75) | 0.76 (0.45–1.27) | >80 |
| [24] | DD | 38 | 63 (35–87) | ||||||||
| 12 | 2019 | Rettenmaier M | 2008–2015 | AOC | retrospective study | HIPEC2 | 64 | 59.9 | 0.72(0.33–1.56) | 0.71 (0.44–1.14) | >80 |
| [25] | IVIP | 81 | 57.8 | 1.11(0.74–1.66) | 0.97 (0.69–1.36) | ||||||
| DD | 100 | 62.9 | |||||||||
| 13 | 2019 | K. Bixel | 2004–2017 | III–IV OC | retrospective study | IVIP | 37 | 59.7 (40–81) | 1.22 (0.77–1.92) | 0.71 (0.46–1.10) | 60 |
| [26] | IV | 97 | 66.3 (21–87) | ||||||||
| 14 | 2018 | Lee J | 2006–2015 | III/IV EOC | retrospective study | IDS + IVIP | 42 | 60.0 (35–76) | 0.61 (0.17–2.22 | 0.79 (0.38–1.64) | >60 |
| [27] | IDS + IV | 24 | 59.0 (46–86) | ||||||||
| PDS + IVIP | 93 | 54.0 (25–81) | 0.43(0.25–0.72) | 0.48 (0.32–0.74) | |||||||
| PDS + IV | 56 | 61.0 (37–84) | |||||||||
| 15 | 2018 | Ceresoli M | 2010–2016 | advanced EOC | retrospective study | HIPEC3 | 28 | 58.99 | 0.73 (0.33–1.60) | 1.41 (0.73–2.73) | 80 |
| [28] | IV | 49 | 63.48 | ||||||||
| 16 | 2018 | van Driel WJ | 2007–2016 | III EOC | RCT | HIPEC1 | 123 | 63 (56–66) | 0.67(0.48–0.94) | NA | 56.4 |
| [29] | IV | 122 | 61 (55–66) | ||||||||
| 17 | 2017 | Mendivil AA | 2008–2015 | AOC | retrospective study | HIPEC2 | 69 | 59.8 | 0.84(0.38–1.84) | 2.10 (1.29–3.42) | >50 |
| [30] | IV | 69 | 62.9 | ||||||||
| 18 | 2017 | Miller EM | 2005–2016 | III/IV OC | retrospective study | IVIP | 49 | 56 ± 12 | 0.31 (0.16–0.62) | NA | 120 |
| [31] | IV | 54 | 58 ± 12 | ||||||||
| 19 | 2017 | Eoh KJ | 2006–2008 | III/IV EOC | retrospective study | CIS-IVIP | 21 | 53 (37–72) | 1.18 (0.46–3.04) | 0.96 (0.52–1.79) | 120 |
| [32] | CAR-IVIP | 16 | 52 (41–65) | 1.7 (0.82–3.54) | 1.41 (0.73–2.73) | ||||||
| IV | 121 | 58 (22–82) | |||||||||
| 20 | 2017 | Provencher DM | IIB–IVA EOC | RCT | CIS-IVIP | 102 | 62 (40–82) | 0.73 (0.57–0.93) | 0.77 (0.59–1.02) | 50 | |
| [33] | CAR-IVIP | 61 | 64.9 ± 10.5 | 0.78 (0.61–1.01) | 0.89 (0.69–1.13) | ||||||
| IV | 72 | 61 (29–78) | |||||||||
| 21 | 2017 | Sioulas VD | 2001–2010 | III EOC | retrospective study | IVIP | 61 | 64.9 ± 10.5 | 0.82 (0.54–1.25) | NA | 53 |
| [34] | IV | 120 | 57.9 ± 9.8 | ||||||||
| 22 | 2017 | Nicoletto MO | 2006–2015 | AOC | retrospective study | IP | 33 | 62.6 | 0.90 (0.65–1.24) | NA | 40 |
| [35] | IV | 66 | 66.7 | ||||||||
| 23 | 2016 | Mueller JJ | 2008–2013 | III–IV OC | retrospective study | IVIP | 48 | 60 (34–76) | 1.07 (0.55–2.09) | 0.93 (0.6–1.45) | >60 |
| [36] | DD | 17 | 64 (36–80) | 1.25 (0.47–3.37) | 1.92 (1.0–3.96) | ||||||
| IV | 63 | 66 (38–86) | |||||||||
| 24 | 2016 | Cascales-Campos P | 2008–2015 | III/IV EOC | retrospective study | HIPEC4 | 60 | 59.43 ± 1.4 | 0.59(0.17–2.03) | NA | >80 |
| [37] | HIPEC1 | 51 | 60.53 ± 1.5 | ||||||||
| 25 | 2014 | Al Mutairi NJ | 2007–2009 | III/IV EOC | retrospective study | IVIP | 16 | 0.74 (0.03–17.44) | 0.91 (0.24–3.44) | 26.2 | |
| [38] | IV | 18 | |||||||||
| 26 | 2014 | Pignata S | 2008–2012 | IC–IV EOC | RCT | IV | 522 | 63 (55–68) | 0.87 (0.73–1.05) | 0.92 (0.78–1.09) | 69 |
| [39] | DD | 523 | 61 (54–67) | ||||||||
| 27 | 2014 | Yoon JY | 2003–2012 | III EOC | retrospective study | IP | 37 | 0.03 (0.01–145.55) | NA | 60 | |
| [40] | IV | 26 | |||||||||
| 28 | 2013 | Katsumata N | 2003–2005 | II–IV OC | RCT | DD | 312 | 0.79 (0.63–0.99) | 0.76 (0.62–0.91) | 76.8 | |
| [41] | IV | 319 | |||||||||
| 27 | 2010 | Kim SW | 2006–2007 | OC | retrospective study | IVIP | 19 | 54 ± 14 | 1.82 (0.57–5.77) | NA | 50 |
| [42] | IV | 34 | 52 ± 13 | ||||||||
| 29 | 2009 | Francisco C | 1997–2004 | III EOC | retrospective study | HIPEC4 | 14 | 54 (28–68) | 1.12 (0.23–5.82) | NA | >60 |
| [43] | IV | 12 | 54 (30–67) | ||||||||
| 30 | 2007 | Bae JH | 1995–2004 | IC-IIIC EOC | retrospective study | HIPEC2 | 45 | 50.1 ± 12.4 | 0.34 (0.24–0.66) | 0.44 (0.24–0.81) | >120 |
| [44] | HIPEC4 | 22 | 49.5 ± 8.6 | 0.20 (0.05–0.82) | 0.28 (0.12–0.67) | ||||||
| IV | 29 | 50.0 ± 11.7 | |||||||||
| 31 | 2006 | Armstrong DK | 1998–2001 | III EOC | RCT | IV | 210 | 0.75 (0.58–0.97) | 0.80 (0.64–1.00) | 60 | |
| [8] | IVIP | 205 | |||||||||
| 32 | 2001 | Markman M | 1992–1995 | III OC | RCT | IVIP | 235 | 0.87 (0.67–1.14) | 0.77 (0.62–0.95) | 60 | |
| [45] | IV | 227 | |||||||||
| Study | Year | Selection | Comparability | Assessment of Outcome | Follow-Up | Adequacy of Follow-Up | Scores | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||||
| e X | 2021 | * | * | * | * | * | * | * | * | 8 |
| Gruner M | 2021 | * | * | * | * | * | * | * | 7 | |
| Manning-Geist BL | 2021 | * | * | * | * | * | * | * | * | 8 |
| Kim SR | 2021 | * | * | * | * | * | * | * | 7 | |
| Lei Z | 2020 | * | * | * | * | * | * | * | * | 8 |
| Ting WH | 2020 | * | * | * | * | * | * | * | * | 8 |
| Shibutani T | 2020 | * | * | * | * | * | * | * | * | 8 |
| Murphy M | 2020 | * | * | * | * | * | * | * | * | 8 |
| Mark A | 2019 | * | * | * | * | * | * | * | * | 8 |
| K. Bixel | 2019 | * | * | * | * | * | * | * | 7 | |
| Lee J | 2018 | * | * | * | * | * | * | * | 7 | |
| Ceresoli M | 2018 | * | * | * | * | * | * | * | 7 | |
| Mendivil AA | 2017 | * | * | * | * | * | * | * | * | 8 |
| Miller EM | 2017 | * | * | * | * | * | * | * | * | 8 |
| Eoh KJ | 2017 | * | * | * | * | * | * | * | * | 8 |
| Mueller JJ | 2017 | * | * | * | * | * | * | * | * | 8 |
| Sioulas VD | 2017 | * | * | * | * | * | * | * | * | 8 |
| Nicoletto MO | 2017 | * | * | * | * | * | * | * | * | 8 |
| Cascales-Campos P | 2016 | * | * | * | * | * | * | * | * | 8 |
| Yoon JY | 2014 | * | * | * | * | * | * | * | * | 8 |
| Al Mutairi NJ | 2014 | * | * | * | * | * | * | * | * | 8 |
| Kim SW | 2010 | * | * | * | * | * | * | * | 7 | |
| Francisco C | 2009 | * | * | * | * | * | * | * | * | 8 |
| Bae JH | 2007 | * | * | * | * | * | * | * | * | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Huang, M.; Zhang, J.; Huang, Z.; Wang, L.; Gong, Z.; Tang, L. Comparative Survival Outcomes of Hyperthermic Intraperitoneal Chemotherapy, Intraperitoneal Chemotherapy and Intravenous Chemotherapy for Primary Advanced Ovarian Cancer: A Network Meta-Analysis. J. Clin. Med. 2023, 12, 1111. https://doi.org/10.3390/jcm12031111
Tang Q, Huang M, Zhang J, Huang Z, Wang L, Gong Z, Tang L. Comparative Survival Outcomes of Hyperthermic Intraperitoneal Chemotherapy, Intraperitoneal Chemotherapy and Intravenous Chemotherapy for Primary Advanced Ovarian Cancer: A Network Meta-Analysis. Journal of Clinical Medicine. 2023; 12(3):1111. https://doi.org/10.3390/jcm12031111
Chicago/Turabian StyleTang, Qin, Mao Huang, Jing Zhang, Zhen Huang, Linlian Wang, Zhengxin Gong, and Liangdan Tang. 2023. "Comparative Survival Outcomes of Hyperthermic Intraperitoneal Chemotherapy, Intraperitoneal Chemotherapy and Intravenous Chemotherapy for Primary Advanced Ovarian Cancer: A Network Meta-Analysis" Journal of Clinical Medicine 12, no. 3: 1111. https://doi.org/10.3390/jcm12031111
APA StyleTang, Q., Huang, M., Zhang, J., Huang, Z., Wang, L., Gong, Z., & Tang, L. (2023). Comparative Survival Outcomes of Hyperthermic Intraperitoneal Chemotherapy, Intraperitoneal Chemotherapy and Intravenous Chemotherapy for Primary Advanced Ovarian Cancer: A Network Meta-Analysis. Journal of Clinical Medicine, 12(3), 1111. https://doi.org/10.3390/jcm12031111






